Abstract
Anemia is the most frequent complication of inflammatory bowel disease (IBD), but anemia, mostly due to iron deficiency, has long been neglected in these patients.
The aim was to briefly present the pathophysiology, followed by a balanced overview of the different forms of iron replacement available, and subsequently, to perform a systematic review of studies performed in the last decade on the treatment of iron-deficiency anemia in IBD.
Given that intravenous therapies have been introduced in the last decade, a systematic review performed in PubMed, EMBASE, the Cochrane Library, and the websites of WHO, FDA, and EMA covered prospective trials investigating the management of iron-deficiency anemia in IBD published since 2004.
A total of 632 articles were reviewed, and 13 articles (2906 patients) with unique content were included. In general, oral supplementation in iron-deficiency anemia should be administered with a target to restore/replenish the iron stores and the hemoglobin level in a suitable way. However, in patients with IBD flares and inadequate responses to or side effects with oral preparations, intravenous iron supplementation is the therapy of choice. Neither oral nor intravenous therapy seems to exacerbate the clinical course of IBD, and intravenous iron therapy can be administered even in active disease stages and concomitantly with biologics.
In conclusion, because many physicians are in doubt as to how to manage anemia and iron deficiency in IBD, there is a clear need for the implementation of evidence-based recommendations on this matter. Based on the data presented, oral iron therapy should be preferred for patients with quiescent disease stages and trivial iron deficiency anemia unless such patients are intolerant or have an inadequate response, whereas intravenous iron supplementation may be of advantage in patients with aggravated anemia or flares of IBD because inflammation hampers intestinal absorption of iron.
INTRODUCTION
Anemia is the most common complication of inflammatory bowel disease (IBD)1,2 both at diagnosis and during flare-ups,3,4 exceeding by far the frequency of extraintestinal manifestations (eg, rheumatic, dermatologic, and ophthalmologic).5,6 Thus, in a systematic review from 2014 the prevalence of anemia in patients treated in tertiary referral centers with Crohn disease (CD) was 27% (95% confidence interval 19–35), and 21% (95% confidence interval 15–27) for ulcerative colitis (UC).1 This huge variation may be due to differences in the study populations (eg, hospitalized patients vs. outpatients) as well as in the definition of anemia. In recent published studies of IBD patients, the calculated mean prevalence was 20% among outpatients7 and 68% among hospitalized patients.8 Furthermore, anemia is more common in CD than in UC, and women with CD are at a higher risk for anemia.9
Anemia in IBD is mostly multifactorial, resulting, on the one hand, from chronic intestinal blood loss from inflamed intestinal mucosa combined with impaired iron absorption mainly as a consequence of inflammation but also in association with intake of proton pump inhibitors, persisting H. pylori infection or reduced food and thus impaired dietary iron uptake. Moreover, cytokines and acute phase proteins being induced upon inflammation impair iron availability for erythropoiesis; cause, a blunted biological activity of erythropoietin, and an inflammation driven impairment of erythroid progenitor cell proliferation.10,11 In general, anemia is found in various chronic inflammatory diseases, including cancer, infection, and autoimmune diseases, and this so-called anemia of chronic disease or anemia of inflammation is more prevalent in patients with advanced disease and those responding poorly to therapy.11 Additionally, anemia in IBD patients occasionally may be induced or aggravated by drugs used for IBD treatment or by vitamin deficiencies, as well as rarely for various other reasons (eg, renal insufficiency, hemolysis, and innate hemoglobinopathies),12 and a recent population based study revealed that patients with IBD have an insufficient intake of iron in their diet.13
Treatment of iron-deficiency anemia is very likely to have a beneficial impact on the affected patients because various organs may be disturbed as a result of the anemia [eg, central nervous system (impaired cognitive function, fatigue, “restless syndrome,” and depression), immune system (impaired reactive oxygen species production and alterations in cell functions), cardiorespiratory system (exertional dyspnea, tachycardia, palpitations, cardiac hypertrophy, systolic ejection murmur, and risk of cardiac failure), vascular system (hypothermia and pallor of skin), genital tract (loss of libido and menstrual problems), and gastrointestinal tract (anorexia, nausea, and motility disorders)].14,15
The aims of this paper are to clarify the pathophysiology of anemia of IBD, to provide a balanced overview of the various forms of treatment, focusing on the approaches to iron replacement that are available for management, and to perform a systematic review to summarize the latest evidence (ie, within the last decade, during which intravenous regimens have been added to the therapeutic armamentarium) with respect to the diagnosis and treatment of choice for anemia in IBD. Finally, based on our systematic review, we aimed to provide an updated decision algorithm for the management of anemia in IBD.
PATHOPHYSIOLOGY OF ANEMIA IN INFLAMMATORY BOWEL DISEASE
Iron deficiency in IBD is caused by numerous factors, including increased iron loss from bleeding due to gastrointestinal inflammation and decreased iron absorption as a consequence of short bowel syndrome, loss of appetite during IBD flares, and inflammation-driven blockage of intestinal iron acquisition and macrophage iron reutilization.16 The average adult harbors at least 3–4 g of stored iron that is balanced between physiologic iron loss and dietary intake. Most iron is incorporated into hemoglobin (Hb), whereas the remainder is stored as ferritin, myoglobin, or within iron-containing enzymes. About 20–25 mg of iron is needed daily for heme synthesis. Approximately 1–2 mg of this requirement comes from dietary intake, and the remainder is acquired by recycling iron from senescent erythrocytes.17,18 Total iron loss averages about 1–2 mg/day, mostly via the feces and cellular desquamation from the skin and intestine, as well as additional losses through menstruation.19,20
Body iron homeostasis is regulated systemically by multiple mechanisms, among which the interaction of the liver-derived peptide hepcidin with the major cellular iron exporter ferroportin is of pivotal importance (Figure 1). The formation and release of hepcidin are induced by iron loading and inflammatory stimuli such as interleukin 1 (IL-1) or IL-6, whereas its synthesis is blocked by iron deficiency, hypoxia, and anemia.21–23 Hepcidin targets ferroportin on the cell surface, resulting in ferroportin internalization and degradation and blockage of cellular iron egress.22 Whereas low circulating hepcidin levels enable an efficient transfer of iron from enterocytes and macrophages to the circulation in order to overcome iron deficiency, iron is retained in these cells when hepcidin levels are high and serum iron levels drop23,24 (Figure 1). Furthermore, inflammatory cytokines can directly inhibit iron absorption and stimulate the uptake and retention of iron in macrophages via hepcidin-independent pathways.21 Of interest, circulating hepcidin levels have an impact on the efficacy of oral iron therapy and can predict nonresponsiveness,25 which is in line with experimental data demonstrating reduced intestinal ferroportin expression and iron absorption in individuals with increased hepcidin levels primarily as a consequence of inflammation.24 As a result, anemia develops and is characterized by low circulating iron levels and an iron-restricted erythropoiesis in the presence of high iron stores in the reticuloendothelial system, reflected by normal or high levels of ferritin. Cytokine-driven induction of hepcidin expression and the direct effects of cytokines on iron trafficking in macrophages play a decisive role in the development of this type of anemia (ie, anemia of chronic disease or the anemia of inflammation), by retaining iron in the reticuloendothelial system and blocking iron absorption, which results in an iron-limited erythropoiesis.11,26 The latter is reflected clinically by a reduced transferrin saturation that according to national guidelines is below 16% or 20%.27 In addition, cytokines and chemokines further contribute to anemia by negatively affecting the activity of erythropoietin, by inhibiting the proliferation and differentiation of erythroid progenitor cells, and by reducing the circulatory half-life of erythrocytes.11
FIGURE 1.
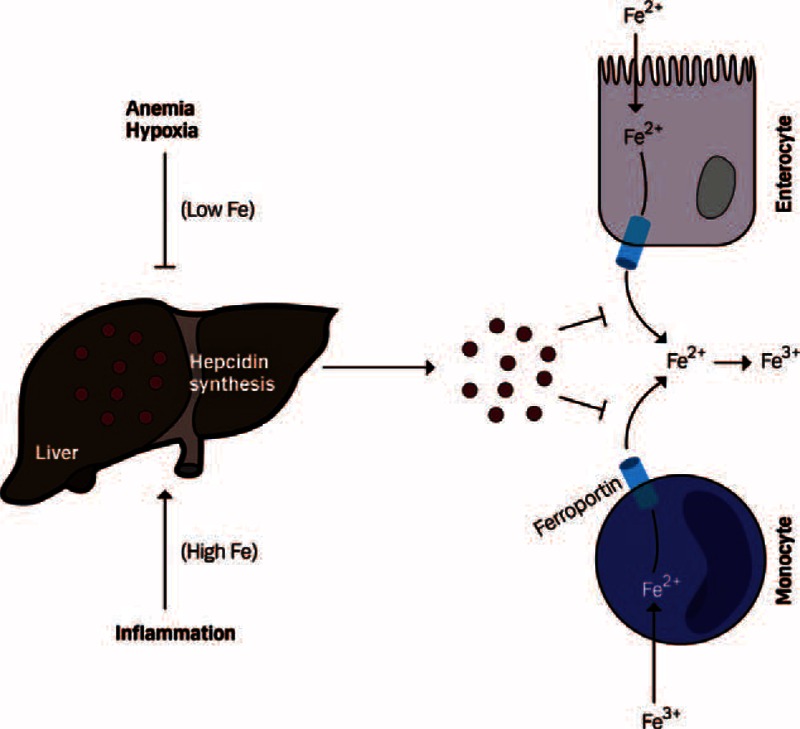
Regulation of systemic iron homeostasis by hepcidin. Enterocytes and monocytes release Fe2+ via the iron exporter ferroportin, which is then oxidized to Fe3+ and transported via the bloodstream. Liver-derived hepcidin inhibits iron efflux from these cells by binding to ferroportin, which promotes ferroportin internalization and degradation. The synthesis of hepcidin in the liver is induced by iron and inflammatory signals and suppressed by iron deficiency, anemia, or hypoxia.
Importantly, patients with active IBD suffer from chronic blood loss due to mucosal bleeding, which often causes true iron deficiency, as reflected by low ferritin levels.28,29 Moreover, true iron deficiency and anemia reduce hepcidin expression. These effects are transmitted by iron-deficiency-mediated inhibition of SMAD signaling in hepatocytes, anemia-induced and erythropoiesis-driven formation of hepcidin inhibitors such as erythroferron and growth differentiation factor 15 (GDF-15), and hypoxia-driven blockade of hepcidin formation via platelet-derived growth factor BB (PDGF-BB) or hypoxia-inducible factors (HIFs).30–34 Thus, in the presence of both inflammation and true iron deficiency due to bleeding in IBD, circulating hepcidin levels decrease because anemia and iron-deficiency regulatory signals dominate inflammation-driven hepcidin induction.34,35 Therefore, truly iron-deficient patients, even in the presence of systemic inflammation, are able to absorb considerable amounts of iron from the intestine.22,24
Furthermore, vitamin deficiencies (eg, vitamin B12, folic acid, and vitamin D) due to either intestinal inflammation or extensive bowel resection contribute to the development of anemia.29,36 Drugs used for the treatment of patients with IBD, such as proton pump inhibitors, sulfasalazine, methotrexate, and thiopurines, as well as functional impairment of the intestine due to inflammation or previous surgery, may aggravate anemia by negatively affecting iron absorption or erythropoiesis.37
TREATMENT OF ANEMIA
The primary treatment of anemia of chronic disease is the cure of the underlying disease which in most cases leads to resolution or at least improvement of anemia unless other pathophysiological factors or deficiencies are involved.4,9,11,12,15 In cases of severe anemia (ie, Hb < 7–8 g/dL),38,39 specifically when it develops rapidly on the basis of acute gastrointestinal bleeding, or if the patient suffers from comorbidities resulting in aggravation of anemia-related symptoms such as coronary heart disease or chronic pulmonary disease, application of blood transfusions might be treatment of choice because this can rapidly correct anemia and increase Hb levels.11,12,15,40 However, the indication for transfusions must be considered carefully as negative effects have been documented.40,41 These include an increased mortality in patients with liberal use of blood transfusions for the treatment of upper gastrointestinal bleeding,42 an increased nosocomial infection rate and mortality among intensive care patients,43 higher frequency of surgical site infections,44 the occurrence of transfusion-related anaphylactic reactions along with a small but residual risk for transmitting infections.45–47 Of note, if other easy-to-treat reasons contributing to anemia have been identified, such as vitamin deficiency, these should be corrected accordingly.
VARIOUS FORMS OF IRON REPLACEMENT
As imbalances of iron homeostasis are the major reason for anemia in IBD, this treatment strategy is in the focus of this review. Before going to the systematic analysis of clinical trials using oral and intravenous regimens, the currently available iron supplementation options are highlighted.
Oral Regimen
Oral iron supplementation is frequently used to treat iron-deficiency anemia partly because of an established safety profile, ease of administration, and a general low cost, although in a pharmacoeconomical setting the cost-effectiveness is more important.16 Oral iron supplements are available as either divalent Fe2+ (ferrous) salts or a trivalent Fe3+ (ferric) form coupled with sugar complexes.48 The most widely used preparations are ferrous sulfate, ferrous gluconate, and ferrous fumarate containing the ferrous form of iron because of the poor solubility of ferric-containing formulations. For absorption by enterocytes, Fe3+ needs to be reduced to Fe2+ (Figure 2), which is catalyzed by a membrane-bound ferric reductase and augmented by ascorbic acid.49 Indeed, ascorbic acid (or vitamin C) facilitates increased absorption of oral iron.50 However, a recent phase III study has reported that oral ferric maltol [a new compound under review by the European Medicines Agency (EMA)] is an efficient alternative treatment option for iron-deficiency anemia in IBD patients who are unresponsive to or intolerant of oral ferrous products.51
FIGURE 2.
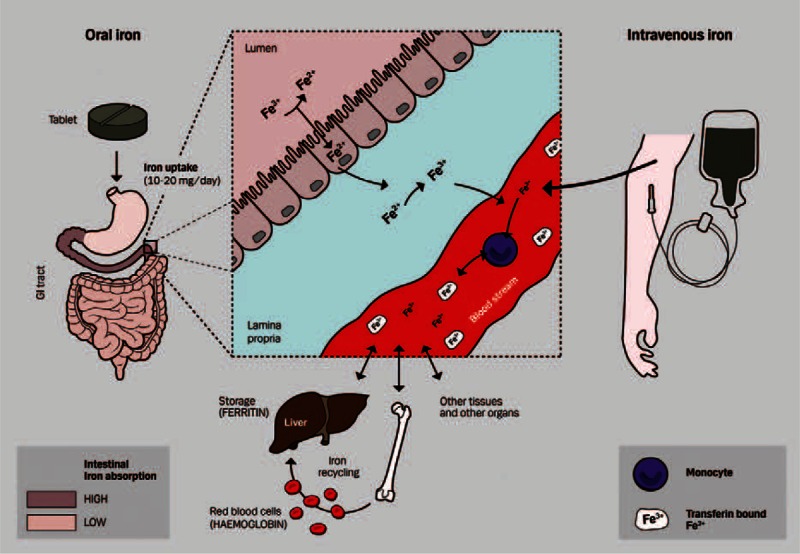
Iron absorption from oral or intravenous iron supplementation. Oral preparations of iron supplements are given as tablets and result in a daily absorption of 10–20 mg elemental iron (predominantly in the duodenum and upper jejunum). The oral iron supplementation mainly consists of the Fe2+ (ferrous) form that can be absorbed directly by enterocytes. Dietary iron, mostly in the Fe3+ (ferric) form, contains 10–30% of heme-bound iron, whereas the majority consists of nonheme iron (Fe3+ form). These 2 dietary iron formulations are taken up by enterocytes via different pathways with subsequent yield of Fe2+ which is exported to the circulation by ferroportin. Here, Fe2+ becomes oxidized to Fe3+ and specifically recognized and bound by transferrin and transported via bloodstream to target cells in the liver, bone marrow, and other tissues and organs for use or storage. Intravenous iron supplementation can be administered as high doses of iron directly in the bloodstream in its trivalent Fe3+ form, which is taken up by circulating monocytes (leading to an increase in their iron content), which redeliver Fe3+ to the blood circulation, where it is bound by plasma transferrin and transported to target cells.
Although the optimal dose in IBD has not been established, the commonly recommended dose of oral iron for the treatment of iron deficiency is 50–200 mg/day of elemental iron once daily,52 but only a maximum of 10–20 mg/day of iron is absorbed in iron-deficient patients.49,53 Given that a high proportion of nonabsorbed ingested iron remains in the gut, oral iron supplementation is associated with gastrointestinal side effects such as nausea, vomiting, diarrhea, abdominal pain, and constipation in up to 20% of patients.3,16 Nausea and abdominal discomfort generally occur 1–2 hours after intake and tend to be dose related, although other gastrointestinal side effects such as constipation and diarrhea are idiosyncratic.52,53 Nonetheless, delayed-release enteric-coated iron tablets may be used in patients reporting such intolerances. However, these tablets may not be absorbed as effectively as standard preparations because they dissolve slowly in the duodenum, where most iron is absorbed (Figure 2).
Most of the anxiety regarding the use of oral iron therapy comes from studies in animal models of IBD that have provided contradictory evidence regarding exacerbation and/or improvement of inflammation.54,55 However, in humans, the evidence has been more controversial, and even though there is no convincing evidence that oral iron given in therapeutic dosages is effective in humans with activate IBD,56,57 it is established that iron availability in the gut has a significant impact on the composition of the microbiome,58 which has a central role in the pathogenesis of IBD.59 The clinical significance of such changes remains speculative, but the evidence suggests that nutritional interventions may influence disease activity in patients with IBD.60
Intravenous Regimen
Parenteral iron administration traditionally has been reserved for patients with intolerance or inadequate response to oral iron and for patients in whom a rapid increase in iron stores (replenishment) is desired (eg, patients scheduled for surgery in the short term).3,4,12 This approach is reflected in the indications approved by the US Food and Drug Administration (FDA) for a number of intravenous iron preparations,61 as well as the EMA.62 Although severe or life-threatening anaphylactic reactions upon intravenous iron administration occur very infrequently and have been mainly observed with high molecular weight dextrans in the past, the risk for such severe adverse reactions is much lower with currently used preparations, including high molecular weight iron components.63 Compared with oral iron, intravenous iron seems to increase Hb and iron storage and improves quality of life more rapidly but not always more effectively.58,64 In addition, disadvantages include—apart from a generally higher cost—a risk of infusion-related adverse reactions, including anaphylaxis, which means that staff should be alerted to manage such potentially life-threatening situations.63 Today, 6 intravenous iron preparations are available, including iron dextran, iron sucrose, and iron gluconate and, the more recently licensed compounds, ferric carboxymaltose, iron isomaltoside 1000, and ferumoxytol16,65,66 (Table 1). The structures of these new preparations are more stable and allow only a low level of labile iron to be released into the circulation, resulting in improved safety profiles.
TABLE 1.
Characteristics of Different Intravenous Iron Preparations
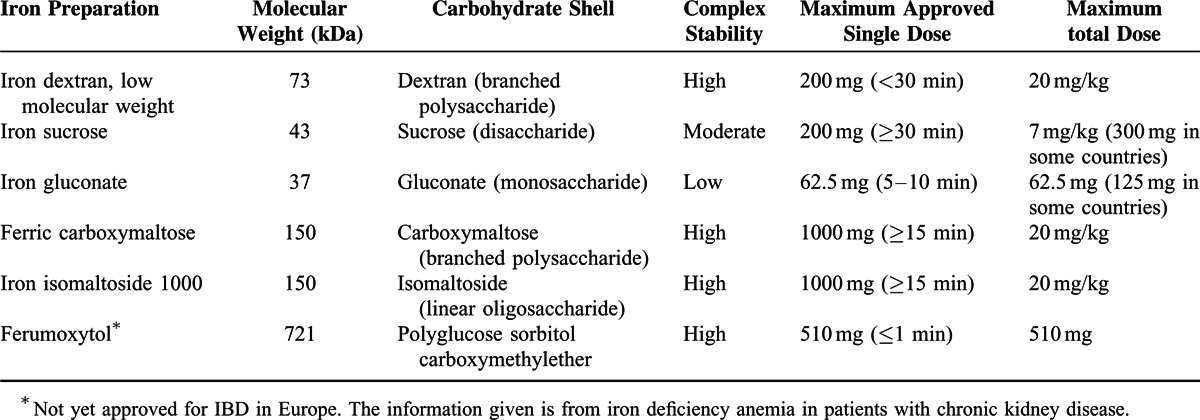
Iron dextran exists in 2 stable forms: a low- (73 kDa) and a high-molecular-weight (165 kDa) complex, although the latter has been associated with an increased risk of anaphylaxis and anaphylactoid reactions.67–70 Thus, only low-molecular-weight iron dextran is currently available in Europe71 and can be given at a maximum single dose of 200 mg over a minimum of 30 min. Given the risk of anaphylactoid reactions, it was previously recommended to administer a test dose of iron dextran (ie, 0.5 mL at a gradual rate over 2–5 min) before giving a full dose, but the EMA no longer recommends this precaution.62
Iron sucrose (43 kDa) and iron gluconate (37 kDa) are less stable and therefore can be administrated in a maximal single dose of only 200 mg (300 mg in some countries) over a (minimum) infusion time of 30 minutes72 or 62.5 mg (in some countries 125 mg) over an infusion time of 5–10 min,73 respectively, without a test dose. However, higher dosages74 or accelerated infusion rates75 are associated with increased adverse events such as transient hypotension due to the release of labile iron. Thus, iron dextran, iron sucrose, and iron gluconate preparations typically require multiple administrations of lower doses to replenish iron stores.
The introduction of more advanced iron formulations, however, has permitted high-dose infusions (without test dosing) with minimal side effects because of the low levels of labile iron released during administration. Ferric carboxymaltose76–80 and iron isomaltoside 100081,82 are highly stable 150-kDa complexes that are approved for clinical use. Their robust structures allow controlled and safe delivery of high-dose iron to the cells. Ferric carboxymaltose can be administered effectively and efficiently with a maximum single dose of 1000 mg over at least 15 minutes at a minimal interval of once per week.81 The structure of iron isomaltoside 1000 allows for administration of high single doses of up to 20 mg/kg of body weight within 15 minutes.81 Currently, there are limited data on iron isomaltoside 1000 in the treatment of iron-deficiency anemia in patients with IBD,82 although clinical trials are currently ongoing. Ferumoxytol is a much larger complex with a molecule weight of 721 kDa, which allows the drug to be given rapidly in relatively large doses. Although ferumoxytol is not yet approved for IBD in Europe, the current recommended intravenous dosing of this drug for patients with iron deficiency anemia due to chronic kidney disease is up to 510 mg in less than 1 min, with a second dose of 510 mg administered 3–8 days later.83 Although limited data are available on ferumoxytol in the treatment of anemia in patients with various gastrointestinal diseases,84 there are indications that the paramagnetic nature of ferumoxytol can interfere with MRI examinations.85 Because MRI is an important diagnostic tool in the management of patients with IBD, this drawback may seriously hamper its use in this patient population. Further, in a recent analysis of different intravenous iron products in the United States, ferumoxytol had the highest rate for adverse events per million units sold of all products,86 impeding its benefit–risk ratio, and since March 2015 it carries a boxed warning by FDA regarding potentially life-threatening allergic reactions.
Although different iron preparations have different side-effect profiles,68 the most frequently reported complaints after infusion of large-molecule iron complexes are itching, dyspnea, wheezing, and myalgias. In this context, it should be noted that acute myalgia at the first administration of intravenous iron (without any other symptoms) that ablates spontaneously within minutes (ie, the so-called Fishbane reaction) does not recur at rechallenge.87 Other, more certain side effects include hypotension, tachycardia, stridor, nausea, dyspepsia, diarrhea, and skin flushing, including periorbital edema. Serious side effects are rare88 and include cardiac arrest,89 but such problems are more common with older, mostly dextran-containing preparations.90 Therefore, close monitoring for signs of hypersensitivity during and for at least 30 min after each administration of an intravenous iron formulation and reduction of the infusion speed on occurrence of discomfort are recommended.62
SYSTEMATIC REVIEW OF CLINICAL TRIALS SINCE 2004 METHODS
Search Strategy
A systematic review was performed adhering to the guidelines established by the PRISMA Statement.91 A bibliographic search was performed in the PubMed and EMBASE databases from January 2004 (ie, before the era of intravenous iron supplementation) to March 2015 using combinations of the following medical subject heading search terms: “inflammatory bowel disease” or “Crohn's disease” or “ulcerative colitis” and “iron deficiency” or “anemia.” No prepublished protocol is accessible. Other sources of information were the Cochrane Library and the websites of WHO, FDA, and EMA. Figure 3 is a flowchart summarizing study identification and selection.
FIGURE 3.
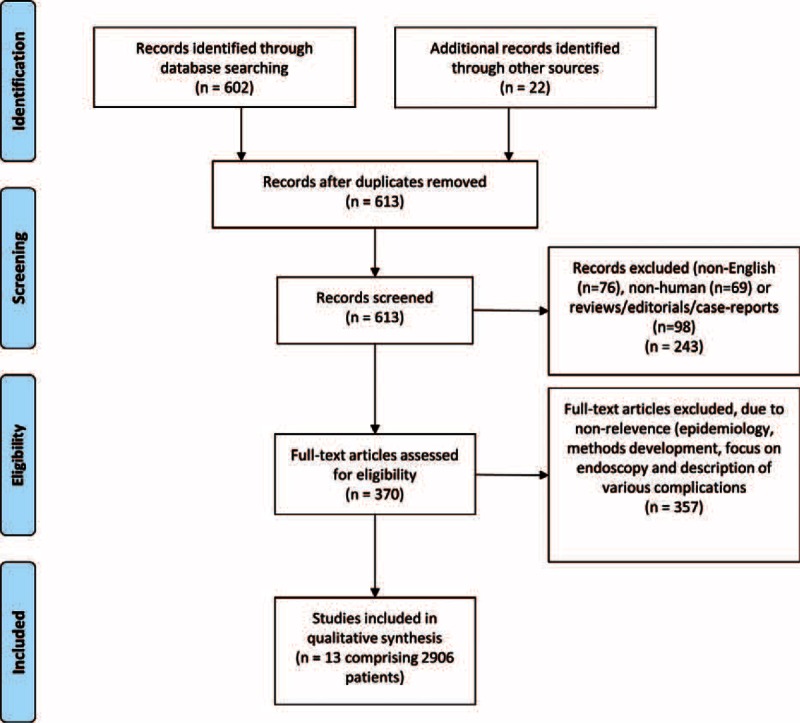
Flowchart of study screening process.
Selection Criteria
For the quality assessment, only original prospective studies evaluating the treatment of iron-deficiency anemia (ie, normalization of Hb concentration) in IBD patients with a minimum observation time of 4 weeks were included. Outcome assessment included correction of iron deficits causing anemia in IBD patients. Only English-language articles, excluding reviews and nonhuman investigations, were evaluated. Subsequently, articles were selected based on clinical relevance, and reference lists of relevant articles were hand searched to identify any additional studies.
Data Abstraction
Two authors (O.H.N. and M.C.) independently identified candidate articles from the results of the initial search on the basis of title and abstract. Subsequently, these 2 authors independently reviewed the full texts of candidate articles to identify interventions and assess study quality. Any discrepancies between the independent searchers were resolved in consensus with the 2 other authors (M.A. and G.W.).
Data Synthesis and Analysis
The literature search identified 13 randomized, controlled studies and prospective studies with and without control groups.51,54,64,69,72,77–80,84,92–94 Because of the considerable diversity in study designs (eg, oral and low- or high-dose intravenous drugs with different compositions), however, the authors were unable to conduct a meta-analysis.
The study was exempt from approval by the Scientific Ethics Committee of the Copenhagen Capital Region because the analysis involved only deidentified data, and all 13 studies included were granted individual ethics approval.
Funding Source
The National Danish and Austrian Research Funds funded the research but had no role in the design, conduct of the study, or preparation of the manuscript.
RESULTS
The systematic search yielded a total of 632 studies, of which 13 prospective trials met the above-mentioned inclusion criteria (Figure 3) and included 2906 patients. Only data acquired from the systematic search are included in this section, and key data from each study are presented in Table 2 with results based on the conclusion of each individual study.
TABLE 2.
Controlled Trials of Iron Deficiency Anemia Since 2004 Included in the Systematic Review

TABLE 2 (Continued).
Controlled Trials of Iron Deficiency Anemia Since 2004 Included in the Systematic Review
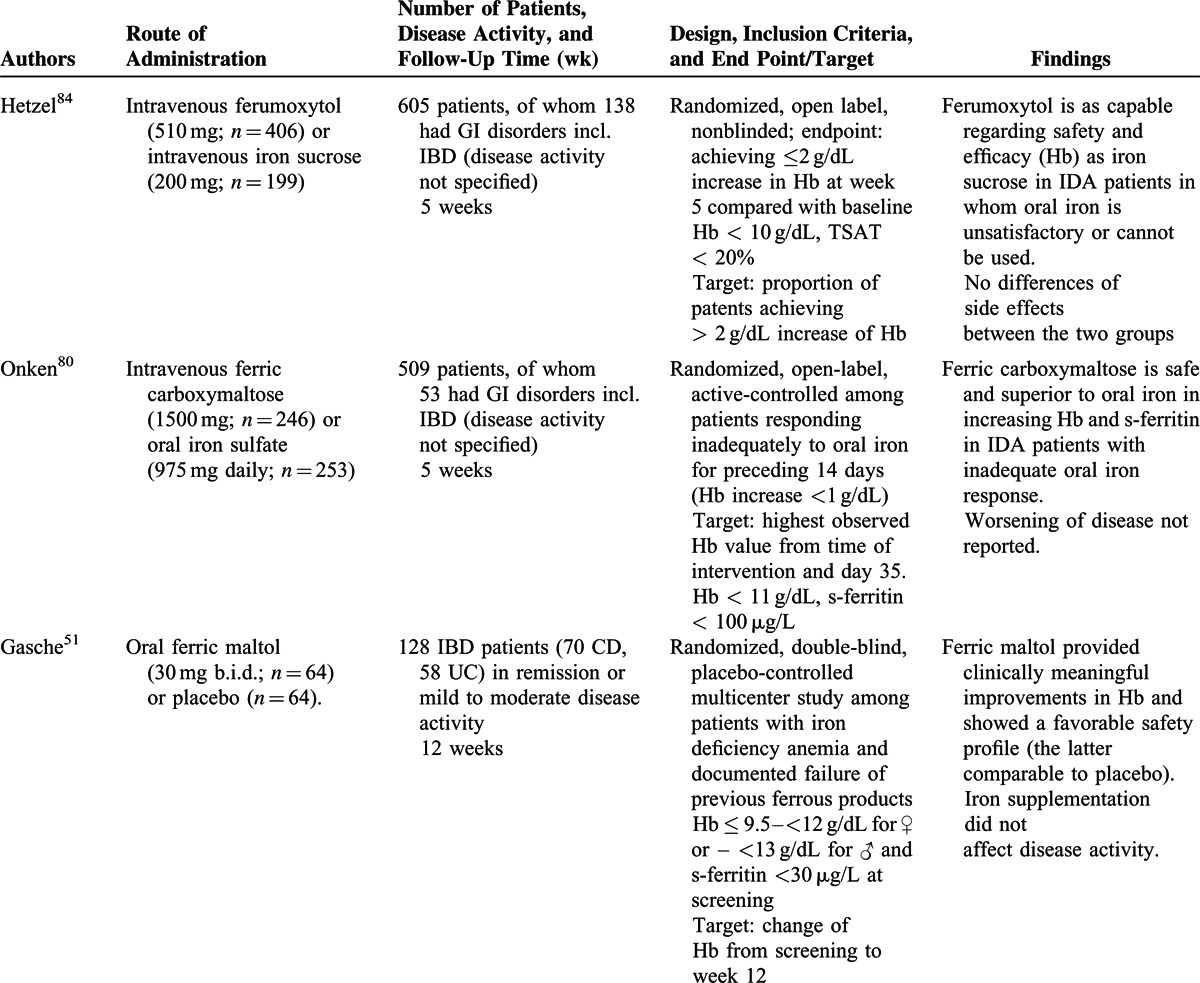
From the systematic review, it was revealed that apart from the WHO definitions of anemia38 (ie, Hb level of <13 g/dL for men and <12 g/dL for women), transferrin saturation [TSAT; ie, the quotient of iron concentration (μmol/L) divided by transferrin concentration (mg/dL) in fasting blood samples multiplied with 70.9 stated as a percentage] of <20%, and serum ferritin concentration of <30 μg/L with a serum C-reactive protein (CRP) level within the normal range or a ferritin concentration of <100 μg/L with an elevated serum CRP level comprised the laboratory tests used in the 13 studies for the diagnosis and assessment of iron-deficiency anemia in IBD.
Only 9 trials including oral iron supplementation in IBD patients have been published since 2004,51,54,64,69,72,79,80,92,94 but oral supplementation is from the published data well tolerated and has a positive effect on both Hb levels and body iron parameters (Table 2 ). From the studies included it seems that milder side effects (ie, abdominal discomfort, diarrhea, nausea, and vomiting) occurred less often after intravenous therapy as compared with oral therapy69,72,79,92,94 although 1 study did not report such differences.64 From the studies included no comparison between side effects to various forms of oral supplementations was, however, performed. From an examination of the available data, it was apparent that there are no data indicating that oral iron supplementation exacerbates symptoms of the underlying IBD. Only 1 study in this systematic review reported worsening of disease activity in 2 of 33 patients with UC (but not in patients with CD).54 However, in this study, the IBD quality-of-life scores improved significantly (P = 0.016) at the same time,54 and when the 8 studies using oral iron supplementation were evaluated, it was apparent that an adequate level of evidence is provided to address the safety of oral iron supplementation in IBD. Of note, a very recent study with oral ferric maltol suggests that this drug may be an alternative for patients who are unresponsive to or intolerant of formulations containing ferrous salts,51 which needs to be confirmed in future studies
In the trials included in the systematic review, it was observed that administration of intravenous iron in IBD patients frequently resulted in higher ferritin levels but not higher hemoglobin concentrations compared with oral iron supplementation in mild anemia (Hb ≥ 10 g/dL) and short-term follow-up,64,79,80,94 whereas in more aggravated iron deficiency anemia intravenous iron supplementation was superior to oral treatment regarding increase of Hb.69,80,92,94 Nevertheless, in all studies included in the systematic review, oral supplementation was administered for a minimum of 4 weeks with the target of normalizing Hb values.
In patients with IBD flares who have an inadequate response to experienced side effects with oral preparations, intravenous iron supplementation is the therapy of choice because it does not seem to exacerbate the clinical course of IBD and, in patients undergoing biological therapy with tumor necrosis factor (TNF) inhibitors, concomitant iron supplementation may be prescribed without affecting the disease course/activity.93
Finally, it was established that the correction of anemia with iron supplementation is associated with a relevant improvement in the patient's quality of life.92
DISCUSSION
For a long time, it was thought that the clinical symptoms of anemia occurred only when the Hb level dropped abruptly95 and, conversely, that patients would adapt to low Hb levels if the anemia developed slowly. This led to the concept of asymptomatic anemia. In truth, the term asymptomatic seems to reflect the fact that impairments in physical condition, quality of life, cardiovascular performance, and cognitive function may be unrecognized by both patients and their physicians. Therefore, the process of adaptation in chronic anemia would seem to be the acceptance/toleration of an impaired quality of life.95 Further, chronic fatigue caused by anemia may debilitate and even worry patients with IBD as much as abdominal pain or diarrhea.95 Therefore, the beneficial effect on quality of life derived from the correction of anemia in patients with IBD may be as important to patients as the control of their abdominal symptoms.95
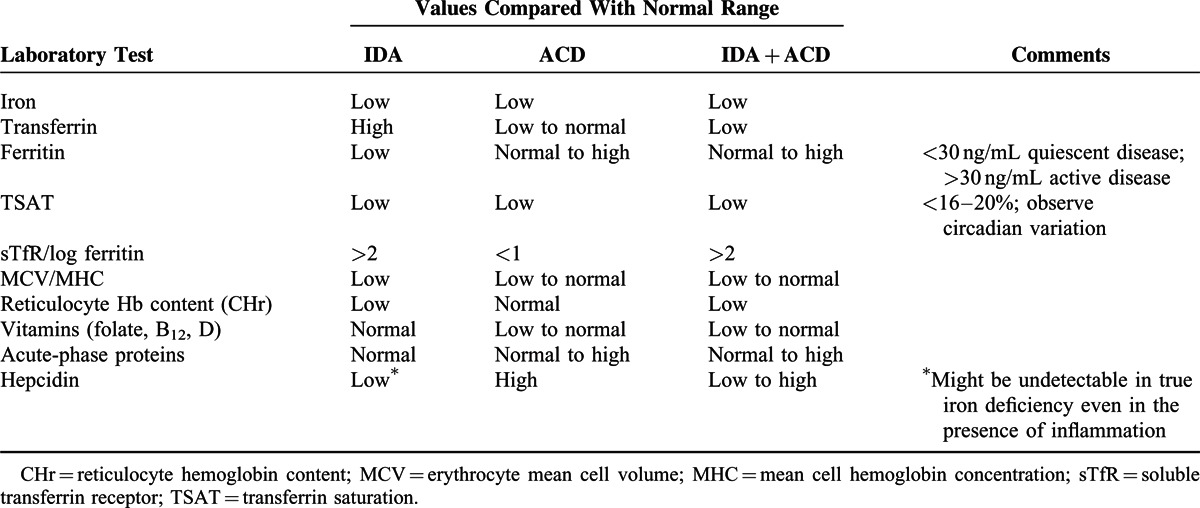
To tailor the most appropriate therapy for iron deficiency and anemia in patients with IBD, some basic diagnostic analyses are mandatory21,27,56 (Table 3). Thus, during active inflammatory stages of IBD, laboratory measures of iron status are more difficult to interpret because inflammation affects the laboratory parameters of iron metabolism.96 In the presence of chronic inflammation, the elevated transferrin levels characterizing iron deficiency may not be found because patients with low albumin levels tend to have lower transferrin concentrations.97 Moreover, the serum ferritin level, the most accessible and well-known surrogate marker of stored iron,98 can be normal or even increased in response to inflammation because ferritin expression is stimulated by several cytokines even in the presence of true iron deficiency.96 Therefore, although ferritin is generally considered to be the best indicator of iron deficiency, this parameter may not be reliable for the stored compartment in the setting of active inflammatory conditions, including IBD.11,96 Of note, both patients with inflammatory anemia and true iron-deficiency anemia have low transferrin saturation, which is a good indicator for a reduced availability of iron for erythropoiesis and thus has been used in a number of studies as a surrogate to determine the time to initiate iron therapy.53,56,95,99,100
TABLE 2 (Continued).
Controlled Trials of Iron Deficiency Anemia Since 2004 Included in the Systematic Review
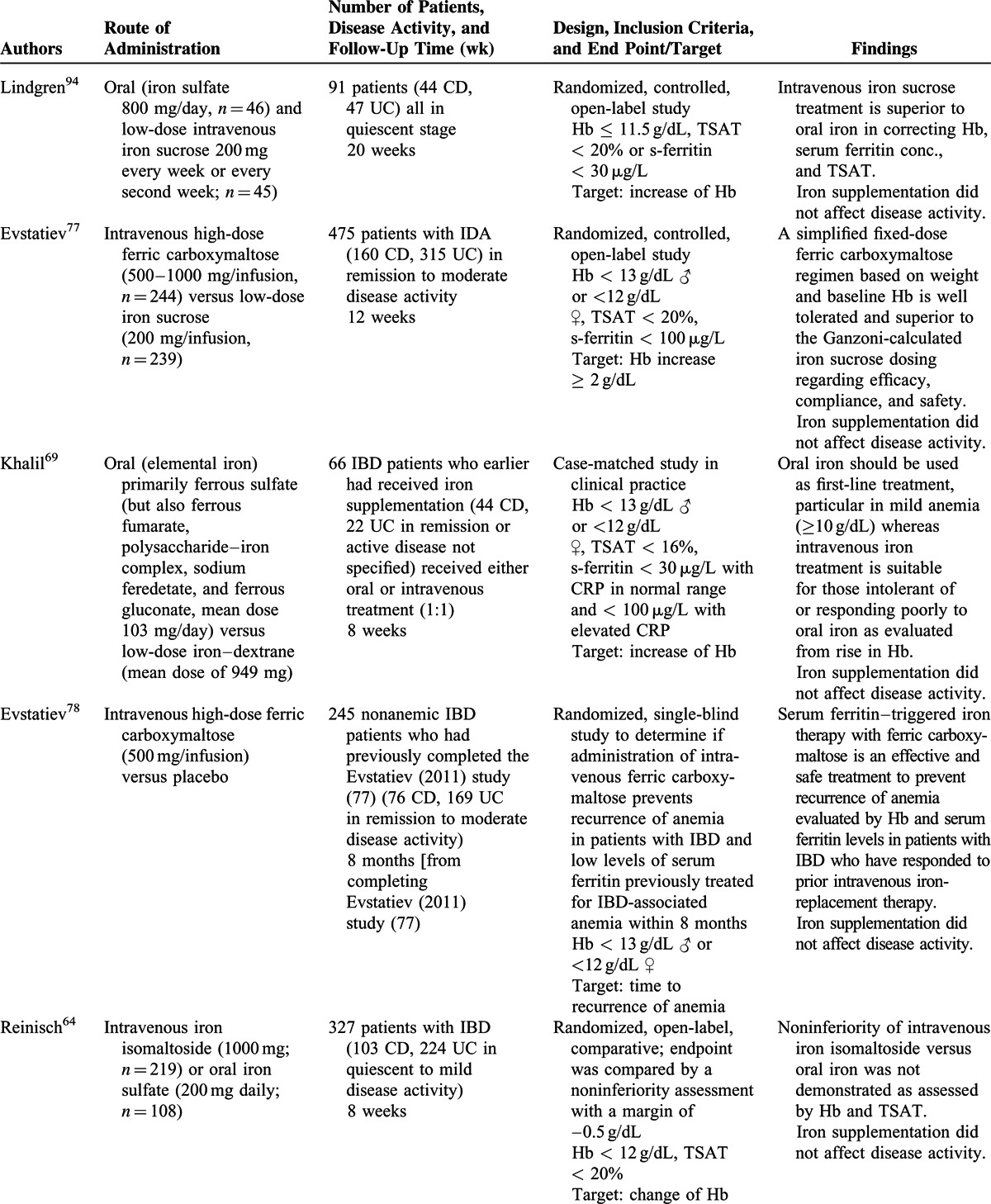
TABLE 3.
Surrogate Markers of Importance for Assessing Anemia Due to Either Possible Coexisting Iron Deficiency (IDA), Chronic Disease (ACD), or Both (IDA + ACD)
Comparative studies of intravenous versus oral iron supplementation in the systematic review did not demonstrate any significant difference in hemoglobin normalization favoring the use of intravenous iron therapy57,64,69,72,79,92 unless considered for patients with intolerance or an inadequate response to oral supplementation or during active disease stages. Moreover, a recent systematic review of randomized, controlled trials with the aim of assessing safety has demonstrated that intravenous iron therapy may increase the risk of infection.101
Because a great number of physicians are uncertain as to which diagnostic procedures and treatment regimen they should prescribe for their patients with iron-deficiency anemia,102 we performed an updated extensive review of the literature published in the last decade (during which novel approaches to intravenous iron supplementation have been introduced). In an independent screening and data extraction of references by 2 authors, only original prospective studies evaluating the treatment of iron-deficiency anemia in patients with IBD with a minimum observation time of 4 weeks were included, and the outcome assessment comprised correction of iron deficits causing anemia in patients with IBD. In terms of limitations, the studies included generally were characterized by small numbers of enrolled patients, were published solely in the English language, and were heterogeneous in design (ie, inclusion criteria and iron compositions administered, such as oral and low- or high-dose intravenous drugs), different enrollment populations as well as different lengths of therapy, and outcomes. Further, in some of the studies, a minimum 4-week treatment period was employed, although it is questionable whether such a short interval is always effective for the correction of iron deficiency in IBD, and thus the efficacy of the oral supplementation may be underestimated compared with a full treatment period of, for example, 3 months.
Based on our systematic review of the evidence, we developed an algorithm to help physicians in identifying IBD patients in need of iron supplementation and in selecting the most appropriate therapy regimen (Figure 4). If intravenous iron supplementation is considered, the use of older low-dose regimens is not recommended from the point of view of clinical practicality because a number of infusions might be needed over several days or weeks. Thus, high-dose regimens result in fewer infusions and increase the convenience and cost-effectiveness of intravenous iron repletion.
FIGURE 4.
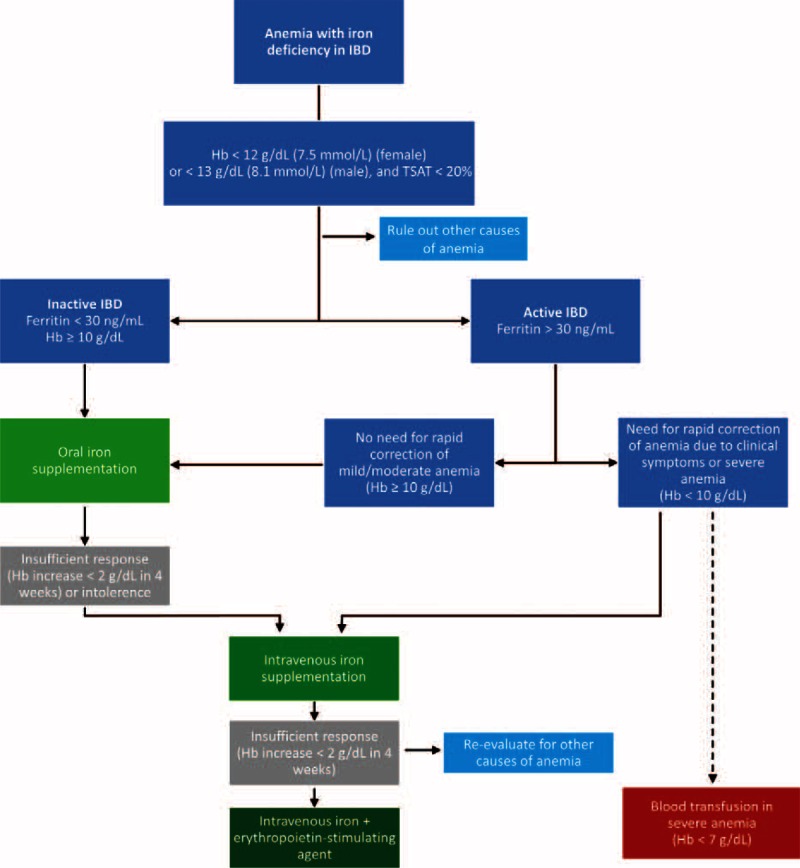
Algorithm for the treatment of anemia with iron deficiency in IBD. The decision tree provides a ready-to-use tool for clinicians to find the most appropriate therapy for patients with anemia/iron deficiency and IBD based on the data contained in this systematic review. Insufficient response is defined as a hemoglobin increase <2 g/dL within 4 weeks. Gastrointestinal intolerance to oral therapy is specified in the text. IBD = inflammatory bowel disease.
The optimal dosing strategy for intravenous compounds depends on the type of preparation and patient body weight and Hb concentration. The amount of iron needed to correct the Hb concentration can be calculated using the Ganzoni equation,103 although this formula might underestimate the iron needed when using a target Hb of 13 g/dL and iron stores of 500 mg.64 Other, more simple schemes for the estimation of total iron need have been published.56,104 It should be mentioned that among patients with iron-deficiency anemia who are unresponsive even to intravenous iron supplementation (Hb increase ≤ 2 g/dL within 4 weeks), treatment with erythropoietin after ruling out other causes of anemia such as vitamin deficiencies, may be an option (Figure 4).105–107
Finally, it should be highlighted that iron deficiency in IBD often relapses after iron replenishment.78 Consequently, periodically monitoring, for example, every 3 months for a year and again after a year once the Hb value is normalized and iron stores are replenished to assess if retreatment is required.98 However, we lack solid data on when to stop iron supplementation therapy in order to avoid iron overloading, which may cause side effects because of the potential of the metal to catalyze the formation of toxic radicals.108 Recent guidelines on the management of anemia in dialysis patients suggest that ferritin levels of up to 500 ng/mL appear to be safe. This limit also appears to be a useful upper a threshold in the management of patients with IBD and anemia.109 Of note, in a recently published prospective single-center study, iron supplementation in chronic kidney disease patients was associated with a significant reduction in overall mortality.110 However, prospective studies will be necessary to clarify the impact of anemia correction and iron supplementation on the course of IBD and patient outcomes,102 as well as the definition of clinical endpoint, in order to optimize anemia management and iron supplementation in IBD patients.
CONCLUSIONS
The control of inflammation is a key objective in the treatment of IBD. Because iron-deficiency anemia has a considerable impact on patient quality of life, a thorough and complete diagnostic and therapeutic strategy should be followed to help patients attain as normal a life as possible.
Given the novel intravenous iron-replacement regimens introduced within the last 10 years, physicians may be uncertain concerning the optimal iron-replacement regimen should be prescribed. Based on the data presented herein, oral iron therapy should be preferred for patients with mild iron deficiency anemia (Hb ≥ 10 g/dL) in quiescent disease stages unless they are intolerant or have an inadequate response (Hb increase < 2 g/dL within 4 weeks),111 whereas intravenous iron supplementation may be of advantage in patients with aggravated iron deficiency anemia or flaring IBD (Hb < 10 g/dL) because inflammation hampers intestinal iron absorption.10,112,113 In our systematic review, only 1 study showed oral iron supplementation to worsen disease activity in 2 patients with UC,54 although quality of life improved significantly in the same group of patients, and intravenous iron supplementation seems to be safe in patients with active IBD. Finally, based on the available data, iron therapy can be administered concomitantly with TNF inhibitors,93 a class of drugs used increasingly in the management of IBD.114
In summary, gastroenterologists treating patients with IBD need to pay attention to the management of anemia and iron deficiency for improvement in the general well-being of their patients a matter which frequently does not gain the attention it deserves. Here, we have presented an evidence-based algorithm for treatment of iron deficiency anemia in patients with IBD, but because of the high risk of anemia recurrence in this cohort, further clinical trials are warranted in an effort to optimize the treatment schedule in these patients.
Acknowledgments
None.
Footnotes
Abbreviations: ACD = Anemia of chronic disease, CD = Crohn's disease, CRP = Creactive protein, EMA = European Medical Agency, FDA = US Food and Drug Administration, Hb = Hemoglobin, IBD = Inflammatory bowel disease, IDA = Iron-deficiency anemia, IL = Interleukin, kDa = Kilo Dalton, MCV = Erythrocyte mean cell colume, MHC = Erythrocyte mean cell hemoglobin concentration, MRI = Magnetic resonance imaging, TFN = Tumor necrosis factor, TSAT = Transferrin saturation, UC = ulcerative colitis.
The National Danish Health Care System and Austrian Research Funds (FWF-TRP188) funded the research but had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
OHN devised the study. OHN, MA, MC, and GW extracted, analyzed, and interpreted the data. wrote the first draft. All authors contributed to subsequent versions and approved the final version of the manuscript.
OHN, MA, and MC have no conflicts of interest to disclose. GW has received lecture honoraria from Vifor Pharma and Pharmacosmos.
REFERENCES
- 1.Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis 2014; 20:936–945. [DOI] [PubMed] [Google Scholar]
- 2.Fiorino G, Allocca M, Danese S. Commentary: anaemia in inflammatory bowel disease: the most common and ignored extra intestinal manifestation. Aliment Pharmacol Ther 2014; 39:227–228. [DOI] [PubMed] [Google Scholar]
- 3.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther 2006; 24:1507–1523. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med 2004; 116:44S–49S. [DOI] [PubMed] [Google Scholar]
- 5.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med 2010; 42:97–114. [DOI] [PubMed] [Google Scholar]
- 6.Goodhand JR, Kamperidis N, Rao A, et al. Prevalence and management of anemia in children, adolescents, and adults with inflammatory bowel disease. Inflamm Bowel Dis 2012; 18:513–519. [DOI] [PubMed] [Google Scholar]
- 7.Bager P, Befrits R, Wikman O, et al. High burden of iron deficiency and different types of anemia in inflammatory bowel disease outpatients in Scandinavia: a longitudinal 2-year follow-up study. Scand J Gastroenterol 2013; 48:1286–1293. [DOI] [PubMed] [Google Scholar]
- 8.Gisbert JP, Gomollon F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol 2008; 103:1299–1307. [DOI] [PubMed] [Google Scholar]
- 9.Hoivik ML, Reinisch W, Cvancarova M, et al. Anaemia in inflammatory bowel disease: a population-based 10-year follow-up. Aliment Pharmacol Ther 2014; 39:69–76. [DOI] [PubMed] [Google Scholar]
- 10.Semrin G, Fishman DS, Bousvaros A, et al. Impaired intestinal iron absorption in Crohn's disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis 2006; 12:1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352:1011–1023. [DOI] [PubMed] [Google Scholar]
- 12.Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis 2007; 13:1545–1553. [DOI] [PubMed] [Google Scholar]
- 13.Vagianos K, Clara I, Carr R, et al. What are adults with inflammatory bowel disease (IBD) eating? A closer look at the dietary habits of a population-based Canadian IBD cohort. JPEN J Parenter Enteral Nutr 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Shander A, Goodnough LT, Javidroozi M, et al. Iron Deficiency Anemia-Bridging the Knowledge and Practice Gap. Transfus Med Rev 2014; 28:156–166. [DOI] [PubMed] [Google Scholar]
- 15.Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol 2010; 7:599–610. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg ND. Iron deficiency anemia in patients with inflammatory bowel disease. Clin Exp Gastroenterol 2013; 6:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of Mammalian iron metabolism. Cell 2010; 142:24–38. [DOI] [PubMed] [Google Scholar]
- 18.Pantopoulos K, Porwal SK, Tartakoff A, et al. Mechanisms of mammalian iron homeostasis. Biochemistry 2012; 51:5705–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coad J, Conlon C. Iron deficiency in women: assessment, causes and consequences. Curr Opin Clin Nutr Metab Care 2011; 14:625–634. [DOI] [PubMed] [Google Scholar]
- 20.Andrews NC. Disorders of iron metabolism. N Engl J Med 1999; 341:1986–1995. [DOI] [PubMed] [Google Scholar]
- 21.Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol 2013; 9:205–215. [DOI] [PubMed] [Google Scholar]
- 22.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306:2090–2093. [DOI] [PubMed] [Google Scholar]
- 23.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011; 62:347–360. [DOI] [PubMed] [Google Scholar]
- 24.Theurl I, Aigner E, Theurl M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 2009; 113:5277–5286. [DOI] [PubMed] [Google Scholar]
- 25.Bregman DB, Morris D, Koch TA, et al. Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol 2013; 88:97–101. [DOI] [PubMed] [Google Scholar]
- 26.Ludwiczek S, Aigner E, Theurl I, et al. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 2003; 101:4148–4154. [DOI] [PubMed] [Google Scholar]
- 27.Thomas C, Thomas L. Anemia of chronic disease: pathophysiology and laboratory diagnosis. Lab Hematol 2005; 11:14–23. [DOI] [PubMed] [Google Scholar]
- 28.Munoz M, Garcia-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol 2011; 64:287–296. [DOI] [PubMed] [Google Scholar]
- 29.Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica 2010; 95:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kautz L, Jung G, Valore EV, et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 2014; 46:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 2007; 117:1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonnweber T, Nachbaur D, Schroll A, et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut 2014; 63:1951–1959. [DOI] [PubMed] [Google Scholar]
- 33.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 2007; 13:1096–1101. [DOI] [PubMed] [Google Scholar]
- 34.Theurl I, Schroll A, Nairz M, et al. Pathways for the regulation of hepcidin expression in anemia of chronic disease and iron deficiency anemia in vivo. Haematologica 2011; 96:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasocki S, Baron G, Driss F, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med 2010; 36:1044–1048. [DOI] [PubMed] [Google Scholar]
- 36.Mullin GE. Micronutrients and inflammatory bowel disease. Nutr Clin Pract 2012; 27:136–137. [DOI] [PubMed] [Google Scholar]
- 37.Hwang C, Ross V, Mahadevan U. Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis 2012; 18:1961–1981. [DOI] [PubMed] [Google Scholar]
- 38.WHO, Geneva, Switzerland. Iron deficinecy anemia: assessment, prevention, and control. A guide for programme managers. 2001. http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf Accessed April 29, 2015. [Google Scholar]
- 39.Gomollon F, Gisbert JP, Garcia-Erce JA. Intravenous iron in digestive diseases: a clinical (re)view. Ther Adv Chronic Dis 2010; 1:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet 2007; 370:415–426. [DOI] [PubMed] [Google Scholar]
- 41.Goodnough LT, Bach RG. Anemia, transfusion, and mortality. N Engl J Med 2001; 345:1272–1274. [DOI] [PubMed] [Google Scholar]
- 42.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RW, Manganaro L, O’Brien J, et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med 2002; 30:2249–2254. [DOI] [PubMed] [Google Scholar]
- 44.Talbot TR, D’Agata EM, Brinsko V, et al. Perioperative blood transfusion is predictive of poststernotomy surgical site infection: marker for morbidity or true immunosuppressant? Clin Infect Dis 2004; 38:1378–1382. [DOI] [PubMed] [Google Scholar]
- 45.Aubron C, Nichol A, Cooper DJ, et al. Age of red blood cells and transfusion in critically ill patients. Ann Intensive Care 2013; 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bihl F, Castelli D, Marincola F, et al. Transfusion-transmitted infections. J Transl Med 2007; 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guinet F, Carniel E, Leclercq A. Transfusion-transmitted Yersinia enterocolitica sepsis. Clin Infect Dis 2011; 53:583–591. [DOI] [PubMed] [Google Scholar]
- 48.Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. Scient World J 2012; 2012:846824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuqua BK, Vulpe CD, Anderson GJ. Intestinal iron absorption. J Trace Elem Med Biol 2012; 26:115–119. [DOI] [PubMed] [Google Scholar]
- 50.Aspuru K, Villa C, Bermejo F, et al. Optimal management of iron deficiency anemia due to poor dietary intake. Int J Gen Med 2011; 4:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasche C, Ahmad T, Tulassay Z, et al. Ferric Maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: results from a phase-3 clinical trial program. Inflamm Bowel Dis 2015; 21:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966; 459:3–10. [DOI] [PubMed] [Google Scholar]
- 53.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol 2005; 18:319–332. [DOI] [PubMed] [Google Scholar]
- 54.de Silva AD, Tsironi E, Feakins RM, et al. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther 2005; 22:1097–1105. [DOI] [PubMed] [Google Scholar]
- 55.Erichsen K, Milde AM, Arslan G, et al. Low-dose oral ferrous fumarate aggravated intestinal inflammation in rats with DSS-induced colitis. Inflamm Bowel Dis 2005; 11:744–748. [DOI] [PubMed] [Google Scholar]
- 56.Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015; 9:211–222. [DOI] [PubMed] [Google Scholar]
- 57.Rizvi S, Schoen RE. Supplementation with oral vs. intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol 2011; 106:1872–1879. [DOI] [PubMed] [Google Scholar]
- 58.Lee TW, Kolber MR, Fedorak RN, et al. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis 2012; 6:267–275. [DOI] [PubMed] [Google Scholar]
- 59.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014; 146:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner T, Wagner SJ, Martinez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut 2011; 60:325–333. [DOI] [PubMed] [Google Scholar]
- 61.FDA: Highlights of Prescription Information: ferric carboxymaltose injection. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203565s000lbl.pdf Accessed April 29, 2015. [Google Scholar]
- 62.EMA: New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/06/news_detail_001833.jsp&mid=WC0b01ac058004d5c1 Accessed April 29, 2015. [Google Scholar]
- 63.Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologia 2014; 99:1671–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reinisch W, Staun M, Tandon RK, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol 2013; 108:1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program 2010; 2010:338–347. [DOI] [PubMed] [Google Scholar]
- 66.Gomollon F, Gisbert JP. Intravenous iron in inflammatory bowel diseases. Curr Opin Gastroenterol 2013; 29:201–207. [DOI] [PubMed] [Google Scholar]
- 67.Gomollon F, Chowers Y, Danese S, et al. Letter: European Medicines Agency recommendations for allergic reactions to intravenous iron-containing medicines. Aliment Pharmacol Ther 2014; 39:743–744. [DOI] [PubMed] [Google Scholar]
- 68.Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 2006; 21:378–382. [DOI] [PubMed] [Google Scholar]
- 69.Khalil A, Goodhand JR, Wahed M, et al. Efficacy and tolerability of intravenous iron dextran and oral iron in inflammatory bowel disease: a case-matched study in clinical practice. Eur J Gastroenterol Hepatol 2011; 23:1029–1035. [DOI] [PubMed] [Google Scholar]
- 70.Koutroubakis IE, Oustamanolakis P, Karakoidas C, et al. Safety and efficacy of total-dose infusion of low molecular weight iron dextran for iron deficiency anemia in patients with inflammatory bowel disease. Dig Dis Sci 2010; 55:2327–2331. [DOI] [PubMed] [Google Scholar]
- 71.Rodgers GM, Auerbach M, Cella D, et al. High-molecular weight iron dextran: a wolf in sheep's clothing? J Am Soc Nephrol 2008; 19:833–834. [DOI] [PubMed] [Google Scholar]
- 72.Schroder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease: a randomized, controlled, open-label, multicenter study. Am J Gastroenterol 2005; 100:2503–2509. [DOI] [PubMed] [Google Scholar]
- 73.Red Book. Pharmacy's Fundamental Reference. 114th ed Montvale, NJ: Thompson Reuters; 2010. [Google Scholar]
- 74.Esposito BP, Breuer W, Sirankapracha P, et al. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 2003; 102:2670–2677. [DOI] [PubMed] [Google Scholar]
- 75.Koskenkorva-Frank TS, Weiss G, Koppenol WH, et al. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 2013; 65C:1174–1194. [DOI] [PubMed] [Google Scholar]
- 76.Beigel F, Lohr B, Laubender RP, et al. Iron status and analysis of efficacy and safety of ferric carboxymaltose treatment in patients with inflammatory bowel disease. Digestion 2012; 85:47–54. [DOI] [PubMed] [Google Scholar]
- 77.Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011; 141:846–853. [DOI] [PubMed] [Google Scholar]
- 78.Evstatiev R, Alexeeva O, Bokemeyer B, et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013; 11:269–277. [DOI] [PubMed] [Google Scholar]
- 79.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol 2008; 103:1182–1192. [DOI] [PubMed] [Google Scholar]
- 80.Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion 2014; 54:306–315. [DOI] [PubMed] [Google Scholar]
- 81.Gozzard D. When is high-dose intravenous iron repletion needed? Assessing new treatment options. Drug Des Devel Ther 2011; 5:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nordfjeld K, Andreasen H, Thomsen LL. Pharmacokinetics of iron isomaltoside 1000 in patients with inflammatory bowel disease. Drug Des Devel Ther 2012; 6:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCormack PL. Ferumoxytol: in iron deficiency anaemia in adults with chronic kidney disease. Drugs 2012; 72:2013–2022. [DOI] [PubMed] [Google Scholar]
- 84.Hetzel D, Strauss W, Bernard K, et al. A phase III, randomized, open-label trial of ferumoxytol compared with iron sucrose for the treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy. Am J Hematol 2014; 89:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schieda N. Parenteral ferumoxytol interaction with magnetic resonance imaging: a case report, review of the literature and advisory warning. Insights Imaging 2013; 4:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm 2012; 69:310–320. [DOI] [PubMed] [Google Scholar]
- 87.Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet 2007; 369:1502–1504. [DOI] [PubMed] [Google Scholar]
- 88.Chertow GM, Winkelmayer WC. On the relative safety of intravenous iron formulations: new answers, new questions. Am J Hematol 2010; 85:643–644. [DOI] [PubMed] [Google Scholar]
- 89.Fishbane S, Ungureanu VD, Maesaka JK, et al. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis 1996; 28:529–534. [DOI] [PubMed] [Google Scholar]
- 90.Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am J Hematol 2008; 83:580–588. [DOI] [PubMed] [Google Scholar]
- 91.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis 2009; 15:1485–1491. [DOI] [PubMed] [Google Scholar]
- 93.Katsanos K, Cavalier E, Ferrante M, et al. Intravenous iron therapy restores functional iron deficiency induced by infliximab. J Crohns Colitis 2007; 1:97–105. [DOI] [PubMed] [Google Scholar]
- 94.Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol 2009; 44:838–845. [DOI] [PubMed] [Google Scholar]
- 95.Gasche C, Lomer MC, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut 2004; 53:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oldenburg B, Koningsberger JC, Van Berge Henegouwen GP, et al. Iron and inflammatory bowel disease. Aliment Pharmacol Ther 2001; 15:429–438. [DOI] [PubMed] [Google Scholar]
- 97.Theurl I, Mattle V, Seifert M, et al. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood 2006; 107:4142–4148. [DOI] [PubMed] [Google Scholar]
- 98.Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. Gut 2000; 46 (Suppl 3–4):IV1–IV5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anker SD, Comin CJ, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 100.Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ 2013; 347:f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stein J, Bager P, Befrits R, et al. Anaemia management in patients with inflammatory bowel disease: routine practice across nine European countries. Eur J Gastroenterol Hepatol 2013; 25:1456–1463. [DOI] [PubMed] [Google Scholar]
- 103.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr 1970; 100:301–303. [PubMed] [Google Scholar]
- 104.Reinisch W, Chowers Y, Danese S, et al. The management of iron deficiency in inflammatory bowel disease: an online tool developed by the RAND/UCLA appropriateness method. Aliment Pharmacol Ther 2013; 38:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Katsanos KH, Tatsioni A, Natsi D, et al. Recombinant human erythropoietin in patients with inflammatory bowel disease and refractory anemia: a 15-year single center experience. J Crohns Colitis 2012; 6:56–61. [DOI] [PubMed] [Google Scholar]
- 106.Liu S, Ren J, Hong Z, et al. Efficacy of erythropoietin combined with enteral nutrition for the treatment of anemia in Crohn's disease: a prospective cohort study. Nutr Clin Pract 2013; 28:120–127. [DOI] [PubMed] [Google Scholar]
- 107.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010; 363:1146–1155. [DOI] [PubMed] [Google Scholar]
- 108.Camaschella C. Iron-deficiency anemia. N Engl J Med 2015; 372:1832–1843. [DOI] [PubMed] [Google Scholar]
- 109.Drueke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int 2012; 82:952–960. [DOI] [PubMed] [Google Scholar]
- 110.Zitt E, Sturm G, Kronenberg F, et al. Iron supplementation and mortality in incident dialysis patients: an observational study. PLoS One 2014; 9:e114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldsmith JR, Sartor RB. The role of diet on intestinal microbiota metabolism: downstream impacts on host immune function and health, and therapeutic implications. J Gastroenterol 2014; 49:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oustamanolakis P, Koutroubakis IE, Messaritakis I, et al. Serum hepcidin and prohepcidin concentrations in inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011; 23:262–268. [DOI] [PubMed] [Google Scholar]
- 113.Ganz T. Systemic iron homeostasis. Physiol Rev 2013; 93:1721–1741. [DOI] [PubMed] [Google Scholar]
- 114.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med 2013; 369:754–762. [DOI] [PubMed] [Google Scholar]


