Abstract
To investigate the relationship between red blood cell distribution width (RDW) and diurnal corrected QT (QTc) variation in patients with coronary heart disease.
This retrospective study included 203 patients who underwent coronary angiography between February 2013 and June 2014. RDW values and dynamic electrocardiography (Holter) results were collected to investigate the relationship between RDW and diurnal QTc variation.
Patients were separated into three groups (A, B, and C) by binning their RDW values in an ascending order. RDW values, coronary artery scores and diurnal QTc variations were significantly different among these groups (P < 0.05). While coronary artery scores gradually rose with increased RDW, diurnal QTc variation decreased. Pearson's correlation analysis was applied to control for confounding factors, and multiple correlation analysis showed that coronary artery score was positively correlated with RDW (r = 0.130, P = 0.020), while it was not correlated with the diurnal QTc variation (r = −0.226, P = 0.681). RDW was negatively correlated with diurnal QTc variation (r = −0.197, P = 0.035).
RDW is independently associated with diurnal QTc variation in patients with coronary heart disease.
INTRODUCTION
Recent studies have found that red blood cell distribution width (RDW) may be a marker for cardiac risk stratification and outcomes evaluation for coronary heart disease (CHD).1–3 RDW elevation was thought to occur when there is inefficient generation of red blood cells (for example, as a result of deficiencies in iron, vitamin B12 or folic acid) or increased erythrocyte destruction.4 In addition, chronic inflammation is a common cause of elevated RDW. Recent studies have shown that increased RDW is associated with cardiovascular disease. Felker et al5 reported for the first time that RDW is a predictor for adverse prognosis of cardiovascular disease, confirming that RDW value is an independent predictor of mortality in patients with chronic heart failure. Subsequent studies have found that RDW is associated with CHD, and can be used as an indicator of risk stratification and outcomes evaluation.6–8
As with RDW, previous studies have shown diurnal corrected QT (QTc) variation to be an effective indicator for diagnosis and prognosis of myocardial ischemia, and that high QTc variation indicates a high risk for sudden cardiac death.9 The two indicators, RDW and QTc variation, share similarities but also have their own characteristics in the diagnosis and prognostic assessment of CHD. However, the relationship between these indicators has not been reported. This retrospective study aimed at exploring the relationship between the RDW and the diurnal QTc variations in diagnosing and prognosticating CHD. We found that increased RDW is independently associated with diurnal QTc variation in patients with CHD. Possible implications of this finding for RDW testing in the diagnosis and prognosis of CHD are discussed.
PATIENTS AND METHODS
Patients
A total of 203 patients admitted to our hospital due to chest pain or suspected acute coronary syndrome that underwent coronary angiography (CAG) and were diagnosed with CHD between February 2013 and June 2014 were selected for this study. CHD was defined as stenosis of ≥ 50% as seen in CAG in the left main coronary artery, the left anterior descending artery, left circumflex, right coronary artery or their main branches. The difference in each clinical indicator between the two groups was compared. The inclusion criteria were as follows: 1) Sinus rhythm. 2) 24 h dynamic electrocardiography (Holter) records available. Exclusion criteria were as follows: 1) CHD accompanied by arrhythmias or non-sinus arrhythmia affecting QT interval. 2) Medication that might affect QT interval within a 2-week interval prior to electrocardiography. 3) Patients with diseases affecting autonomic nervous function such as diabetes and hyperthyroidism etc. This study was approved by the ethics committee of the affiliated hospital of Taishan Medical College.
METHODS
Clinical and Laboratory Examination
Patients’ clinical and laboratory examination results were collected and entered into a computer database independently by two cardiovascular specialists. The data included patients’ disease histories: age, gender, diabetes, hypertension, hyperlipidemia, smoking, and laboratory results which included: RDW, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, fasting plasma glucose and clinical indicators (severity of coronary disease). For all patients, 3 ml fasting blood was collected from the medial cubital vein and stored in Eppendorf tubes. An automatic hematology analyzer was used to test RDW, and a Hitachi 7170-automatic biochemical analyzer and its corresponding reagents were used to measure fasting plasma glucose, total cholesterol, triglycerides, HDL-C, LDL-C and other indicators. Based on the RWD grouping method by Özcan et al (2013)10 cases were divided into three groups (A, B and C, respectively), by sorting the RDW values in an ascending order.
Dynamic Electrocardiography
Dynamic electrocardiography was conducted on all study participants during hospitalization, and the data were recorded using a MDS 300–4A ECG recorder (DMS Corporation, USA). The playback recordings were performed by professionals using software for advanced dynamic analysis of electrocardiograms by Cardioscan Luxury (Tim software (Beijing) Limited). Artifacts or interference were eliminated manually, and related data were analyzed and calculated. The analysis process strictly followed the single-blind principle, whereby the data analyzers were blinded to the case grouping, and the clinicians were blinded to the data analysis. Diurnal QTc calculations were done automatically by the analysis software, including correction of QT intervals using Bazett's formula (QTc = QTRR). Daytime was set from 06:00 to 22:00 and nighttime from 22:00 to 06:00, and the diurnal QTc variation of each patient was obtained by subtracting mean daytime QTc from mean nighttime QTc.
Coronary Angiography and Interpretation of Results
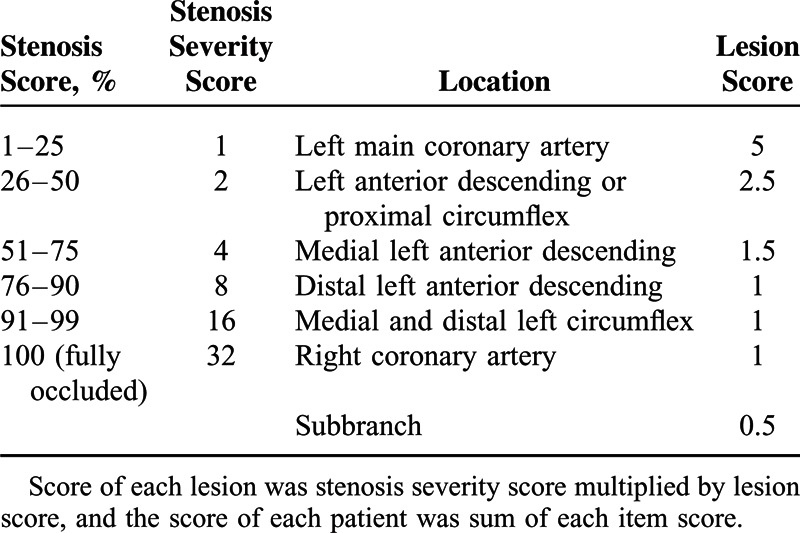
All study participants underwent CAG during hospitalization, in which the Seldinger method for radial artery catheterization was adopted. The CAG procedure and the evaluation of results were performed by two to three cardiovascular specialists, and the Gensini grading method was applied to assess the severity of coronary artery stenosis and the blood vessels involved11 (Table 1)
TABLE 1.
Gensini Grading Method
Statistical Analysis
The measurement data were expressed as mean ± standard deviation, and measurement data were expressed as frequency or percentages. Independent samples t-test was used to compare the measurement data between two groups, and variance analysis was used for comparison among groups. Fisher's Least Significant Difference and Student–Newman–Keuls tests were used for multiple comparisons of mean values. Pearson's correlation analysis was used to analyze correlation of coronary artery score, RDW and diurnal QTc variation. Receiver operating characteristic (ROC) curves were used to determine the optimal threshold RWD value for the diagnosis of acute coronary syndrome, and areas under ROC curves and 95% confidence intervals (CI) were calculated to compare the diagnostic efficiencies. All statistical analyses were conducted using SPSS 20.0 software, and P < 0.05 was considered statistically significant.
RESULTS
Comparison of Baseline Data
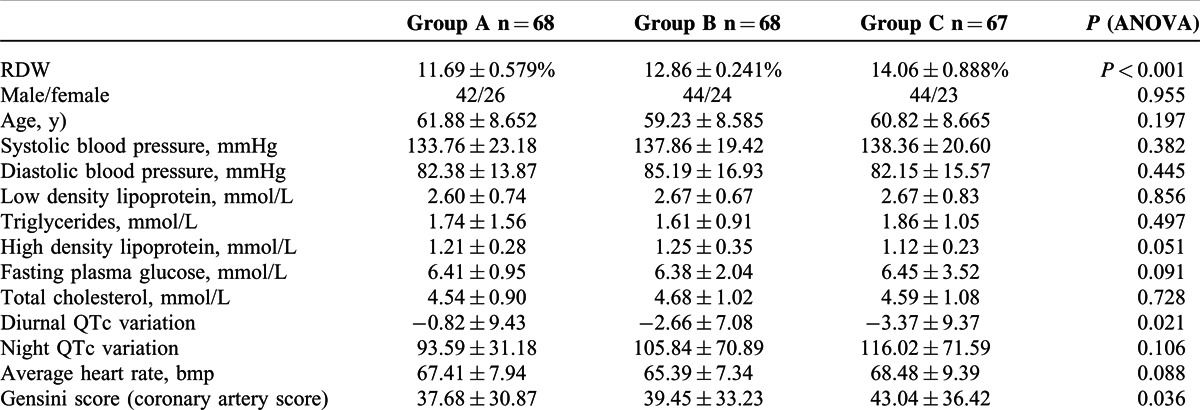
By sorting RDW values in an ascending order, the patients were divided into three groups, denoted as A, B and C groups. The sex ratio, age, blood pressure, blood glucose, blood lipids, night QTc and average heartbeat had no significant differences among these groups (P > 0.05). However, the RDW value, coronary artery score and diurnal QTc variation of these groups were significantly different (P < 0.05). The coronary artery score (Gensini score) gradually increased with RDW, while diurnal QTc variation decreased with RDW value, as illustrated in Table 2.
TABLE 2.
Baseline Data of the 3 Groups
Pearson's Correlation Analysis
Pearson's correlation analysis was applied to assess possible effects of other factors, and then multiple correlations among coronary artery score, RDW, and diurnal QTc variation were analyzed. Results showed that the coronary artery score was positively correlated with RDW (r = 0.130, P = 0.020) but was not correlated with the diurnal QTc variation (r = −0.226, P = 0.681), while the RDW was negatively correlated with the diurnal QTc variation (r = −0.197, P = 0.035), as shown in Figure 1.
FIGURE 1.
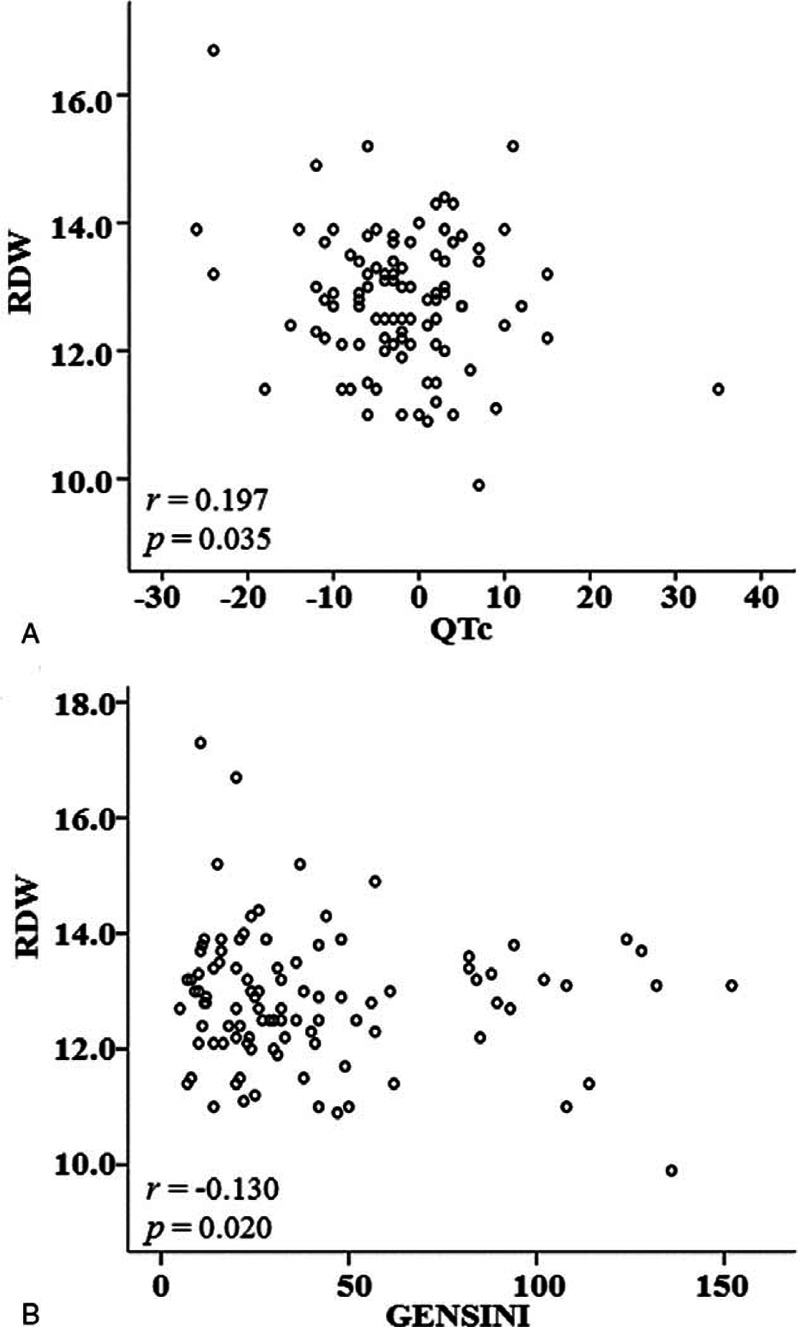
Correlation between diurnal QTc variation and RDW.
Selection of the Best Cutoff Value for RDW in Acute Coronary Syndrome Patients
The RDW values in patients with or without acute coronary syndrome were analyzed to determine the predictive value of RDW for the diagnosis of acute coronary syndrome. The ROCs curves suggest that RDW (area under the curve (AUC) 0.816 [95% CI 0.752–0.880]) has better sensitivity and specificity for predicting acute coronary syndrome than diurnal QTc variation (AUC 0.570 [95% CI 0.490–0.651] (Figure 2).
FIGURE 2.
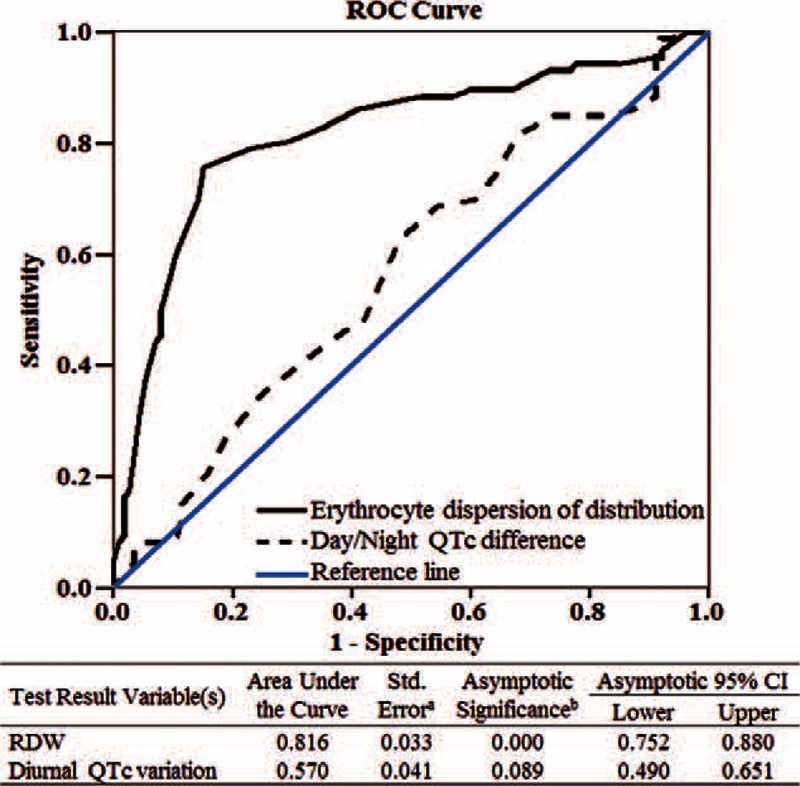
Receiver operating characteristic curve (ROC) analysis showing that RWD predicts the incidence of coronary heart disease patients.
DISCUSSION
In the present study we explored a potential relationship between the RDW and the diurnal QTc variations and its utility in diagnosing and prognosticating CHD. Validating our dataset, We confirmed the previous finding4,12 that the coronary artery score was positively correlated with RDW. Several previous studies have shown that the RDW predictor is independent of hemoglobin levels, indicating that the poor prognosis of cardiovascular disease associated with increased RDW may not be related to anemia. The mechanism of RDW elevation in patients with CHD is still unclear, but was proposed to be related to chronic inflammation, oxidative stress, renal dysfunction, and neuroendocrine system activation.13–19
Some studies have suggested that long-term activation of the neuroendocrine system can suppress bone marrow hematopoiesis and result in increased RDW. It was suspected that in CHD patients, increased RDW is associated with myocardial ischemia activating the sympathetic nervous system, which partially reflects the extent of myocardial ischemia. But this theory has not been supported by clinical and laboratory evidence. Recently, Özcan et al10 studied the correlation between heart rate variability (HRV) and RDW by conducting 24 h dynamic ECG examination and detecting RDW in blood samples of 180 patients with confirmed systolic heart failure. They found that RDW was independently negatively correlated with parameters of heart rate variability in patients with heart failure, where HRV parameters (SDNN, SDANN, RMSSD) decreased with increase in RDW, suggesting that RDW elevation coincided with autonomic nerve dysfunction.
Although QTc is normalized for heart rate, it can still reflect the status of autonomic nerve function to a certain extent. Normal individuals exhibit activated sympathetic nerves, weakened vagal nerves, faster heart rate and shorter QTc at daytime. The opposite pattern occurs during the night thus resulting in negative diurnal QTc variation. Myocardial ischemia in patients with coronary artery disease may lead to impaired autonomic nerve function and changes in cardiac ion channel properties, resulting in prolonged duration and increased dispersion of ventricular repolarization, thereby inducing malignant ventricular arrhythmia. Animal experiments and clinical studies have confirmed that diurnal QTc variation in subjects with CHD is more significant than in the healthy population.20–22 The present study showed that increased RDW values were related with the diurnal QTc variation decreased, which was the first reported. We also found that the differences in the night QTc variation among the three groups was not significant, indicating that the increased diurnal QTc variation may be related to increased sympathetic activity during daytime, especially its sudden increase in early morning.23
This retrospective study was conducted to explore the relationship of the RDW and diurnal QTc variation in patients with CHD, and the two indicators were found to be negatively correlated (r = −0.197, P = 0.035). With increased RDW values, the diurnal QTc variation decreased, indicating that increased RDW may be accompanied by autonomic nerve dysfunction in these patients.
After controlling the effects of other factors, Pearson's correlation analysis showed that there was no correlation between coronary artery score and diurnal QTc variation (r = −0.226, P = 0.681), indicating that there could be more complex mechanisms governing the severity of coronary stenosis and autonomic nerve dysfunction. Limitations of this retrospective study include lack of repeated RDW data collection, possible errors in QTc measurement, and the relatively small sample size. This is the first report of a correlation between RDW and diurnal QTc variation, and further clinical and basic studies are required to substantiate this finding.
CONCLUSIONS
In conclusion, increased RDW is independently associated with diurnal QTc variation in patients with CHD. An easily obtained, cheap RDW test may aid in the diagnosis and prognostic assessment of CHD.
Acknowledgments
The authors gratefully acknowledge the contributions of Dr. Haifeng Hou to data analysis.
Footnotes
Abbreviations: CAG = coronary angiography, CHD = coronary heart disease, CI = confidence intervals, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, QTc = corrected QT, RDW = red blood cell distribution width, ROC = receiver operating characteristic.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Dzieciatkowski T, Przybylski M, Rusicka P, et al. Usefulness of wide-range microbiological diagnostics proceedings in case of simultaneous infection with four herpesviruses after allogeneic haematopoietic stem cell transplantation—a case report. Med Dosw Mikrobiol 2014; 66:23–28. [PubMed] [Google Scholar]
- 2.Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117:163–168. [DOI] [PubMed] [Google Scholar]
- 3.Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011; 22:138–144. [DOI] [PubMed] [Google Scholar]
- 4.Zalawadiya SK, Veeranna V, Niraj A, et al. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol 2010; 106:988–993. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007; 50:40–47. [DOI] [PubMed] [Google Scholar]
- 6.Celik A, Koc F, Kadi H, et al. Relationship between red cell distribution width and echocardiographic parameters in patients with diastolic heart failure. Kaohsiung J Med Sci 2012; 28:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isik T, Uyarel H, Tanboga IH, et al. Relation of red cell distribution width with the presence, severity, and complexity of coronary artery disease. Coron Artery Dis 2012; 23:51–56. [DOI] [PubMed] [Google Scholar]
- 8.Nishizaki Y, Yamagami S, Suzuki H, et al. Red blood cell distribution width as an effective tool for detecting fatal heart failure in super-elderly patients. Intern Med 2012; 51:2271–2276. [DOI] [PubMed] [Google Scholar]
- 9.Gadaleta F, Llois S, Kaski JC. Corrected QT interval: a prognostic marker in patients with non-ST-segment elevation acute coronary syndrome? Trends Cardiovasc Med 2011; 21:129–135. [DOI] [PubMed] [Google Scholar]
- 10.Özcan F, Turak O, Avci S, et al. Heart rate variability and red cell distribution width in patients with systolic left heart failure. Scand Cardiovasc J 2013; 47:225–229. [DOI] [PubMed] [Google Scholar]
- 11.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51:606. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Filippozzi L, Montagnana M, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med 2009; 47:353–357. [DOI] [PubMed] [Google Scholar]
- 13.Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis 2010; 209:278–282. [DOI] [PubMed] [Google Scholar]
- 14.Lippi G, Targher G, Montagnana M, et al. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest 2008; 68:745–748. [DOI] [PubMed] [Google Scholar]
- 15.Lorente L, Martin MM, Abreu-Gonzalez P, et al. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PloS One 2014; 9:e105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009; 169:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail 2002; 4:681–686. [DOI] [PubMed] [Google Scholar]
- 18.Solak Y, Yilmaz MI, Saglam M, et al. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am J Med Sci 2014; 347:118–124. [DOI] [PubMed] [Google Scholar]
- 19.Torre-Amione G, Anker SD, Bourge RC, et al. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): a placebo-controlled randomised trial. Lancet 2008; 371:228–236. [DOI] [PubMed] [Google Scholar]
- 20.Hansen S, Rasmussen V, Torp-Pedersen C, et al. QT intervals and QT dispersion determined from a 12-lead 24-hour Holter recording in patients with coronary artery disease and patients with heart failure. Ann Noninvasive Electrocardiol 2008; 13:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccirillo G, Moscucci F, D’Alessandro G, et al. Myocardial repolarization dispersion and autonomic nerve activity in a canine experimental acute myocardial infarction model. Heart Rhythm 2014; 11:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yetkin E, Senen K, Ileri M, et al. Diurnal variation of QT dispersion in patients with and without coronary artery disease. Angiology 2001; 52:311–316. [DOI] [PubMed] [Google Scholar]
- 23.Arntz HR, Willich SN, Oeff M, et al. Circadian variation of sudden cardiac death reflects age-related variability in ventricular fibrillation. Circulation 1993; 88:2284–2289. [DOI] [PubMed] [Google Scholar]


