Abstract
Alpha-fetoprotein (AFP) has not played a large role in the surveillance of hepatocellular carcinoma due to inadequate sensitivity and specificity for active chronic hepatitis or cirrhosis. The aim of this study was to evaluate the diagnostic accuracy of AFP in small hepatocellular carcinomas after hepatitis C virus eradication to determine the optimal cutoff value.
We conducted a case–control study of 29 cases and 58 controls, matched for age, gender, and platelet counts.
The AFP cutoff was 5 ng/mL in patients after hepatitis C virus eradication and 17 ng/mL in those without hepatitis C virus eradication. The areas under the receiver operating characteristic curve were 0.86 (95% confidence interval, 0.76–0.96) in patients after hepatitis C virus eradication and 0.83 (95% confidence interval, 0.74–0.91) in those without hepatitis C virus eradication. In patients after hepatitis C virus eradication, the sensitivity and specificity of AFP levels were 24.1% and 100%, respectively, using a cutoff value of 17 ng/mL. Using a lower cutoff value of 5 ng/mL, the sensitivity increased to 75.9%, although the specificity decreased to 89.0%.
AFP is a specific tumor marker for the diagnosis of hepatocellular carcinoma after hepatitis C virus eradication when using the optimal cutoff value of 5 ng/mL.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and the leading cause of cancer-related death.1 Hepatitis C virus (HCV) infection is one of the major causes of chronic liver disease and associated with an increased risk of HCC.2,3 In recent years, effective direct-acting antiviral agents have been developed that result in a sustained viral response (SVR) in most patients.4–6 Although HCV eradication has been documented to reduce the risk of HCC,7–9 HCC still develops in some patients after achieving an SVR,7,10,11 which necessitates cancer surveillance after HCV eradication.
Alpha-fetoprotein (AFP) is widely used as a serum biomarker for the diagnosis of HCC.12–14 However, elevated AFP levels are also sometimes seen in patients with chronic viral hepatitis and cirrhosis who do not have HCC.15–17 The use of AFP for detecting early HCC lacks adequate sensitivity and specificity.18,19 Thus, AFP testing is not recommended for HCC surveillance by the practice guidelines of the American Association for the Study of Liver Diseases.20
After HCV eradication, serum alanine aminotransferase (ALT) levels are usually decreased within normal limits, and hepatitis flares occur rarely, which might lead to decreased AFP levels without HCC. Thus, the specificity of AFP for the diagnosis of HCC after HCV eradication could be improved. The aims of the current study were to evaluate the diagnostic accuracy of AFP in small HCCs after HCV eradication compared with patients without HCV eradication to identify the optimal cutoff value for serum AFP and to differentiate patients with HCC from those without HCC after HCV eradication.
MATERIALS AND METHODS
Patients
This retrospective study was conducted according to the ethical guidelines for epidemiological research of the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor, and Welfare. The study design was included in a comprehensive protocol for retrospective studies at the University of Tokyo Hospital, Department of Gastroenterology (Tokyo, Japan) and was approved by the University of Tokyo Medical Research Center Ethics Committee (approval number, 2058).
Four cohorts were enrolled based on the presence of HCC or HCV status. Patients with positive serology for hepatitis B surface antigen were excluded. Inclusion criteria were as follows:
Cohort 1 included patients who developed HCC after HCV eradication using interferon (IFN)-based therapy. These patients were enrolled from January 1990 to December 2012 at the Department of Gastroenterology of the University of Tokyo Hospital. Of the 37 patients who developed HCC after HCV eradication, 29 were defined as early stage HCC (defined below);
Cohort 2 included patients who did not develop HCC after HCV eradication using IFN-based therapy. These 179 patients, who were enrolled from January 1990 to December 2012, achieved SVR, confirmed as the absence of HCC during follow-up for more than 1 year;
Cohort 3 included patients who developed HCC without HCV eradication, consisting of 1185 chronic hepatitis C patients who developed HCC treated initially with radical therapies (percutaneous ethanol injection therapy, percutaneous microwave coagulation therapy, or radiofrequency ablation) from January 1990 to December 2009 at the same institution, excluding those who achieved SVR before HCC development; and
Cohort 4 included patients without either HCC or HCV eradication. These patients were extracted from the follow-up cohort, which was analyzed for hepatitis C-related HCC development, as reported previously.21
Diagnosis of HCC
HCC was diagnosed by dynamic computed tomography (CT) or magnetic resonance imaging (MRI) with hyperattenuation in the arterial phase and washout in the late phase.22 As the diagnosis of HCC was not definite by CT or MRI, an ultrasound-guided tumor biopsy was performed and pathological diagnosis made based on the Edmondson-Steiner criteria.23
Early stage HCC was defined as a tumor number ≤3 with each lesion ≤3 cm in diameter with no evidence of vascular invasion or extrahepatic metastasis.
Virological Testing
Anti-HCV antibody was used in a chemiluminescent immunoassay (Dainabot, Tokyo, Japan). HCV RNA was measured quantitatively using the Amplicore HCV RNA Monitor Kit Version 2.0 (Roche Diagnostics; Indianapolis, IN) or COBAS TaqMan HCV Test (Roche Diagnostics), both of which were also used to qualitatively confirm seronegative HCV RNA. Hepatitis B virus surface antigen was examined using a chemiluminescent immunoassay (Dainabot).
Matched Case–Control Study
A matched case–control study was conducted to compare the diagnostic accuracy of AFP between SVR cohorts 1 and 2 and non-SVR cohorts 3 and 4 to minimize the influence of non-HCC factors on AFP levels. Details of the matching method are described in Figure 1. The first step was to extract patients without HCC into an SVR cohort (cohort 2) to match age, gender, and platelet counts with those of early-stage HCC patients after HCV eradication (cohort 1). The next step was to extract patients with HCC in the non-SVR cohort (cohort 3) to match age, gender, platelet counts, tumor number, and tumor diameter with those of early-stage HCC patients in an SVR cohort (cohort 1). The third step was to extract patients without HCC in a non-SVR cohort (cohort 4) to match age, gender, and platelet count with those of HCC patients in a non-SVR cohort (cohort 3). In both SVR and non-SVR cohorts, two non-HCC cases were selected for each HCC case. To ensure that non-HCC patients did not have subclinical HCC, patients who developed HCC during the following year were excluded.
FIGURE 1.
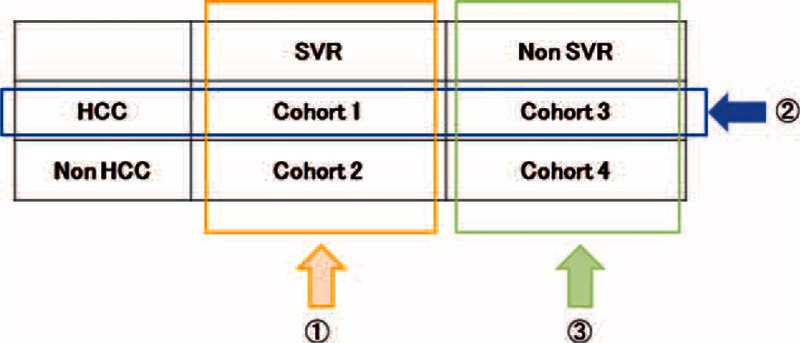
Schematic of the matching process. Cohort 1 included patients who developed hepatocellular carcinoma after hepatitis C virus eradication; cohort 2 included patients without hepatocellular carcinoma development after hepatitis C virus eradication; cohort 3 included patients who developed hepatocellular carcinoma without hepatitis C virus eradication; and cohort 4 included patients without hepatocellular carcinoma development or hepatitis C virus eradication. The first step in the matching process was to match age, gender, and platelet counts between hepatocellular carcinoma cases and controls after hepatitis C virus eradication. The second step was to match age, gender, platelet counts, tumor number, and tumor diameter between hepatocellular carcinoma cases after hepatitis C virus eradication and those without hepatitis C virus eradication. The third step was to match age, gender, and platelet counts between hepatocellular carcinoma cases and controls without hepatitis C virus eradication. HCC = hepatocellular carcinoma, HCV = hepatitis C virus, SVR = sustained viral response.
Statistical Analyses
Data were presented as means and standard deviations or as medians and interquartile ranges for quantitative variables. Qualitative variables were expressed as numbers and percentages. Statistical differences between subgroups were analyzed using the Student t-test or Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. To analyze the diagnostic accuracy of AFP for differentiating HCC cases from controls, an area under the receiver operator characteristic curve (AUROC) was calculated. The optimal cutoff value was validated by calculating the Youden index.24 All tests were 2-tailed with a P < 0.05 considered to indicate a significant difference. Statistical analyses were performed using R 2.13.0 (http://www.R-project.org).
RESULTS
Patient Profiles
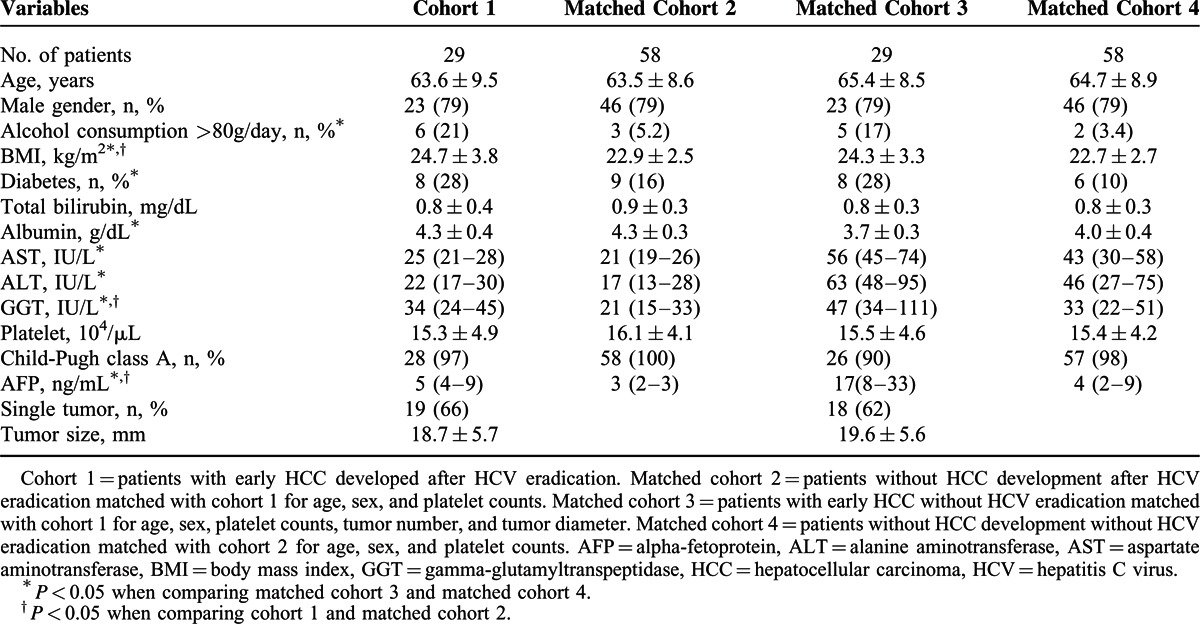
Cohort 1 included 29 total patients who were diagnosed with early stage HCC after HCV eradication. The mean age of these patients at initial HCC development was 63.6 ± 9.5 years, and 79% were male. The median time between HCC diagnosis and achieving SVR was 4.7 years (interquartile ranges, 2.4–8.5). HCC was solitary in 19 patients (66%), and the mean tumor size was 18.7 ± 5.7 mm. Matched cohorts were extracted according to the matching process described in Figure 1. The baseline characteristics of these patients are shown in Table 1. Each cohort was matched for age, gender, platelet counts, tumor size, and tumor number. When compared with the non-HCC cohorts, the HCC cohorts showed significantly elevated serum AFP levels (cohort 1, 5 [range 4–9] vs matched cohort 2, 3 [range, 2–3] ng/mL; matched cohort 3, 17 [8–33] vs matched cohort 4, 4 [2–9] ng/mL, P < 0.001). Serum gamma-glutamyl transpeptidase levels were significantly higher in HCC cohorts than in non-HCC cohorts (cohort 1, 34 [24–45] vs matched cohort 2, 21 [15–33] IU/L, P < 0.001; matched cohort 3, 47 [34–111] vs matched cohort 4, 33 [22–51] IU/L, P = 0.01). Body mass index was significantly higher in the HCC cohorts than in the non-HCC cohorts (cohort 1, 24.7 ± 3.8 vs matched cohort 2, 22.9 ± 2.5 kg/m2, P = 0.009; matched cohort 3, 24.3 ± 3.3 vs matched cohort 4, 22.7 ± 2.7 kg/m2, P = 0.04), and there was a greater number of patients who consumed >80 g/day alcohol in the HCC cohorts than in the non-HCC cohorts (cohort 1, 21% vs matched cohort 2, 5.2%, P = 0.06; matched cohort 3, 17% vs matched cohort 4, 3.6%, P = 0.04).
TABLE 1.
Baseline Patient Characteristics in Each Cohort
Performance of AFP Level in Differentiating HCC Cases From Controls
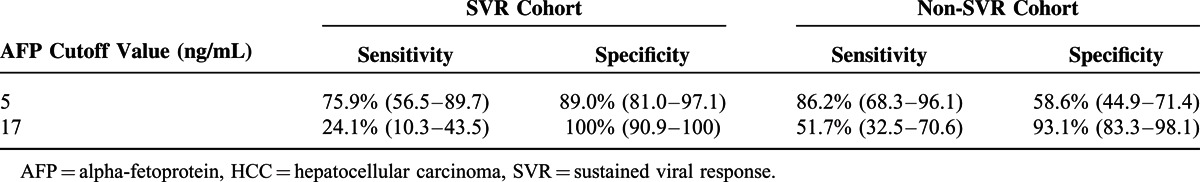
The Receiver operating characteristic (ROC) curve for AFP levels in the SVR cohorts is shown in Figure 2. The optimal cutoff value validated by calculating the Youden index was 5 ng/mL. The AUROC was 0.86 (95% confidence interval [CI]: 0.76–0.96). The ROC curve for AFP levels in the non-SVR cohorts is shown in Figure 3. The optimal cutoff value was 17 ng/mL. The AUROC was 0.83 (95% CI: 0.74–0.91). Table 2 shows the sensitivity and specificity of AFP levels for differentiating HCC patients from non-HCC patients at these two cutoff values. Using the cutoff value of 17 ng/mL, the sensitivity and specificity of AFP levels in the SVR cohorts were 24.1% and 100%, respectively. Using the lower cutoff value of 5 ng/mL, the sensitivity increased to 75.9%, whereas the specificity decreased to 89.0%. At both lower and higher cutoff values, the specificity of the AFP levels was higher in the SVR cohorts than in the non-SVR cohorts.
FIGURE 2.
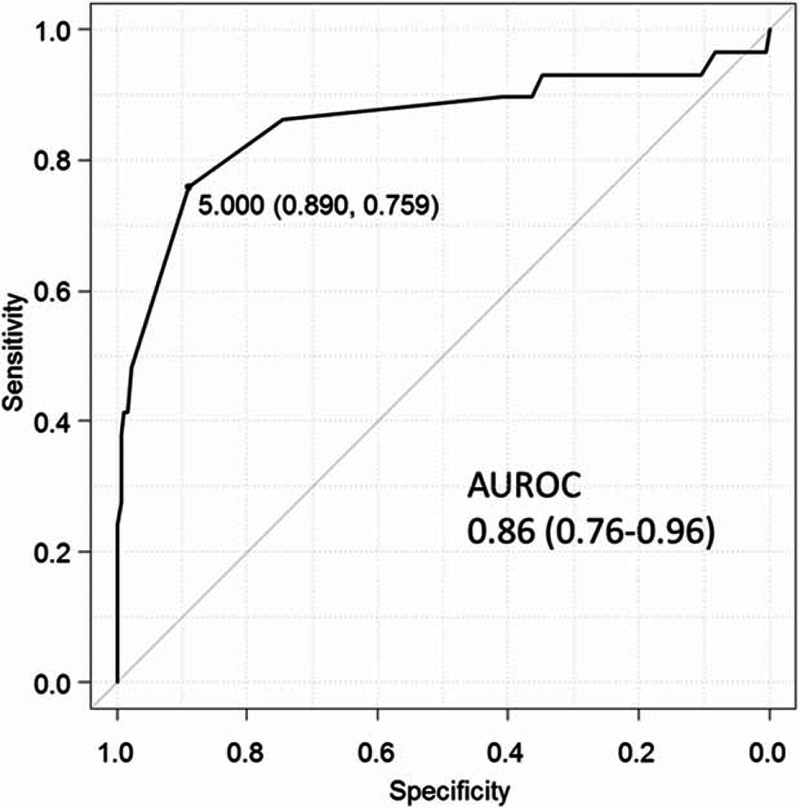
Receiver operating characteristic curve of alpha-fetoprotein levels differentiating hepatocellular carcinoma cases from controls after hepatitis C virus eradication. AUROC = areas under the receiver operating characteristic curve.
FIGURE 3.
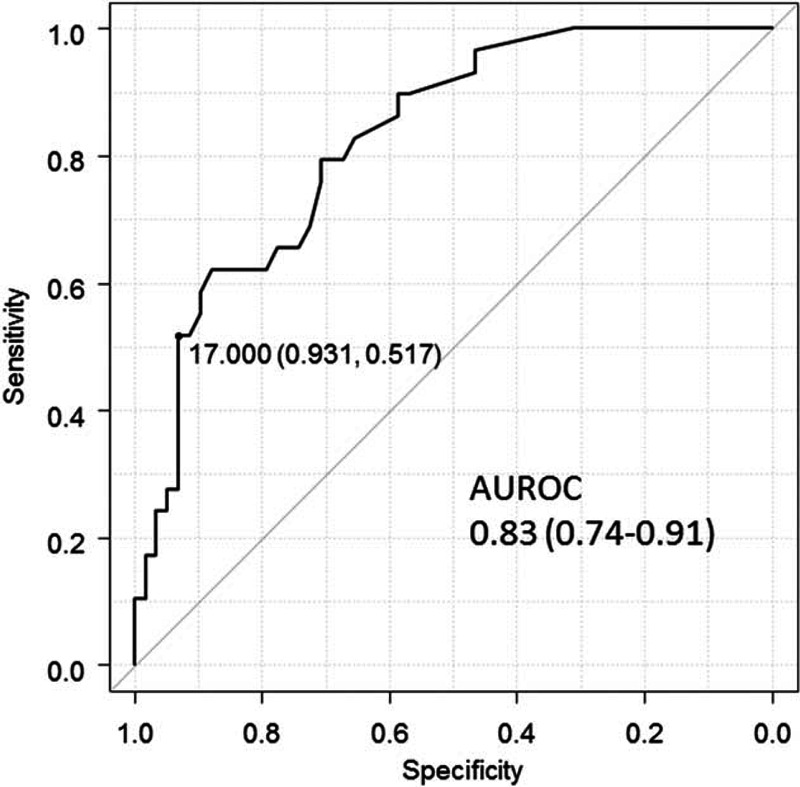
Receiver operating characteristic curve of alpha-fetoprotein levels differentiating hepatocellular carcinoma cases from controls without hepatitis C virus eradication. AUROC = areas under the receiver operating characteristic curve.
TABLE 2.
Sensitivity and Specificity of AFP for Differentiating HCC From Non-HCC Patients in SVR and Non-SVR Cohorts
Role of AFP in Differentiating HCC Cases After HCV Eradication Stratified by Albumin Level, ALT Level, and Platelet Counts
Subgroup analysis of the role of AFP in differentiating HCC from controls after HCV eradication is shown in Figure 4. Sensitivity and specificity were similar between patients with low (<4.3 g/dL) and high albumin levels (≥4.3 g/dL) (75.0% vs 76.5%; 92.0% vs 90.9%, respectively). The specificity was higher in patients with low (<20 IU/L) compared with high ALT levels (≥20 IU/L) (94.3% vs 87.0%), as well as in patients with high (≥15 × 104/μL) compared with low platelet counts (<15 × 104/μL) (96.8% vs 85.2%). In contrast, sensitivity was lower in patients with high (68.8%) compared with low platelet counts (84.6%).
FIGURE 4.
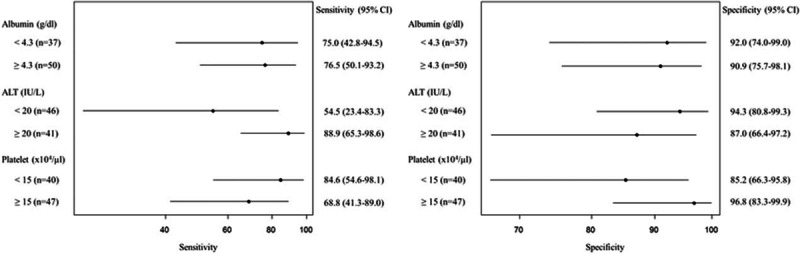
Forest plot of the sensitivity and specificity of alpha-fetoprotein levels for differentiating hepatocellular carcinoma cases from controls after hepatitis C virus eradication, using a cutoff value of 5 ng/mL, stratified by albumin level, alanine aminotransferase level, and platelet counts. ALT = alanine aminotransferase.
DISCUSSION
This study demonstrated AFP to be a specific tumor marker for the diagnosis of HCC after HCV eradication compared with patients without HCV eradication. This could occur because liver inflammation stabilizes after HCV eradication, which decreases false elevations in AFP levels. It was reported previously that AFP was a specific marker for the diagnosis of HCC in chronic hepatitis B patients receiving entecavir.25 In this study, AFP was also a specific marker for HCC after HCV eradication. As shown in Table 2, the AFP level was more specific in SVR cohorts compared with non-SVR cohorts at any of the cutoff values used. Using a cutoff value of 5 ng/mL, the AFP level had a specificity of 89.0% and a sensitivity of 75.9%, compared with the higher cutoff value of 17 ng/mL, which was optimal in non-SVR cohorts with improved specificity as high as 100% but decreased sensitivity to 24%.
AFP testing is not recommended for HCC surveillance by the American Association for the Study of Liver Diseases practice guidelines because of the lack of adequate sensitivity and specificity of AFP for detecting HCC.20 Meta-analysis showed that AFP provided no additional benefit to surveillance using ultrasonography.26 However, ultrasonography might be difficult in obese patients or cirrhotic patients,27 and ultrasonography performance for HCC detection depends on the skills of the operator and the quality of the equipment. Therefore, effective tumor markers complementing ultrasonography are needed. In patients after HCV eradication, AFP could provide a marker that differentiates HCC cases from controls because of the high specificity of AFP levels. This could lead to further imaging studies by CT or MRI, even if ultrasound findings are negative, to confirm the diagnosis of HCC. In addition, when using the lower cutoff value of 5 ng/mL, improving sensitivity to 76% was achieved with a mild decrease in specificity to 89%.
Because IFN-based therapies are often associated with various adverse events and low antiviral efficacy, they are less likely to be recommended for aged patients or cirrhotic patients.28,29 Cirrhotic patients are indicated for IFN-free regimens because of drug safety and high efficacy.4,5 As reported previously, elevated serum AFP was associated with progressive liver fibrosis or aspartate aminotransferase levels.17 Therefore, the AFP specificity for differentiating HCC cases from controls among cirrhotic patients or patients with higher ALT levels after HCV eradication was expected to be lower than in noncirrhotic patients or those with lower ALT levels. In the current study, Figure 4 demonstrates lower specificity in patients with lower platelet counts or higher ALT levels after HCV eradication, albeit remaining as high as 85.2% or 87.0%, respectively.
The strength of this study was the use of well-matched cases and controls. AFP levels are influenced by nontumoral factors such as etiology of chronic liver disease and progression of cirrhosis.12,17 Therefore, we matched age and gender, as well as platelet counts, which reflect liver fibrosis.30,31 Without this matching process, the HCC cases would have comprised patients with more progressive liver fibrosis, which is associated with elevated AFP levels, leading to an overestimation of the diagnostic accuracy of AFP levels. Another advantage of our study is that it demonstrated the usefulness of AFP levels for the diagnosis of early stage HCCs after HCV eradication, which could lead to curative therapies.
There are several limitations of the current study. First, longitudinal data for AFP level measurements in HCC patients after HCV eradication were not available. This is because our hospital is a tertiary care center, and HCC patients after HCV eradication consisted primarily of patients referred to our hospital after diagnosis of HCC and after HCV eradication in other hospitals. Second, the number of HCC cases after HCV eradication was relatively small. However, eradication of HCV was proven to be associated with reduced incidence of HCC, and HCCs after HCV eradication are expected to occur less frequently. Third, in the matching process, 2 non-HCC patients were selected per HCC patients, which did not reflect the actual incidence rate of HCC after HCV eradication. Forth, it could not be assessed in this study design whether the use of AFP improves efficiency in HCC surveillance based on ultrasound.
In conclusion, AFP is a specific tumor marker for the diagnosis of HCC after HCV eradication using an optimal cutoff value of 5 ng/mL.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, ALT = alanine aminotransferase, AUROC = areas under the receiver operating characteristic curve, CI = confidence interval, CT = computed tomography, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, MRI = magnetic resonance imaging, SVR = sustained viral response.
The English in this document has been checked by at least 2 professional editors, both are native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/ZNtCg0
This work was supported by Health Sciences Research Grants of The Ministry of Health, Labour and Welfare of Japan (Research on Hepatitis). No additional external funding received for this study.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Kiyosawa K, Umemura T, Ichijo T, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology 2004; 127:S17–S26. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti RG, Camma C, Fiorello F, et al. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis. A case-control study. Ann Intern Med 1992; 116:97–102. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370:1973–1982. [DOI] [PubMed] [Google Scholar]
- 6.Zeuzem S, Berg T, Gane E, et al. Simeprevir Increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a Phase IIb Trial. Gastroenterology 2014; 146:430–441. [DOI] [PubMed] [Google Scholar]
- 7.Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010; 52:518–527. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med 1999; 131:174–181. [DOI] [PubMed] [Google Scholar]
- 9.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–2593. [DOI] [PubMed] [Google Scholar]
- 10.Enokimura N, Shiraki K, Kawakita T, et al. Hepatocellular carcinoma development in sustained viral responders to interferon therapy in patients with chronic hepatitis C. Anticancer Res 2003; 23:593–596. [PubMed] [Google Scholar]
- 11.Nagaoki Y, Aikata H, Miyaki D, et al. Clinical features and prognosis in patients with hepatocellular carcinoma that developed after hepatitis C virus eradication with interferon therapy. J Gastroenterol 2011; 46:799–808. [DOI] [PubMed] [Google Scholar]
- 12.Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 2001; 34:570–575. [DOI] [PubMed] [Google Scholar]
- 13.Gebo KA, Chander G, Jenckes MW, et al. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology 2002; 36:S84–S92. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen MH, Garcia RT, Simpson PW, et al. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology 2002; 36:410–417. [DOI] [PubMed] [Google Scholar]
- 15.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005; 43:434–441. [DOI] [PubMed] [Google Scholar]
- 16.Di Bisceglie AM, Hoofnagle JH. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer 1989; 64:2117–2120. [DOI] [PubMed] [Google Scholar]
- 17.Hu KQ, Kyulo NL, Lim N, et al. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol 2004; 99:860–865. [DOI] [PubMed] [Google Scholar]
- 18.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 2010; 138:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tateishi R, Yoshida H, Matsuyama Y, et al. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008; 2:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009; 49:1954–1961. [DOI] [PubMed] [Google Scholar]
- 22.Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 1999; 30:889–893. [DOI] [PubMed] [Google Scholar]
- 23.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954; 7:462–503. [DOI] [PubMed] [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 25.Wong GL, Chan HL, Tse YK, et al. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology 2013. [DOI] [PubMed] [Google Scholar]
- 26.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009; 30:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Tateishi R, Yoshida H, et al. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol Int 2009; 3:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki Y, Ikeda H, Araki Y, et al. Limitation of combination therapy of interferon and ribavirin for older patients with chronic hepatitis C. Hepatology 2006; 43:54–63. [DOI] [PubMed] [Google Scholar]
- 29.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965. [DOI] [PubMed] [Google Scholar]
- 30.Karasu Z, Tekin F, Ersoz G, et al. Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Dig Dis Sci 2007; 52:1535–1539. [DOI] [PubMed] [Google Scholar]
- 31.Lu SN, Wang JH, Liu SL, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer 2006; 107:2212–2222. [DOI] [PubMed] [Google Scholar]


