Abstract
This open-label, randomized phase II trial was performed to compare the efficacy and safety of nimotuzumab plus S-1 and cisplatin (NCS) versus S-1 and cisplatin (CS) alone in patients with untreated unresectable or metastatic gastric cancer in the first-line setting.
Eligible participants were randomly assigned (1:1) to receive either NCS or CS. The treatment consisted of 3-week cycles of twice-daily S-1 40 mg/m2 (on days 1–14) and intravenous cisplatin 30 mg/m2 (on days 1, 2), with or without weekly nimotuzumab (200 mg/m2). The primary endpoint was objective response rate (ORR). The second endpoint included progression-free survival (PFS), overall survival (OS), safety and association between efficacy and tumor epidermal growth factor receptor (EGFR) expression.
Between October, 2009, and February, 2012, we enrolled 62 patients in Cancer Hospital Chinese Academy of Medical Sciences (CAMS). The ORR for 31 patients allocated NCS was 54.8% compared with 58.1% for 31 patients who were allocated to receive CS alone (P = 0.798). Median PFS for patients in CS arm was significantly improved than that in NCS arm [7.2 months vs. 4.8 months HR = 2.136 (95% CI 1.193–3.826), P = 0.011]. There was also a trend toward better overall survival for patients in CS arm compared with NCS arm [14.3 months vs. 10.2 months; HR = 1.776 (95% CI 0.972–3.246), P = 0.062]. In the EGFR 2+/3+ subgroup, adding nimotuzumab also failed to show additional benefit than chemotherapy alone. Both groups were well tolerated. Less than 10% of patients in both arms developed grade 3/4 toxicity.
Combination of nimotuzumab and S-1-cisplatin provided no additional benefit than chemotherapy alone in the first-line treatment of unresectable or metastatic gastric cancer.
INTRODUCTION
Gastric and oesophageal cancers are one of the most common causes of cancer-related death worldwide. For patients with locally unresectable or metastatic disease, although combination chemotherapy improves the prognosis, which extended the median overall survival from 3–4 months with best supportive care to about 10–13 months,1,2 the 5-year survival of patients is still less than 10%, and therefore a high and unmet require exists for more effective treatment. Currently, there is no universally accepted standard treatment for advanced gastric cancer while several combination regimens have been used as first-line treatment including cisplatin-S-1,3 cisplatin–capecitabine,4 and epirubicin–oxaliplatin–capecitabine.2
To improve the poor outcomes, addition of molecular-targeted drugs to chemotherapies with acceptable toxicities is necessary. Epidermal growth factor receptor (EGFR) has been identified to be expressed in various tumors.5 Approximately 20%–30% of gastric cancers are reported to show EGFR over-expression. Several studies have demonstrated that EGFR positive was associated with unfavorable prognosis for patients with gastric cancer.6,7 However, this correlation was not observed in a recent study.8 Moreover, EGFR signaling pathways is frequently disordered in gastric cancer, thereby considering as candidate therapeutic targets.
However, two prospective randomized phase III studies (EXPAND, REAL-3) failed to prove that adding anti-EGFR agents such as cetuximab and panitumumab to chemotherapy could improve clinical outcome, neither response rate, PFS nor OS, in patients with advanced or metastatic gastric cancer. Exact reasons underlying such results are unclear and it is assumed that the negative synergistic effect between anti-EGFR agents and capecitabine may be responsible for the disappointing results.9,10
In addition to capecitabine, S-1 is an oral anticancer agent that combines tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP), and potassium oxonate (Oxo) at a molar ratio of 1:0.4:1 that concurrently has dual actions, that is, effect-enhancing activity and adverse reaction-reducing activity. FT is a metabolically activated prodrug of 5-FU. CDHP, as the first modulator, reinforced the pharmacological activity of 5-FU by restraining its degradation. The second modulator, Oxo, localizing in mucosal cells of the gastrointestinal tract after oral administration, reduces the digestive toxicities by inhibiting the activity of 5-FU in the gastrointestinal tract.11 Phase III clinical trials showed that S-1 was noninferior to intravenous 5-FU,12 and that cisplatin plus S-1 was superior to S-1 monotherapy.3 Therefore, CS regimen is considered a standard first-line treatment for AGC in many Asian countries including Japan, Korea, and China.
Nimotuzumab is another recombinant humanized monoclonal antibody against human EGFR, which is diverse from cetuximab and panituzumab in the following aspects. First, the parental antibody of nimotuzumab was produced from BALB/c mice immunized with a purified placenta fraction enriched in EGFR. Moreover, nimotuzumab demonstrates different pharmacokinetic trait compared with other anti-EGFR antibodies, which displays a prolonged half-life and elevated area under the curve than cetuximab at the same dose level.13 Besides, safety data showed that nimotuzumab rarely caused severe dermatological toxicity, which is the most common adverse events resulting from cetuximab and panitumumab, and therefore, it is expected to improve the quality of life.14
Randomized studies have demonstrated that nimotuzumab, combined with irradiation or chemoradiotherapy, could provide an improvement in prognosis to patients with head and neck cancer,14 gliomas,15 and esophagus squamous cell carcinoma.16 Additionally, a noticeable synergistic antitumor effect of combined anti-EGFR antibodies and S-1 was observed in gastric cancer cell lines overexpressing EGFR.17,18
Therefore, we conduct the phase 2 randomized clinical trial to assess the efficacy and safety of addition nimutuzumab to S-1 and cisplatin in patients with locally advanced unresectable or metastatic gastric adenocarcinoma in the first-line setting.
METHODS
Study Design
This was a prospective, open-label, randomized phase II clinical trial carried out in the Cancer Hospital and Institute, Chinese Academy of Medical Sciences. Eligible patients with unresectable locally advanced or metastatic cancer adenocarcinoma in gastric or gastroesophageal junction (GEJ) without prior treatment for metastatic disease were randomized (1:1) to receive either CS regimen (cisplatin: 30 mg/m2 iv. infusion on days 1, 2 plus S-1: 40 mg/m2 bid 2 weeks on,1week off) or NCS regimen (cisplatin: 30 mg/m2 iv. infusion on days 1, 2 plus S-1: 40 mg/m2 bid, 2 weeks on, 1 week off plus nimotuzumab 200 mg infusion on days 1, 8, 15) every 3 weeks until disease progression, unacceptable toxicity, or patient consent withdrawal.
In both treatment groups, the dose of each drug was reduced to approximately 80%, if the neutrophil count was <500/mm3, the platelet count was <25,000/mm3 or ≥grade 3 febrile neutropenia, diarrhea, stomatitis developed. The dose of cisplatin was reduced in the event of grade 3 anorexia suspected to be caused by cisplatin.
Randomisation was done centrally with an interactive voice response system. We used a stratified, permuted, block randomisation procedure (variable block size) with the following strata: age (<60 vs. ≥60 years); Eastern Cooperative Oncology Group (ECOG) performance (0 vs. 1); primary tumor location (GEJ vs. gastric); disease stage (M0 vs. M1). EGFR expression level (2+/3+ vs. 0+/1+)
The primary end point was to evaluate and compare the ORR between the 2 groups; the secondary end points included PFS, OS and safety. Image examination such as computed tomography scanning or magnetic resonance imaging was required every 4 weeks during treatment and each 2 months following discontinuation of study treatment. Tumor response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 guidelines. Adverse events were graded in accordance with the National Cancer Institute Common Toxicity Criteria for adverse events, version 3.0 (NCI-CTCAE 3.0). This clinical trial was performed following the Declaration of Helsinki and approved by the ethical committee (Approval No. 11-16/451). All the patients have signed informed consent. The study is registered with ClinicalTrials.gov, number NCT02370849. All the data were kept at Cancer Hospital Chinese Academy of Medical Sciences.
Patient Selection
Eligible patients were aged ≥18 years with histologically or cytologically confirmed advanced or metastatic gastric or GEJ junction adenocarcinoma. Patients had to have at least 1 measurable lesion, which has not been exposed to radiotherapy previously. Patients should not receive prior systemic chemotherapy in metastatic settings, while previous adjuvant chemotherapy was allowed. Additional eligibility criteria included ECOG performance status 0–1, adequate organ function, life expectancy >3 months, no concurrent uncontrolled medical condition, no other active malignancy, no known brain metastasis and no history of allergic reactions to nimotuzumab, cisplatin, leucovorin, or 5-FU.
Exploratory Biomarker Analysis
EGFR protein expression analysis was conducted in tumor specimens from patients who had provided informed consent for further biomarker exploration, with the immunohistochemistry (IHC) staining kit (EGFR PharmDX; Dako, Copenhagen, Denmark). The tumor tissues were tested collectively and then classified into 4 groups (0, 1+, 2+, and 3+).
Statistical Considerations
PFS was defined as the time from the start of the treatment until disease progression or death. OS was calculated from the date of first administration of treatment to death by any cause or censored at the last date the patient was known to be alive. When the study was designed, a 22.4% increase of response rate was expected in NCS group compared with CS group. To detect the difference between groups with a power of 80% and 2-sided significance level at 0.05, at least 75 patients were needed in each group. Patients’ characteristics and response rates of different regimens were compared with χ2 test or Fisher exact test. Median PFS, OS were all estimated by the Kaplan–Meier method compared by log-rank test. In exploratory analyses, subgroup efficacy and multivariate analyses were performed on stratification factors and demographic factors in the Cox proportional hazards model.
RESULTS
Patients’ Characteristics
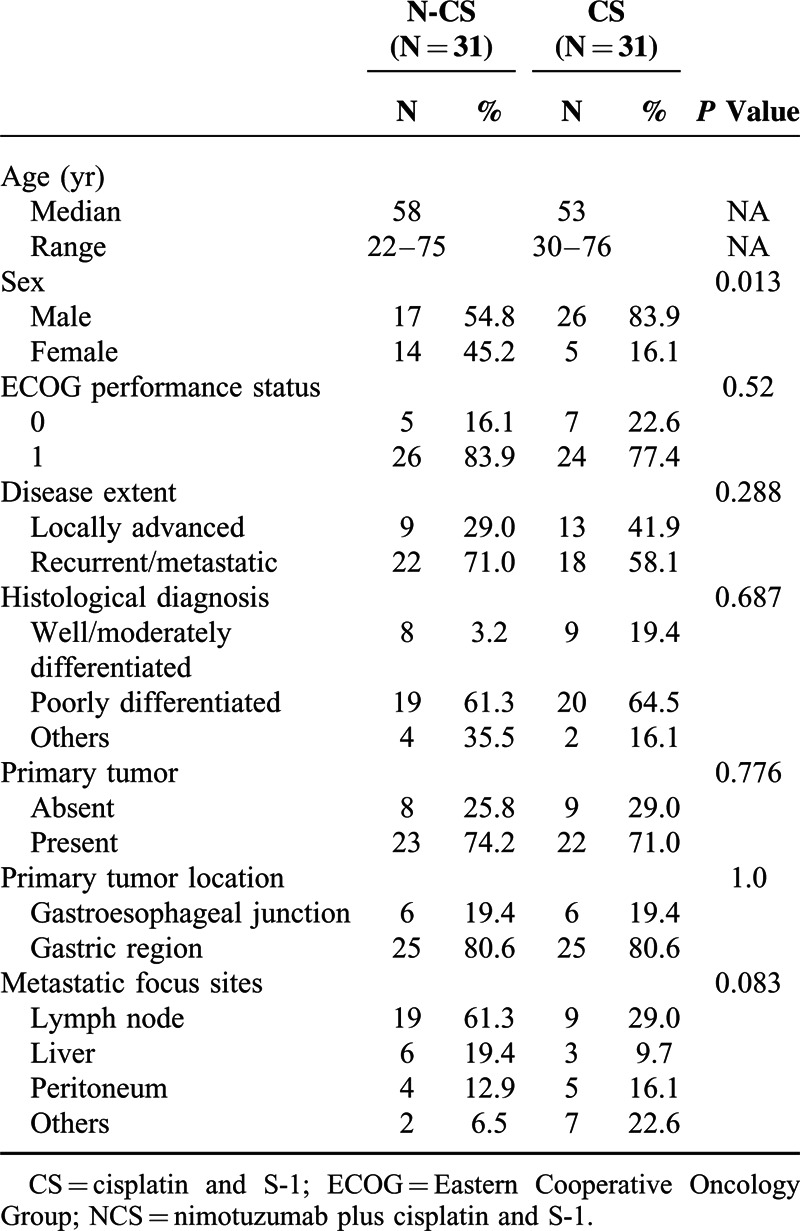
A total of 64 patients were randomized from October 2009 to February 2012. All the patients belonged to Chinese Han population. Of these patients, 62 patients were included in the efficacy and safety analysis set (2 patients in CS group were excluded because they did not receive S-1). At the 32-month follow-up after registration of the last patient for the study, the median treatment exposure in the NCS group and CS group was 84 days (range, 42–210) and 84 days (range, 21–168), respectively. Generally, the baseline characteristics such as age, ECOG performance status, histological grade, and primary tumor status were well balanced between 2 groups. Significantly, more male were enrolled in CS arm than that in NCS arm (83.9% vs. 54.8%, P = 0.013). Besides, with regard to the disease extent, more patients in NCS arm had metastatic disease than CS arm (71.0% vs. 58.1%, P = 0.288) (Table 1).
TABLE 1.
Baseline Characteristics of Patients
Of the 62 patients, 27 patients had provided informed consent for exploratory biomarker analysis and submitted tumor samples. The EGFR protein expression level was detected in the assessable tumor tissues of 27 patients (43.5% of the full analysis set population), with 16 patients classified as 0+, 4 patients as 1+, 4 patients as 2+, and 3 patients as 3+.
Treatment Delivery
The relative dose intensity was 82.4% [interquartile range (IQR), 64.3–100] for cisplatin, 90.5% (IQR 78.5–100) for S-1 and 95.1% (IQR 84.1–100) for nimotuzumab in NCS; it was 81.7% (IRQ 68.6–100) for cisplatin and 91.8% (IQR 76.3–100) for S-1 in CS.
In NCS, 18 of 31 (58.1%) patients who discontinued treatment received second-line chemotherapies: taxanes-containing regimens in 8/18 (44.4%) patients, irinotecan-containing regimens in 12/18 (66.7%) patients, and epirubicin-containing regimens in 5/18 (27.8%) patients. In CS, 16/31 (51.6%) patients who discontinued treatment received second-line chemotherapies: taxanes-containing regimens in 6/16 (37.5%) patients, irinotecan-containing regimens in 10/16 (62.5%) patients, and epirubicin-containing regimens in 3/16 (18.8%) patients.
Efficacy
A total of 62 patients (31 in NCS group vs. 31 in CS group) were evaluable for radiologic tumor responses. As shown in Table 2, there was no significant difference in ORR or disease control rate (DCR) at the 32-month follow-up between the treatment groups (ORR, 54.8% in the NCS group vs. 58.1% in the CS group P = 0.798; DCR, 90.3% in the NCS group vs. 90.3% in the CS group P = 1.0). In addition, CS regimen significantly prolonged the median PFS versus NCS regimen [7.2 months vs. 4.8 months; HR = 2.136 (95% CI 1.193–3.826), P = 0.011] (Figure 1A). Also, a trend toward better OS favoring CS group compared with NCS group was observed, although there was not statistical significance between treatment groups. [14.3 months vs. 10.2 months; HR = 1.776 (95% CI, 0.972–3.246), P = 0.062] (Figure 1B).
TABLE 2.
Response Rate
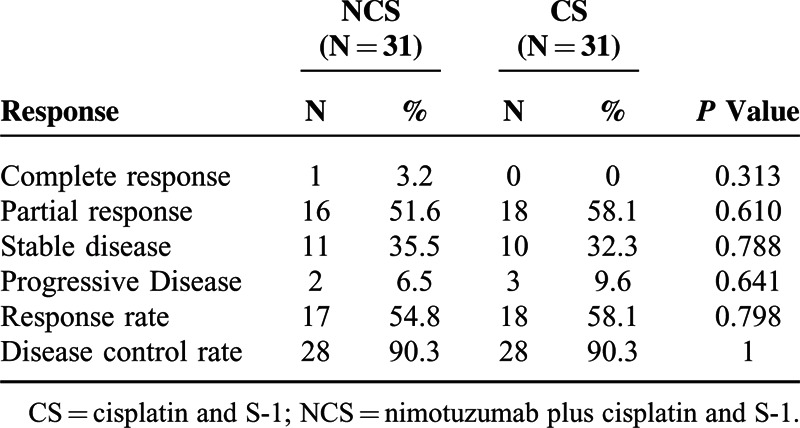
FIGURE 1.
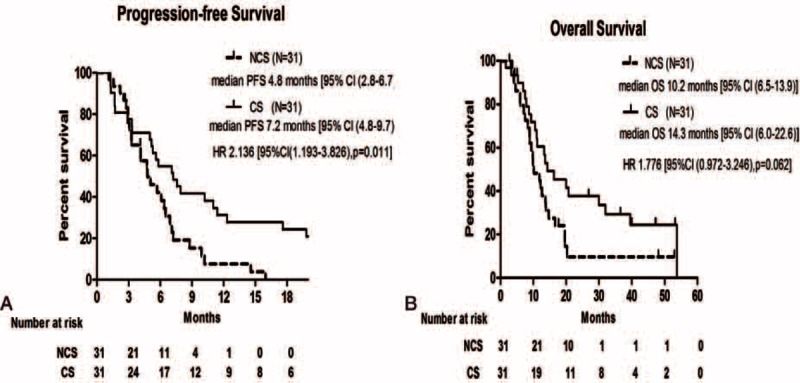
Kaplan–Meier estimates of progression-free survival (A) and overall survival (B) in the intention-to-treat population.
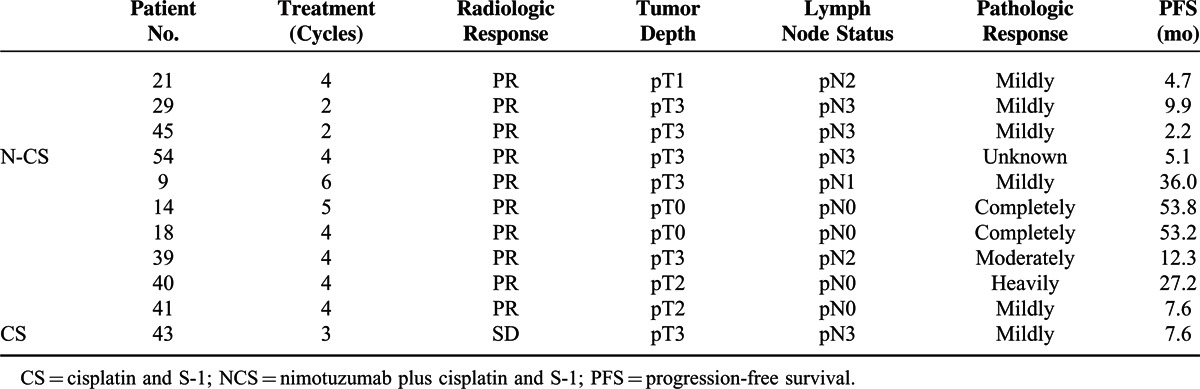
A total of 11 patients underwent surgery after systemic treatment (4 in NCS arm, 7 in CS arm, respectively). By radiological assessment, patients in both arms achieved high response rate (100% [4/4] in NCS arm, 85.7% [6/7] in CS arm). However, by the pathologic assessment of an independent pathologist, 75% (3/4) of patients in NCS group was evaluated as mild response while in CS group the overall pathologic response rate was 100% (7/7), especially including 2 patients with complete pathologic response. This superiority in pathologic response also transferred into survival advantage of CS arm over NCS arm in this subset of patients (median PFS, 4.7m in NCS arm vs. 27.2m in CS arm) (Table 3).
TABLE 3.
Characteristics and Outcomes of Patients Undergoing Surgery
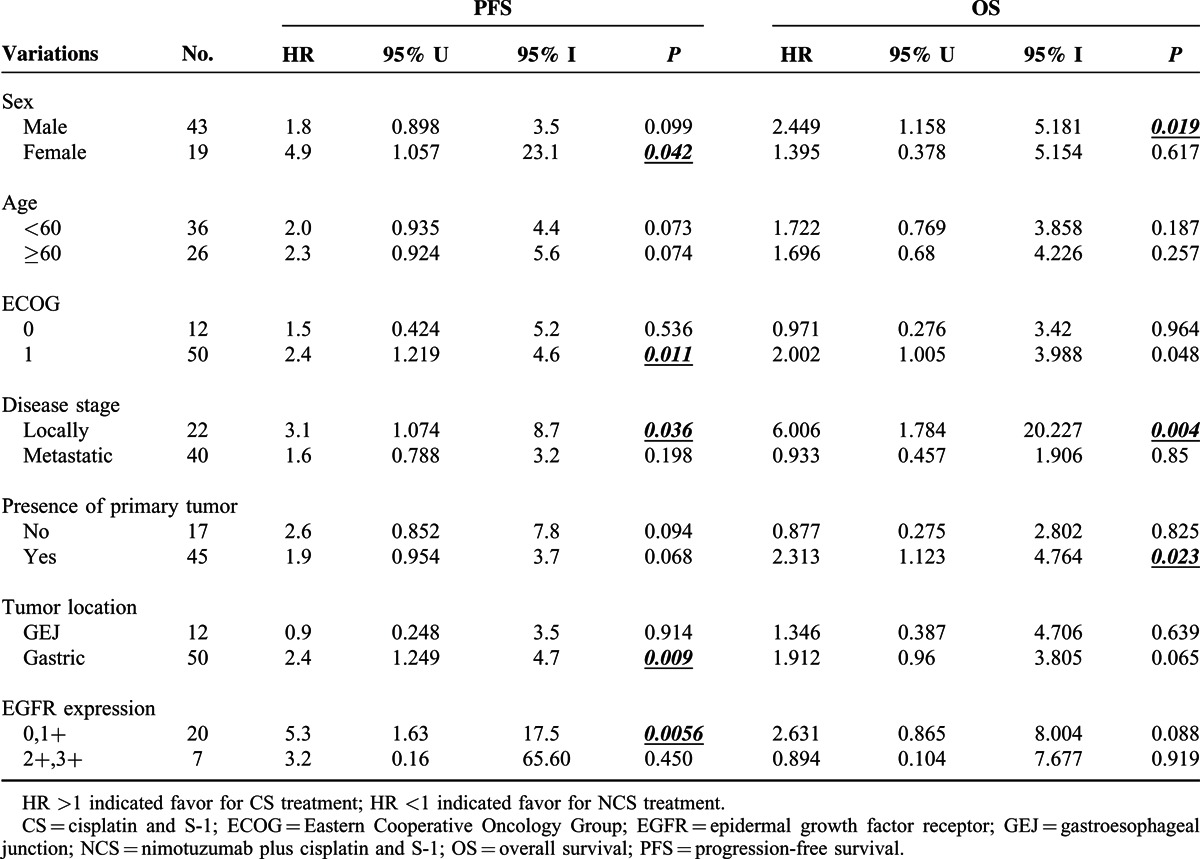
In general, we noted a consistent trend toward a better PFS or OS in the CS group compared with NCS group, in an analysis of subgroups based on predefined factors. In the EGFR 2+/3+ subgroup, adding nimotuzumab did not provide additional benefit to the CS combination. The median PFS was 7.735 months for 6 patients in the CS arm versus 5.07 months for 1 patient in the NCS arm. (HR = 3.203, 95% CI 0.156–65.6, P = 0.449). In addition, the median OS was 10.85 months for 6 patients in the CS arm versus 13.7 months for 1 patient in the NCS arm (HR = 0.894, 95% CI 0.104–7.677, P = 0.919). On the other hand, patients with locally advanced gastric cancer seemed to have significantly benefited in terms of PFS and OS after receipt of S-1 and cisplatin versus S-1, cisplatin plus nituzumab (Table 4).
TABLE 4.
Summary of Progression-Free Survival and Overall Survival in a Prespecified Subgroup Analysis of the Intention-to-Treat (ITT) Population
Safety
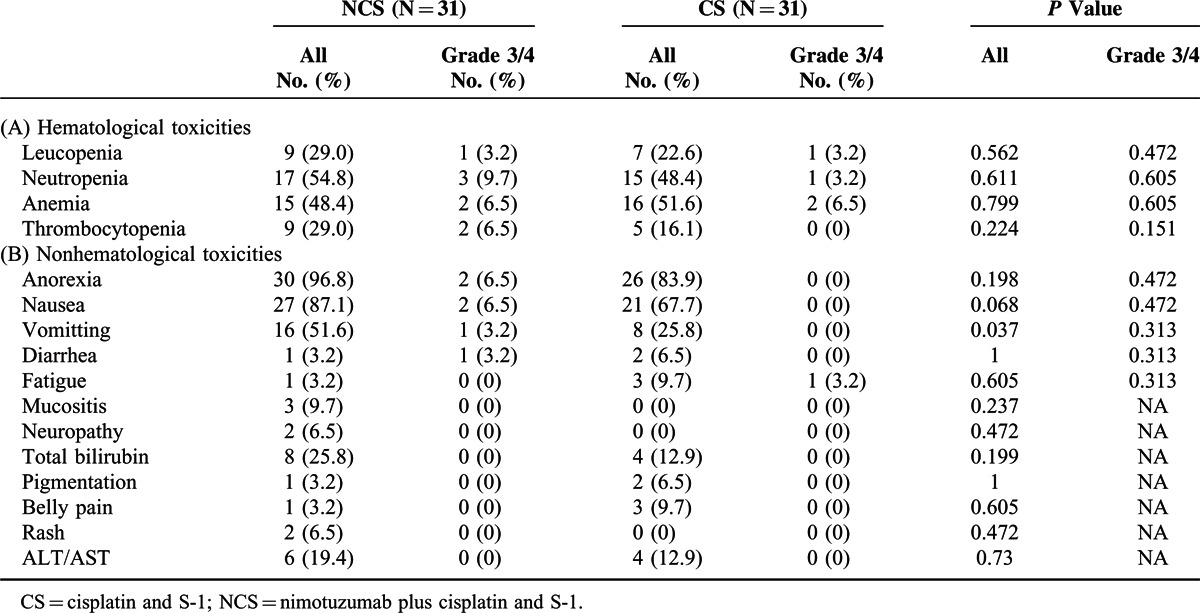
Adverse events were reported in all 62 patients. Table 5 shows the incidence, by treatment group, of main adverse events occurring. The most common adverse events (>50% in at least 1 group) were anorexia, nausea, neutropenia, vomiting, and anemia. Significantly more vomiting of all grades was observed in NCS group compared with CS group (51.6% vs. 25.8%, P = 0.037) but no statistically difference was detected in Grade 3/4 vomiting (3.2% in NCS vs. 0 in CS, P = 0.313). A rash occurred in 6.5% (2/31) and 0 % (0/31) of patients in the NCS and CS groups, respectively. There were no cases with severe (≥grade 3) skin toxicity, including severe acne-like rash. The most common grade 3 or higher adverse events were neutropenia, nausea, anorexia, anemia, and thrombocytopenia. Blood transfusion was required by 2 patients (1 in NCS arm, 1 in CS arm) due to grade 3 anemia. The incidence of adverse events causing in discontinuation or dose reduction of cisplatin was 9.7% (3/31) in the NCS group and 3.2% (1/31) in the CS group, with no significant difference between the 2 groups. The incidence of adverse events resulting in discontinuation of nimotuzumab was 3.2% (1/31) in the NCS treatment.
TABLE 5.
Adverse Events in the Safety Population
DISCUSSION
The present work is the first randomized study assessing the efficacy and toxicity of adding nituzumab to cisplatin and S-1, which was commonly adapted in Asian patients with advanced or metastatic gastric adenocarcinoma in the first-line setting. It is also one of randomized clinical trials assessing the combination of anti-EGFR monoclonal antibodies and chemotherapy in first-line for esophagogastric cancer. Based on the findings of our work, giving nimotuzumab in combined with CS was not supposed to be recommended in an unselected population with advanced or metastatic GEJ or gastric adenocarcinoma due to the association with inferior PFS and overall survival. This trial does, however, confirm the efficacy of the CS control group in this setting, with median overall survival and PFS results that are consistent with those previously reported in SPIRITS study (13.0 months for overall survival and 6.0 months for PFS).3
In our work, the median PFS and OS of NCS group was 4.8 months and 10.2 months, respectively. The results were consistent with previous studies that evaluate the efficacy of adding anti-EGFR antibodies to chemotherapy (In EXPAND study, PFS and OS in experimental arm is 4.4 months and 9.4 months, respectively. In REAL3 study, PFS and OS in experimental group is 6.0 months and 8.8 months, respectively),9,10 suggesting a comparable effect of nimuzumab compared with cutuximab and panituzumab when combined with chemotherapy in patients with advanced gastric cancer.
To our knowledge, there is fairly few randomized study aiming to test the synergistic effect between anti-EGFR antibodies and S-1-containing regimens in patients with advanced gastric cancer. The disappointing findings from our work suggested that there may be a negative interaction between nimutuzumab and S-1, which contribute to the detrimental outcome in patients receiving N-CS versus CS alone. This is also in agreement with previous evidence, such as EXPAND and REAL3 study, indicating the combination of anti-EGFR antibodies and oral fluoropyrimidine-based chemotherapy may weaken the antitumor effect in clinical setting.9,10 Similarly, in patients with KRAS wild-type metastatic colorectal cancer, recent randomized studies showed that combination of cetuximab and first-line chemotherapy regimens containing an oral fluoropyrimidine, or bolus fluorouracil does not provide additional benefit in patients versus chemotherapy alone.19,20 The reasons for this effect are unclear, and might be caused by a negative pharmacokinetic interaction between oral fluoropyrimidine and anti-EGFR antibodies.
Recently, cell-line data suggest that greater synergy might exist between anti-EGFR therapy and irinotecan. However, the results of randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer showed that adding nimotuzumab did not provide additional benefit to irrinotecan alone, in terms of PFS, as well as the OS and ORR in the 5-fluorouracil-refractory population.21
It is demonstrated that the percentage of EGFR-positive gastric cancer in Eastern Asia vary greatly depending on the different definition of EGFR-positive. For example, Terashima et al7 retrospectively evaluated EGFR expression in 829 patients with gastric cancer and EGFR positivity was defined as an IHC score of 3+. As a result, the EGFR were positive in 75 (9.0%) patients. On the other hand, Kim et al6 found that EGFR overexpression rate by IHC was 27.4% in a total of 511 patients with gastric cancer, where the EGFR positivity was defined as an IHC score of 2+ and 3+. This is consistent with our data showing a EGFR positive rate of 25.9% (7/27) using the same definition.
EGFR expression was considered one of the candidate predictive factors of anti-EGFR antibody such as nimotuzumab. However, in the subgroup analysis of the present study, EGFR 2+/3+ patients in NCS arm did not obtain additional benefit from nimotuzumab compared with corresponding subgroup in CS arm. It is contrast to the previous study of Satoh T et al21 where patients with higher EGFR expression (EGFR 2+/3+) seemed to achieve improved PFS and ORR after receiving nimotuzumab and irinotecan versus those who received irinotecan alone. It is noticeable that as submission of tumor tissue was not mandatory in both studies, EGFR protein expression was only detected in a small proportion of the full analysis set population. Therefore, the subset analysis based on the EGFR expression level could not yield any conclusive results yet.
As a matter of fact, the role of EGFR expression as a robust predictive biomarker for anti-EGFR antibodies has not been established due to the controversial results from different studies. Preclinical studies have suggested that there is apparent synergistic antitumor effect of combined cetuximab and S-1 in gastric cancer cell lines overexpressing EGFR.18 Besides, it has been well documented that patients with none small cell lung cancer (NCSLC) whose tumor was EGFR antigen positive could achieve additional benefit from combination of cetuximab and chemotherapy.22 Nevertheless, subgroup analysis in EXPAND study failed to distinguish substantial differences for PFS and OS between treatment groups irrespective of EGFR expression score.9 Similarly, in the CRYSTAL and EXTREME studies, EGFR expression level was also not reported to be a clinically useful predictive biomarker in patients with metastatic colorectal cancer and metastatic or recurrent squamous-cell carcinoma of the head and neck.23 Another candidate biomarker, KRAS mutation, has generally accepted as an effective biomarker for predicting the benefit of anti-EGFR antibodies in metastatic colorectal cancer.24,25 However, plenty data showed that KRAS mutation was infrequently detected in human gastric adenocarcinoma (approximately 3%),26,27 and this low prevalence of mutation hinders the further application in clinical practice.
Our toxicity profile was much the same as previous reported: addition of nimotuzumab to S-1 and cisplatin did not produce any unexpected adverse events. Besides, the introduction of nimotuzumab did not cause increasing incidence of skin rash, which was the specific toxicity of cetuximab and petuximab. Vomiting of grade 1/2 was slightly more frequent in the NCS group compared with the CS group but it did not decrease the dose intensity in NCS arm.
Of course, there are some limitations of this open-label, phase II study. The virtual number of patients enrolled was less than that we initially planned. First, the recruit was relatively slow, which was partly attributable to the very high cost of nimotuzumab in China. Second, adding nimotuzumab to CS did not show any advantage than CS arm. What is more, when 62 patients were enrolled, the analysis showed that NCS treatment seemed to even weaken the antitumor effect of CS regimen. Therefore, we decided to stop the recruit ahead of the schedule. Of course, based on the present data, it is not rigorous to conclude that adding nimotuzumab to chemotherapy would impair the benefit of chemotherapy. However, at least, we deemed that it is acceptable to conclude that the addition of nimotuzumab would not provide additional benefit to standard chemotherapy. Another issue is that the status of HER2 was not tested and we could not know its exact influence on our results. However, plenty of data from previous studies showed that tumor HER2 status has neither prognostic nor predictive value for anti-EGFR agents in patients with gastric cancer.9,28 Besides, in the second-line treatment, none of patients in this study received trastuzumab. Therefore, the potential heterogeneous between patients seemed not to impact on comparing efficacy and toxicity between the two arms.
Overall, our data suggested that addition of nimotuzumab to first-line S-1 and cisplatin in unselected patients with advanced or metastatic gastric cancer produced no benefit versus chemotherapy alone. These results were generally unanimous between subgroups. Further understanding of this heterogeneous disease based on molecular biology might be required before promising advantages in treatment outcomes can be expected.
Acknowledgments
All authors were involved in data acquisition or data analysis/interpretation, drafting of the manuscript and/or its critical review, and all read and approved the final version for submission. The work was presented in part at the 2012 American Society of Clinical Oncology, June 1–5, 2012, Chicago, Illinois and the 2011 American Society of Clinical Oncology, June 3–7, 2011, Chicago, Illinois.
Footnotes
Abbreviations: CAMS = Chinese Academy of Medical Sciences, CDHP = 5-chloro-2,4-dihydroxypyridine, CS = cisplatin and S-1, DCR = disease control rate, ECOG = Eastern Cooperative Oncology Group, EGFR = epidermal growth factor receptor, GEJ = gastroesophageal junction, IHC = immunohistochemistry, IQR = interquartile range, NCI-CTCAE = National Cancer Institute Common Toxicity Criteria for Adverse Events, NCSLC = none small cell lung cancer, NCS = nimotuzumab plus cisplatin and S-1, ORR = objective response rate, OS = overall survival, oxo = potassium oxonate, PFS = progression-free survival, RECIST = Response Evaluation Criteria in Solid Tumors.
Funding: Lunan Pharmaceutical Group Corporation and BioTech Pharmaceuticals Co., Ltd.
All authors declare that they have no conflicts of interest.
REFERENCES
- 1.Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993; 72:37–41. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358:36–46. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008; 9:215–221. [DOI] [PubMed] [Google Scholar]
- 4.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009; 20:666–673. [DOI] [PubMed] [Google Scholar]
- 5.Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995; 19:183–232. [DOI] [PubMed] [Google Scholar]
- 6.Kim MA, Lee HS, Lee HE, et al. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 2008; 52:738–746. [DOI] [PubMed] [Google Scholar]
- 7.Terashima M, Kitada K, Ochiai A, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res 2012; 18:5992–6000. [DOI] [PubMed] [Google Scholar]
- 8.Fuse N, Kuboki Y, Kuwata T, et al. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer 2015. [DOI] [PubMed] [Google Scholar]
- 9.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14:490–499. [DOI] [PubMed] [Google Scholar]
- 10.Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 2009; 39:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009; 10:1063–1069. [DOI] [PubMed] [Google Scholar]
- 13.Crombet T, Torres L, Neninger E, et al. Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother 2003; 26:139–148. [DOI] [PubMed] [Google Scholar]
- 14.Crombet T, Osorio M, Cruz T, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 2004; 22:1646–1654. [DOI] [PubMed] [Google Scholar]
- 15.Ramos TC, Figueredo J, Catala M, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther 2006; 5:375–379. [DOI] [PubMed] [Google Scholar]
- 16.Ma NY, Cai XW, Fu XL, et al. Safety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagus. Int J Clin Oncol 2014; 19:297–302. [DOI] [PubMed] [Google Scholar]
- 17.Kobunai T, Watanabe T, Fukusato T. Antitumour activity of S-1 in combination with cetuximab on human gastric cancer cell lines in vivo. Anticancer Res 2011; 31:3691–3696. [PubMed] [Google Scholar]
- 18.Fukuda K, Saikawa Y, Takahashi M, et al. Antitumor effect of cetuximab in combination with S-1 in EGFR-amplified gastric cancer cells. Int J Oncol 2012; 40:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011; 377:2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012; 30:1755–1762. [DOI] [PubMed] [Google Scholar]
- 21.Satoh T, Lee KH, Rha SY, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirker R, Pereira JR, von PJ, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012; 13:33–42. [DOI] [PubMed] [Google Scholar]
- 23.Licitra L, Storkel S, Kerr KM, et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer 2013; 49:1161–1168. [DOI] [PubMed] [Google Scholar]
- 24.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011; 22:1535–1546. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29:2011–2019. [DOI] [PubMed] [Google Scholar]
- 26.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 2010; 102:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moehler M, Mueller A, Trarbach T, et al. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 2011; 22:1358–1366. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Zheng L, Wang Y, et al. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol 2014; 35:5315–5321. [DOI] [PubMed] [Google Scholar]


