Abstract
Available evidence shows that metabolic syndrome (Mets) has clear adverse effects for middle-aged and pre-elderly adults; however, the effect of Mets on mortality among elderly adults remains unclear. In addition, the comparative utility of Mets and its component for predicting mortality among the elderly has not been clearly established. Using data from a large Taiwanese cohort, we evaluated the effect of Mets and its components on subsequent all-cause and cause-specific mortality overtime among the elderly.
A total of 73,547 elders (age ≥65 years) participated in the Taipei Elderly Health Examination Program from 2007 to 2010. Mets was diagnosed using the adult treatment panel III criteria, and mortality was ascertained by using national death records. Time-dependent analysis was used to evaluate associations of Mets and its components with all-cause mortality, cardiovascular disease (CVD) mortality, and expanded CVD mortality.
This retrospective cohort study found that 42.6% of elders had Mets. During 194,057 person-years of follow-up, 2944 deaths were observed. After adjusting for sociodemographic characteristics and comorbidities, Mets was associated with increased risk of expanded CVD mortality (hazard ratio [HR], 1.27; 95% CI, 1.10–1.46) but not all-cause or CVD mortality. Among Mets components, decreased high-density lipoprotein cholesterol (HDL-C, HR 1.25, 95% CI 1.13–1.37) and hyperglycemia (HR 1.21, 95% CI 1.12–1.31) were associated with a significant increase in all-cause mortality. Hypertension and low HDL-C were predictors of CVD mortality and expanded CVD mortality, and, as compared with Mets, were associated with a higher risk of expanded CVD mortality.
The present findings indicate that, in elderly adults, individual components of Mets are better predictors of all-cause and cause-specific mortality than is Mets as a whole. Our results suggest that future efforts should focus on preventing and managing individual risk factors (particularly hypertension, low HDL-C, and hyperglycemia) rather than on “diagnosing” Mets in elders.
INTRODUCTION
The metabolic syndrome (Mets) refers to a cluster of metabolic abnormalities, including obesity, hyperglycemia, dyslipidemia, and hypertension. Although the adverse effects of Mets in middle-aged and pre-elderly adults are clear,1–3 the association of Mets with mortality among elderly adults remains uncertain.4–10 Although some reports indicate that Mets increases the risk of all-cause mortality in the elderly,4,9,10 other studies have found no significant association.5,7,8 Mets has also been found to increase the risk of cardiovascular disease (CVD) mortality4,8 and coronary heart disease mortality,6,10 but one study found no significant association with either.7
Although Mets is used as predictor of mortality in the elderly, some concerns have been voiced regarding this practice. First, while obesity—a component of Mets—significantly increased mortality risk in adults, it did not increase this risk in elderly adults.11 Second, some Mets components (eg, hyperglycemia and low high-density lipoprotein cholesterol [HDL-C]) predicted mortality in the elderly, while other components (eg, hypertriglyceridemia) did not.12,13 Finally, it is not clear whether Mets as a whole or its components better predict mortality.14,15
In this large cohort study of elderly Taiwanese, we evaluated the effects of Mets and its components on subsequent all-cause and cause-specific mortality and analyzed whether Mets or components of Mets were better predictors of mortality.
METHODS
Study Population
This retrospective cohort study used the Taipei Elderly Health Examination Database, which is collected by the city government of Taipei, Taiwan. The subjects were Taiwanese elderly (age ≥65 years) who participated in an annual physical examination program during 2007 to 2010. This research was approved by the Institutional Review Board of Taipei City Hospitals. The requirement for written informed consent was waived by the approving Institutional Review Board, because personally identifying information was not included in the dataset.
Data Collection
The Taipei Elderly Health Examination Program is free for adults aged 65 years or older. Elders participating in the Health Examination Program were interviewed by trained case managers using a structured questionnaire to inquire about demographics (eg, age, sex, marital status, and education level), lifestyle behaviors (eg, smoking history, alcohol consumption, and exercising habit), and medications (eg, antihypertension drugs, antidiabetes drugs). Blood pressure was measured during the medical check-up. Overnight fasting blood was collected for the measurement of serum blood sugar, triglyceride (TG), glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), albumin, blood creatinine, uric acid, hemoglobin, and platelets. All elderly in the Health Examination Program were followed until death or December 31, 2010.
Outcome Variables
The outcome variable in this study was death, which was determined by the National Death Certification Registry in Taiwan.16 During study period, the Taiwan mortality registry was consulted to identify underlying causes of death among the elderly in the Health Examination Program. According to Taiwan law, a death certificate must be registered within 30 days after the death of a resident. Since trained managers review all death certificates in the central office of the National Death Certification Registry, the cause-of-death coding has been considered very accurate in Taiwan.17
Death in this study was classified as all-cause (International Classification of Diseases [ICD]-9: 001–998; ICD-10: A00-Z99), CVD (ICD-9: 390–459; ICD-10: I00–I99), and expanded CVD deaths (CVD plus diabetes, ICD-9: 250; ICD-10: E10–E14, plus kidney diseases, ICD-9: 580–589; ICD-10: N00–N29). Data related to individual identification were not included before all data were released to the researchers.
Main Explanatory Variable
The main explanatory variable was Mets, which was diagnosed using the adult treatment panel III criteria,18,19 namely, the presence of at least 3 of the following 5 risk factors: central obesity (waist circumference ≥90 cm in Asian men or ≥80 cm in Asian women), low HDL-C (fasting HDL-C ≤40 mg/dL for men or ≤50 mg/dL for women), elevated blood pressure (systolic ≥130 mm Hg and/or diastolic ≥85 mm Hg, or antihypertensive drug treatment in a patient with a history of hypertension), hypertriglyceridemia (fasting plasma TGs ≥150 mg/dL or drug treatment for elevated TGs), and hyperglycemia (fasting glucose level ≥100 mg/dL or drug treatment for elevated glucose).
Control Variables
The control variables included subject sociodemographic characteristics, lifestyle behaviors, and comorbidities. Education level was categorized as uneducated, elementary school, high school, and university or higher. Hyperuricemia was defined as a uric acid level of >7.0 mg/dL in men or 6.0 mg/dL in women.20 Hypoalbuminemia was defined as an albumin level of ≤3.5 g/dL. Anemia was defined as a hemoglobin level of <13 g/dL in men or <12 g/dL in women, according to the World Health Organization definition of anemia.21 Thrombocytopenia was defined as a platelet count of <150 × 103/μL,22 and thrombocytosis as a platelet count of >450 × 103/μL.23 In accordance with a previous report,24 the upper limit of normal (ULN) of GOT was 31 IU/L for men and women, and the ULN for GPT was 45 IU/L for men and 29 IU/L for women. On the basis of GOT and GPT data, subjects were divided into 3 groups: those with results that were less than the ULN, 1–2 times the ULN, and >2 times the ULN.
Statistical Analyses
First, the demographic characteristics of the study subjects were analyzed. Continuous data are presented as mean (standard deviation), and the 2-sample t test was used for comparisons between groups. Categorical data were analyzed by the Pearson χ2 test, where appropriate.
We analyzed all data on Mets and its components, which were recorded for all subjects at annual healthy examination programs. Since Mets (and its components) might have changed for each subject throughout the study period, this study used time-dependent Cox proportional hazards models to determine the associations of Mets and its components with all-cause and cause-specific mortality.25 In these models, time-dependent covariables25 included Mets and its components, and fixed covariates included age, sex, smoking, alcohol consumption, physical activity, and comorbidities at baseline. Adjusted hazard ratios with 95% confidence intervals (CIs) are reported, to indicate the strength and direction of associations.
To examine the interaction between Mets and other covariates in the multivariate analysis, we conducted subgroup analyses after stratifying study subjects by sex, age, and comorbidities. All data management and analyses were performed using the SAS 9.4 software package (SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
During the 4-year follow-up, 73,547 elders (age ≥65 years) participated in the annual physical examination program in Taipei: 48.6% (35,722) participated in the program once, 27.9% (20,518) twice, 13.1% (10,228) 3 times, and 9.6% (7,079) 4 times. Overall, 42.6% (31,307) of the study subjects had Mets at baseline. Mean age was 76.0 years (range 65–109 years), and 46.2% were male. During 194,057 person-years of follow-up, 2944 deaths were observed. Among the 31,307 participants with Mets, there were 1212 (3.9%) all-cause deaths, 303 (1.0%) CVD deaths, and 426 (1.4%) expanded CVD deaths during the follow-up period (Figure 1). Among the 42,240 participants without Mets, there were 1732 (4.1%) all-cause deaths, 401 (1.0%) CVD deaths, and 468 (1.1%) expanded CVD deaths during the follow-up period.
FIGURE 1.
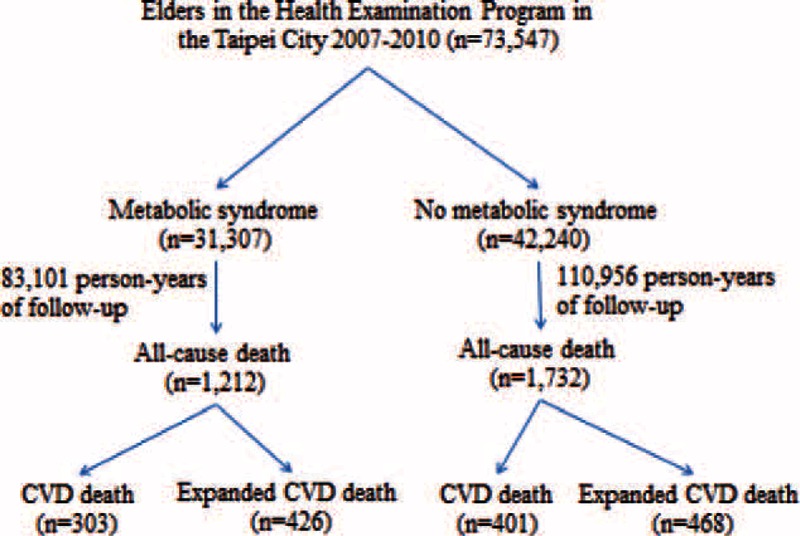
Study flow diagram. CVD = cardiovascular disease.
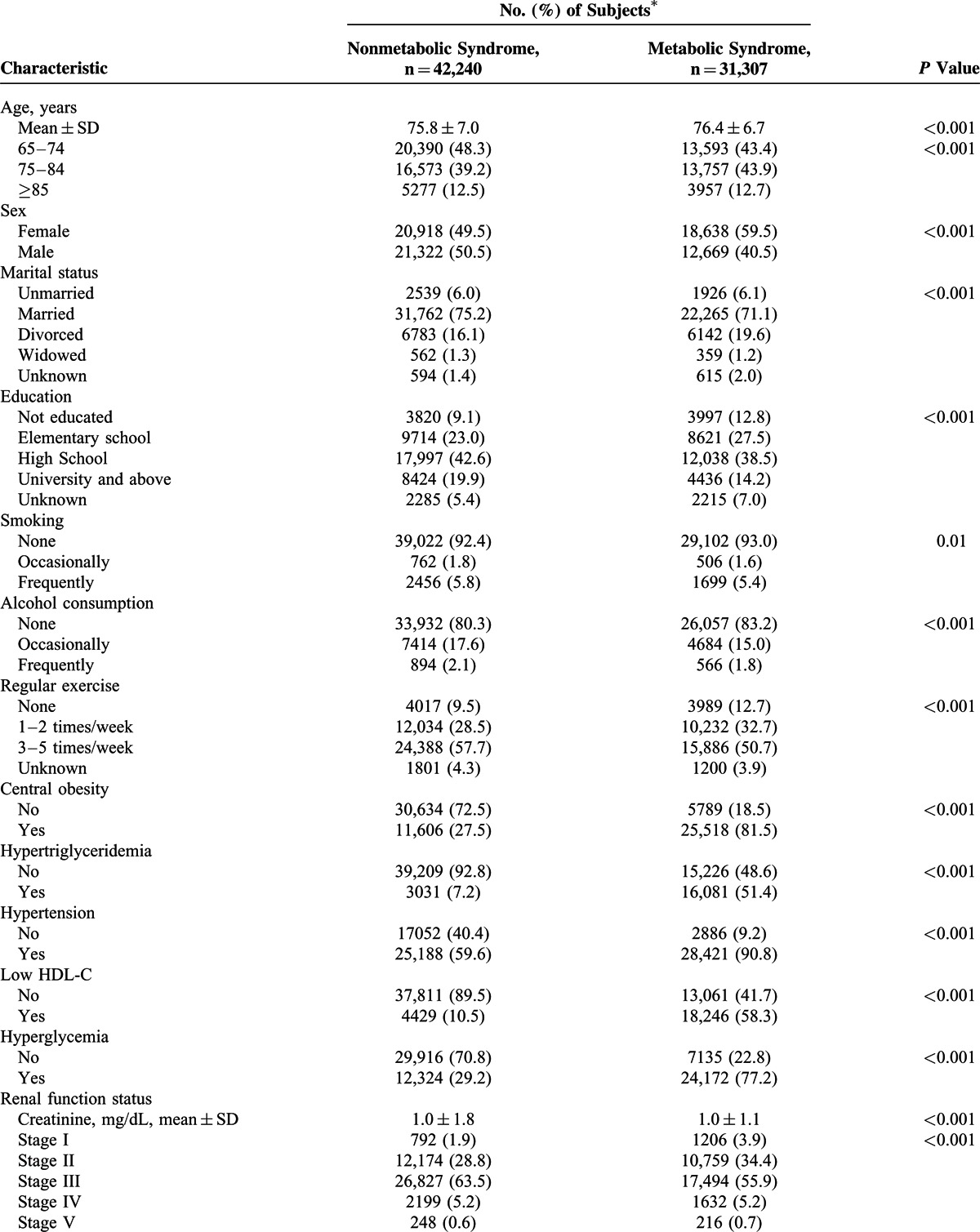
The demographic characteristics and laboratory data of the 2 groups are shown in Table 1 . Subject with Mets were slightly older than those without Mets (76.4 vs 75.8 years). There were significant differences between the groups in laboratory parameters, including albumin, creatinine, uric acid, hemoglobin, platelet, GOT, and GPT.
TABLE 1.
Characteristics of Elderly Adults With and Without Metabolic Syndrome
Association Between Mets and Mortality
A time-dependent Cox proportional hazards model was used to identify independent risk factors for all-cause and cause-specific mortality (Table 2 ). After adjusting for sociodemographic characteristics and comorbidities, Mets was not significantly associated with all-cause or CVD mortality but was significantly associated with increased risk of expanded CVD mortality (HR, 1.26; 95% CI, 1.09–1.46).
TABLE 1 (Continued).
Characteristics of Elderly Adults With and Without Metabolic Syndrome
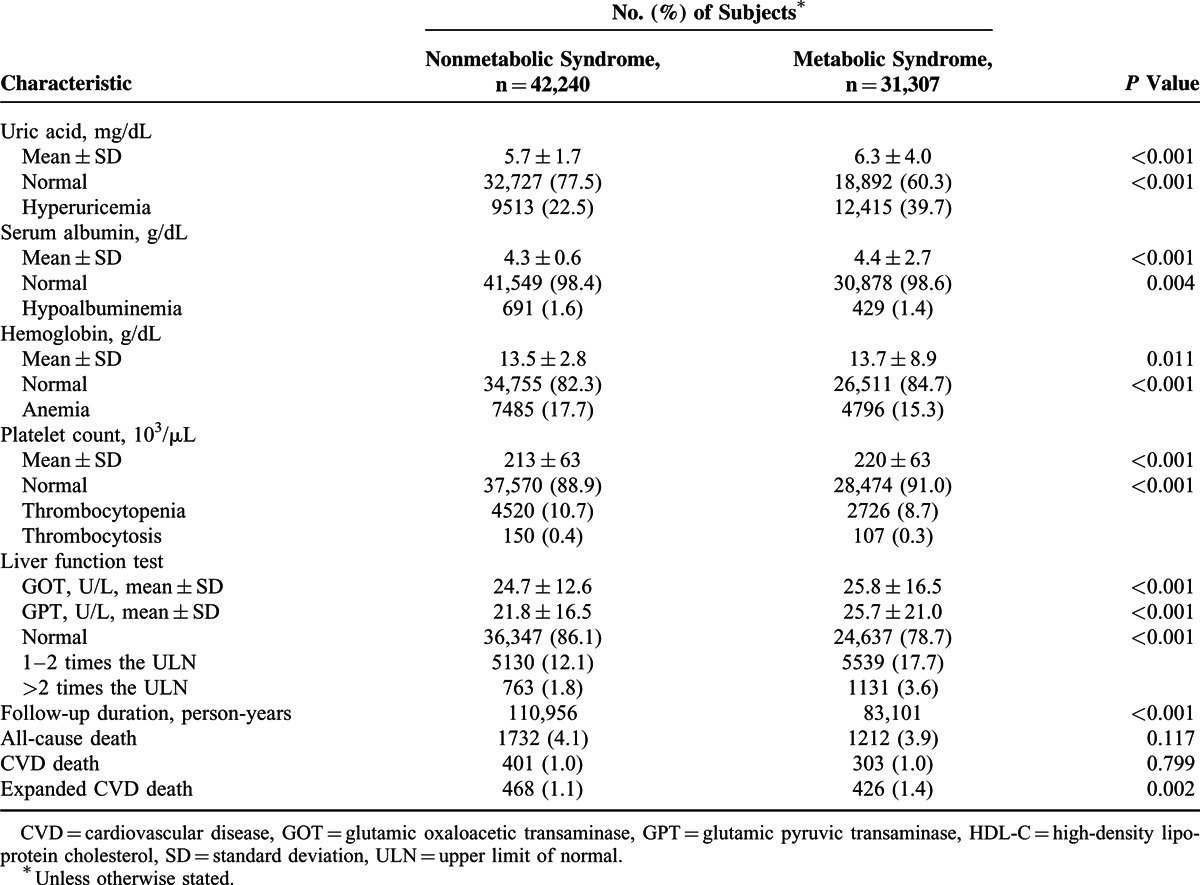
TABLE 2.
Cox Proportional Hazards Model of Factors Associated With All-Cause, CVD, and Expanded CVD Mortality
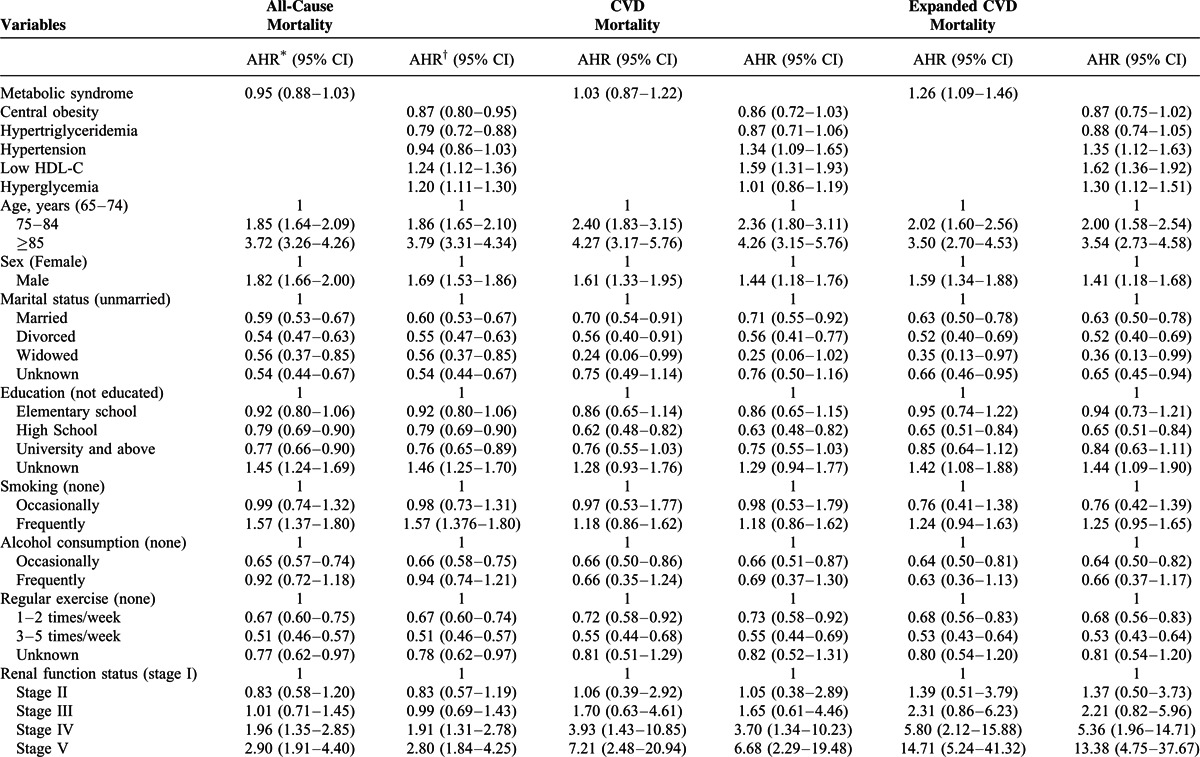
TABLE 2 (Continued).
Cox Proportional Hazards Model of Factors Associated With All-Cause, CVD, and Expanded CVD Mortality
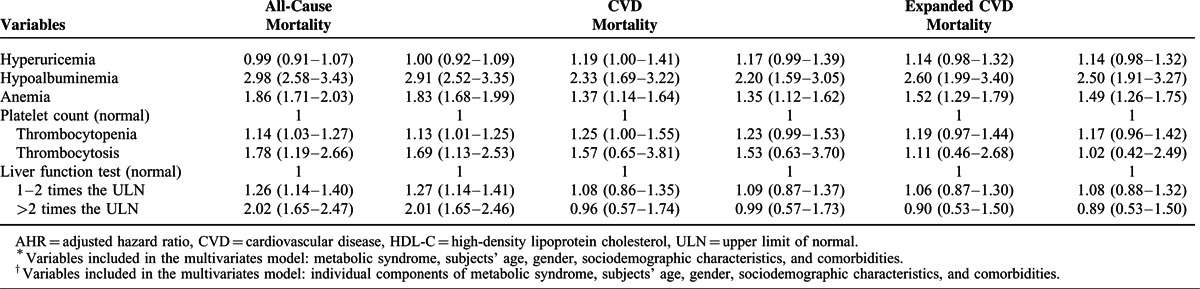
Association of Mets Components With Mortality
The Cox proportional hazards model revealed that central obesity (HR 0.87, 95% CI 0.80–0.95) and hypertriglyceridemia (HR 0.79, 95% CI 0.72–0.88) were associated with lower risk of all-cause mortality, and that low HDL-C (HR 1.24, 95% CI 1.12–1.36) and hyperglycemia (HR 1.20, 95% CI 1.11–1.30) were associated with higher risk of all-cause mortality (Table 2). Hypertension (HR 1.34, 95% CI 1.09–1.65) and low HDL-C (HR 1.59, 95% CI 1.31–1.93) increased the risk of CVD mortality. Furthermore, hypertension (HR 1.35, 95% CI 1.12–1.63), low HDL-C (HR 1.62, 95% CI 1.36–1.92), and hyperglycemia (HR 1.30, 95% CI 1.12–1.51) were associated with higher risk of expanded CVD mortality.
Sensitivity Analysis of the Association Between Mets and Mortality
Figure 2 presents the results of sensitivity analysis of the association between Mets and mortality, after stratification by age group, sex, and comorbidities. Mets was not associated with higher risk of all-cause or CVD mortality in any subgroup, except in age group 65 to 74 years. Moreover, Mets was significantly positively associated with risk of expanded CVD mortality in most subgroups including age group 65 to 74 years and elders with hypertension, central obesity, hypoalbuminemia, or anemia.
FIGURE 2.

Sensitivity analysis of the association between Mets and mortality in subgroups, after adjustment for demographic characteristics and comorbidities. Values greater than 1.0 indicate increased risk. AHR = adjusted hazard ratio, CVD = cardiovascular disease, HDL-C = high-density lipoprotein cholesterol.
Sensitivity Analysis of the Associations Between Mets Components and Mortality
Figure 3 presents the results of sensitivity analysis of the associations between Mets components and mortality, after stratification by age group and sex. Cox regression analysis showed that low HDL-C and hyperglycemia were significantly positively associated with risk of all-cause mortality in age groups 65 to 74 and 75 to 84 years and in men and women. Hypertension and low HDL-C were associated with higher risks of CVD death and expanded CVD death in both men and women.
FIGURE 3.
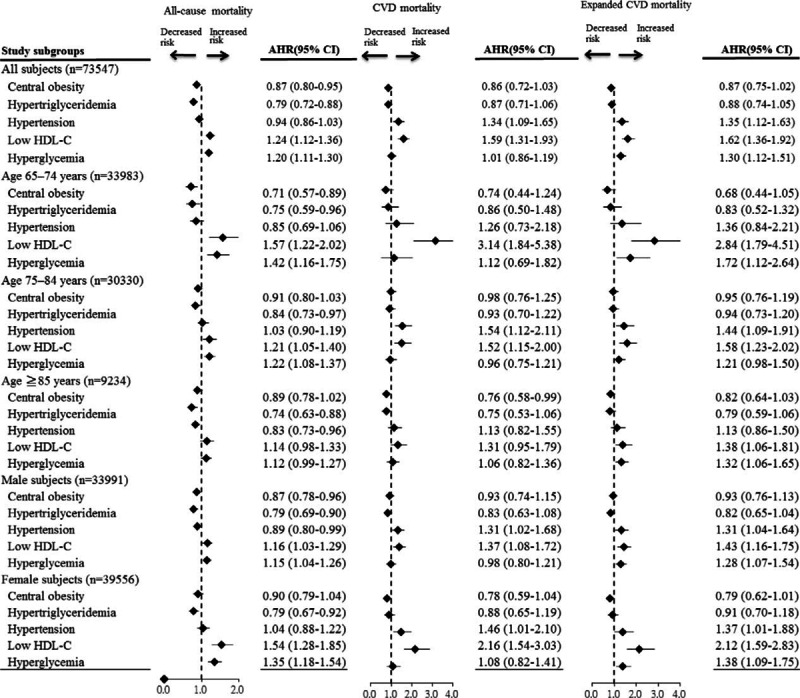
Sensitivity analysis of the associations between Mets components and mortality in subgroups, after adjustment for demographic characteristics and comorbidities. Values greater than 1.0 indicate increased risk. AHR = adjusted hazard ratio, CVD = cardiovascular disease, HDL-C = high-density lipoprotein cholesterol.
DISCUSSION
This retrospective cohort study found that 42.4% of elderly adults in Taipei had Mets. During 194,057 person-years of follow-up, 2944 deaths were observed. After adjusting for sociodemographic characteristics and comorbidities, Mets was positively associated with a higher risk of expanded CVD mortality but not all-cause or CVD mortality. The associations of Mets components with mortality varied. Low HDL-C and hyperglycemia significantly increased the risk of all-cause mortality. In addition, hypertension and low HDL-C were associated with higher risks of CVD mortality and expanded CVD mortality.
Our study showed robust associations between Mets, its components, and mortality after stratifying subjects by age, sex, and comorbidities, respectively. Mets was not associated with higher risks of all-cause or CVD mortality, except in the age group 65 to 74 years. However, Mets significantly increased the risk of expanded CVD mortality in most subgroups, including the age group 65 to 74 years and in subjects with hypertension, central obesity, hypoalbuminemia, or anemia. Mets components such as low HDL-C and hyperglycemia significantly increased all-cause mortality risk in age groups 65 to 74 and 75 to 84 years and in men and women. Hypertension and low HDL-C were associated with higher risks of CVD mortality and expanded CVD mortality in men and women.
The adverse effect of Mets on CVD mortality has been well demonstrated in adults.1–3 However, the association of Mets with CVD mortality among the elderly remains uncertain.4,7,8 Two studies found that Mets increased the risk of CVD mortality,4,8 but 1 study found no significant association.7 The present study showed that Mets was not significantly associated with a higher risk of CVD mortality. The uncertain prediction of Mets on CVD mortality in the elderly may be partially because the underweight elderly was predisposed to a higher risk of mortality.11 Furthermore, some Mets components (eg, diastolic blood pressure and hypertriglyceridemia) in the elderly population are not clearly associated with poor health outcomes later in life.12
Previous studies have showed that the prediction of individual Mets components on mortality varied in elderly population.4,6,8,9 Although hyperglycemia4,6,8,9 and hypertension4 were associated with a higher risk of all-cause mortality, low HDL-C increased the risk of CVD mortality.8 However, high TGs and central obesity were not associated with all-cause or CVD mortality in the elderly population.4,6,8,9 This study simulated a real scenario of Mets change overtime by repeatedly measuring Mets and showed that low HDL was the predictor of all-cause, CVD, and expanded CVD mortality in the elderly population. Moreover, hypertension and hyperglycemia were associated with a higher risk of expanded CVD mortality. Elderly with hypertension, low HDL-C, or hyperglycemia should be the target population for managing their individual risk factors.
The comparative utility of Mets and Mets components for predicting mortality in the elderly has not been extensively studied, and the existing evidence is inconsistent. In the Health ABC study (mean patient age, 74 years)7 and Italians aged ≥65 years,5 Mets was not associated with higher risks of all-cause or CVD mortality;5,7 the relationships of Mets components with mortality were not reported.5,7 In nondiabetic Finns aged 65 to 74 years, Mets was a predictor of CVD mortality but was not significantly associated with all-cause mortality. In addition, impaired fasting glucose and low HDL-C significantly increased CVD mortality risk.8 In the Cardiovascular Health Study (age ≥65 years),4 Progetto Veneto Anziani Study (age ≥65 years),9 French elders (age ≥65 years),6 and Australian older adults (age ≥49 years),10 Mets was associated with higher risks of all-cause4,6,9 and CVD mortality.6,9,10 Mets components, including hyperglycemia4,6,9 and hypertension,4 were predictors of all-cause mortality. The present study showed that Mets significantly increased the risk of expanded CVD mortality but not all-cause or CVD mortality. Low HDL-C and hyperglycemia were predictors of all-cause mortality. In addition, hypertension and low HDL-C were predictors of CVD mortality and expanded CVD mortality, and, as compared with Mets, were associated with a higher risk of expanded CVD mortality. These findings suggest that, in elder adults, Mets components are better predictors of all-cause and cause-specific mortality than is Mets as a whole. Thus, greater effort should be directed toward preventing and managing individual risk factors (particularly hypertension, low HDL-C, and hyperglycemia) rather than to “diagnosing” Mets in elders.
We believe that there are 2 possible explanations regarding why Mets is less useful than its components in predicting mortality among the elderly. First, the cut-off values for Mets components were mostly derived from middle-aged populations26 and might not be appropriate for elderly populations. Second, obesity is an important component of Mets and is associated with a higher risk of mortality in adults.11 However, 1 study found that obesity was not significantly associated with mortality risk in the elderly.11 In elderly adults, underweight might be a greater mortality risk than overweight.11,27 The present study also showed that central obesity and hypertriglyceridemia were significantly associated with lower risk of all-cause mortality in the elderly. The inverse associations of obesity and hypertriglyceridemia with all-cause mortality might lessen the predictive ability of Mets in the elderly.
Several limitations should be considered when interpreting the findings of this study. First, the adult treatment panel III criteria are not the only standardized definition for Mets. In elderly adults, however, they are better than other criteria for Mets in predicting clinically important outcomes.28 Second, the present subjects voluntarily participated in the annual examination programs and therefore might not be representative of the general population. However, because the risk assessment was based on internal comparisons, the calculated relative risks are reasonable estimates of those in the general population. Third, because of retrospective cohort study design, this study did not have the information regarding the uncalculated person-time risk of the subjects with Mets before enrollment. As the status of Mets might change overtime,10 this study simulated a real scenario of Mets change overtime by repeatedly measuring Mets in annual elderly examination programs. However, this study only followed up study subjects for 4 years. Future studies with longer periods of follow-up will be needed to evaluate the effect of Mets on mortality. Fourth, the Taipei Elderly Health Examination Database did not include the detailed information regarding the subjects’ medications (eg, antiplatelet agent). More studies will be needed to evaluate the impact of this confounder on the association between Mets and mortality. Fifth, since this study was a retrospective cohort study, unknown data in some variables were found in this report. However, the association between Mets, its components, and mortality were the same if subjects with missing data were excluded in the multivariate analysis. Finally, information bias regarding subject characteristics and comorbidities cannot be avoided in studies using self-reported questionnaires. However, the comorbidities in this study were laboratory parameters, which would reduce the possibility of recall bias.
The strengths of this study include the fact that the large numbers of events (2944 deaths) provided substantial statistical power and precision, allowing detection of HRs as low as 1.21. In addition, the study participants were repeatedly evaluated for Mets in the annual elderly examination program. This better represents real-life conditions, in which Mets status changes overtime. Longitudinal studies that do not account for changes in an exposure overtime do not yield precise estimates of the effect of an exposure on outcomes.25
CONCLUSION
This retrospective cohort study found that 42.4% of elderly adults in Taipei had Mets. Mets was associated with a higher risk of expanded CVD mortality but not all-cause or CVD mortality. Among Mets components, low HDL-C and hyperglycemia increased the risk of all-cause mortality. Hypertension and low HDL-C were predictors of CVD mortality and expanded CVD mortality, and, as compared with Mets, were associated with a higher risk of expanded CVD mortality. These findings suggest that, in the elderly, Mets components are better predictors of all-cause and cause-specific mortality than is Mets as a whole. Future efforts should focus on preventing and managing individual risk factors (particularly hypertension, low HDL-C, and hyperglycemia) rather than on “diagnosing” the syndrome in elderly adults.
Acknowledgements
The authors are grateful to the members of the Research Office for Health Data, Department of Education and Research, Taipei City Hospital, Taiwan for their valuable contributions in data management and statistical analysis. The authors are also grateful for statistical consultation at the Biostatistical Consultation Centre, National Yang-Ming University, Taipei, Taiwan.
Footnotes
Abbreviations: CI = confidence intervals, CVD = cardiovascular disease, GOT = glutamic oxaloacetic transaminase, GPT = glutamic pyruvic transaminase, HDL-C = high-density lipoprotein cholesterol, ICD = International Classification of Diseases, Mets = metabolic syndrome, TG = triglyceride, ULN = upper limit of normal.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Sundstrom J, Riserus U, Byberg L, et al. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 2006; 332:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas GN, Schooling CM, McGhee SM, et al. Metabolic syndrome increases all-cause and vascular mortality: the Hong Kong Cardiovascular Risk Factor Study. Clin Endocrinol (Oxf) 2007; 66:666–671. [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Qiao Q, Tuomilehto J, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med 2004; 164:1066–1076. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Kamineni A, Prineas RJ, et al. Metabolic syndrome and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med 2008; 168:969–978. [DOI] [PubMed] [Google Scholar]
- 5.Ravaglia G, Forti P, Maioli F, et al. Metabolic syndrome: prevalence and prediction of mortality in elderly individuals. Diabetes Care 2006; 29:2471–2476. [DOI] [PubMed] [Google Scholar]
- 6.Akbaraly TN, Kivimaki M, Ancelin ML, et al. Metabolic syndrome, its components, and mortality in the elderly. J Clin Endocrinol Metab 2010; 95:E327–E332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol 2006; 47:1595–1602. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Ruotsalainen S, Moilanen L, et al. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J 2007; 28:857–864. [DOI] [PubMed] [Google Scholar]
- 9.Zambon S, Zanoni S, Romanato G, et al. Metabolic syndrome and all-cause and cardiovascular mortality in an Italian elderly population: the Progetto Veneto Anziani (Pro.V.A.) Study. Diabetes Care 2009; 32:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaem Maralani H, Tai BC, Wong TY, et al. Metabolic syndrome and mortality in the elderly: a time-dependent association. Diabetes Res Clin Pract 2013; 99:209–216. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867. [DOI] [PubMed] [Google Scholar]
- 12.Burke GL, Arnold AM, Bild DE, et al. Factors associated with healthy aging: the cardiovascular health study. J Am Geriatr Soc 2001; 49:254–262. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA 1998; 279:585–592. [DOI] [PubMed] [Google Scholar]
- 14.Franks PW, Olsson T. Metabolic syndrome and early death: getting to the heart of the problem. Hypertension 2007; 49:10–12. [DOI] [PubMed] [Google Scholar]
- 15.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health: Vital Statistics in Taiwan. Department of Health, Executive Yuan, Taiwan; http://www.mohw.gov.tw/CHT/DOS/ [accessed 31 January 2015]. [Chinese]. [Google Scholar]
- 17.Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol 2000; 29:336–343. [DOI] [PubMed] [Google Scholar]
- 18.Shen HC, Zhao ZH, Hu YC, et al. Relationship between obesity, metabolic syndrome, and nonalcoholic fatty liver disease in the elderly agricultural and fishing population of Taiwan. Clin Interv Aging 2014; 9:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Promotion Administration, Ministry of Health and Welfare (2015) (The definition of metabolic syndrome), Taiwan: http://www.hpa.gov.tw/BHPNet/Web/News/News.aspx?No=200712250398. (Accessed February 22, 2015) (Chinese). [Google Scholar]
- 20.Chuang SY, Wu CC, Hsu PF, et al. Hyperuricemia and incident atrial fibrillation in a normotensive elderly population in Taiwan. Nutr Metab Cardiovasc Dis 2014; 24:1020–1026. [DOI] [PubMed] [Google Scholar]
- 21.Bross MH, Soch K, Smith-Knuppel T. Anemia in older persons. Am Fam Physician 2010; 82:480–487. [PubMed] [Google Scholar]
- 22.Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician 2012; 85:612–622. [PubMed] [Google Scholar]
- 23.Vannucchi AM, Barbui T. Thrombocytosis and thrombosis. Hematology Am Soc Hematol Educ Program 2007; 363–370. [DOI] [PubMed] [Google Scholar]
- 24.Lee TH, Kim WR, Benson JT, et al. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008; 47:880–887. [DOI] [PubMed] [Google Scholar]
- 25.Collett D. Modelling Survival Data in Medical Research, 2nd ed., Chapman & Hall; 2003; Unites States of America. [Google Scholar]
- 26.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 27.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010; 363:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scuteri A, Najjar SS, Morrell CH, et al. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care 2005; 28:882–887. [DOI] [PubMed] [Google Scholar]


