Abstract
The spleen is a crucial organ in humans. Little is known about the association between stroke and splenic injury or splenectomy. The aim of this study was to determine the risk of stroke in patients with splenic injury and splenectomy.
A nationwide cohort study was conducted by analyzing the National Health Insurance Research Database in Taiwan. For comparison, control patients were selected and matched with splenic injury patients in a ratio of 4:1 according to age, sex, and the year of hospitalization. We analyzed the risks of stroke using a Cox proportional-hazards regression analysis.
A total of 11,273 splenic injury patients, including 5294 splenectomized and 5979 nonsplenectomized patients, and 45,092 control patients were included in this study. The incidence rates of stroke were 8.05, 6.53, and 4.25 per 1000 person-years in splenic injury patients with splenectomy, those without splenectomy, and the control cohort, respectively. Compared with the control cohort, splenic injury patients with splenectomy exhibited a 2.05-fold increased risk of stroke (95% confidence interval [CI] 1.8–2.34), whereas those without splenectomy exhibited a 1.74-fold increased risk (95% CI 1.51–2). Splenectomy entailed an additional 1.21-fold increased risk of stroke compared with nonsplenectomy in patients with splenic injury.
This study revealed that splenic injury and splenectomy were significantly associated with an increased risk of hemorrhagic and ischemic strokes. The results of this study may alert physicians and patients to the complications of splenic injury and splenectomy.
INTRODUCTION
The spleen is a reticuloendothelial organ that constitutes a crucial part of the hematologic and immunologic system. Splenic injury is commonly associated with blunt abdominal trauma. The standard management of splenic injury has shifted from surgical splenectomy to spleen-preserving nonoperative procedures.1,2 However, approximately 22,000 all-cause splenectomies are still performed per year in the United States.3–5 The most common indications for splenectomy are hematologic disorders, such as hemolytic anemia, autoimmune hemolytic anemia, sickle cell disease, β-thalassemia, and immune thrombocytopenic purpura.3 Although splenectomy in trauma patients has decreased in recent years, this procedure is still recommended for severe splenic injury (grade III–V) because of the high failure rate of nonoperative management.1,6,7 Moreover, emergent splenectomy remains a life-saving technique for patients who exhibit life-threatening hemorrhage caused by splenic injury.6,7
Several complications of splenectomy, including bacterial infections, thrombosis, thromboembolism, pulmonary arterial hypertension, and cancer, have been described.3,8–11 Thrombosis and thromboembolism may complicate ischemic heart disease, ischemic stroke, hemorrhagic stroke, pulmonary embolism, deep vein thrombosis, and portal vein thrombosis.3,8,10,11 However, the association of thrombosis and thromboembolism with splenectomy has been inconsistently reported in previous studies.8,10,12,13
Splenic injury can impair the function of the spleen by causing the loss of functional splenic tissue.14 However, no study has evaluated the increased risk of stroke in patients with splenic injury. In addition, most studies have investigated vascular complications of splenectomy that was indicated for hematologic disorders.12,13,15–17 Therefore, we conducted a nationwide retrospective cohort study to determine the risk of ischemic and hemorrhagic strokes in patients with splenic injury and splenectomy by analyzing the National Health Insurance Research Database (NHIRD) of Taiwan.
METHODS
Data Source
The Taiwan National Health Insurance (NHI) program is a single-payer universal insurance program that was initiated in 1995 and covers approximately 99% of the Taiwanese population (>25 million people; http://www.nhi.gov.tw/english/index.aspx). The government of Taiwan has authorized the National Health Research Institutes (NHRI) to establish the NHIRD and release decoded and scrambled data for research purposes. In this study, we analyzed the hospitalization claims data, which constitute a subdataset of the NHIRD, containing information on sex, age, dates of admission and discharge, diagnoses, surgical procedures, discharge status, and medical expenditures. Diagnoses and management were classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. This study was approved by the Ethics Review Board of China Medical University (CMU-REC-101–012).
Sampled Patients
The study patients were selected from the inpatients claims of patients who were diagnosed with splenic injury (ICD-9-CM code 865) between 1998 and 2010. Patients aged 20 years or older with splenic injury who underwent splenectomy (ICD-9-CM procedure codes 41.2, 41.3, 41.5) were classified as the splenectomized cohort, and patients with splenic injury who did not undergo splenectomy were classified as the nonsplenectomized cohort. The dates of the first hospitalization for splenic injury were defined as the index dates. To create a control cohort, patients without splenic injury and splenectomy were randomly selected and matched with splenic injury patients in a 4:1 ratio according to age group with a 5-year interval, sex, and the year of the index date. The exclusion criteria were age <20 years, a history of stroke (ICD-9-CM codes 430–438) before the index date, and a lack of complete information.
Outcome and Comorbidities
Hemorrhagic (ICD-9-CM codes 430–432) and ischemic strokes (ICD-9-CM codes 433–438) were defined as the endpoint of this study. All of the included patients were followed from the index date to the occurrence of the endpoint, withdrawal from the NHI, or the end of 2011. To evaluate the potential risk and account for confounding factors, we explored the comorbidities of each patient, namely hypertension (ICD-9-CM codes 401–405), diabetes mellitus (ICD-9-CM code 250), chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, 496), hyperlipidemia (ICD-9-CM code 272), liver cirrhosis (ICD-9-CM code 571), chronic renal failure (ICD-9-CM code 585), autoimmune diseases (ICD-9-CM codes 710.0, 710.1, 710.2, 710.3, 714), cancer (ICD-9-CM codes 140–208), atrial fibrillation (ICD-9-CM code 427.31), and coronary artery disease (ICD-9-CM codes 410–414).
Statistical Analysis
The χ2 test was used for categorical variables, whereas the Student t test was used for continuous variables. Cumulative incidences of stroke in the splenectomized, nonsplenectomized, and control cohorts were explored using the Kaplan–Meier method, and the differences were determined using log-rank tests. The incidence rates of stroke stratified by sex, age, and comorbidity were calculated for the splenectomized, nonsplenectomized, and control cohorts, respectively. Univariable and multivariable Cox proportional hazard regression models were used to determine the risk for stroke, shown as a hazard ratio (HR) with a 95% confidence interval (CI). All P values were 2-tailed, and a P value <0.05 was considered significant. Data management and analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
RESULTS
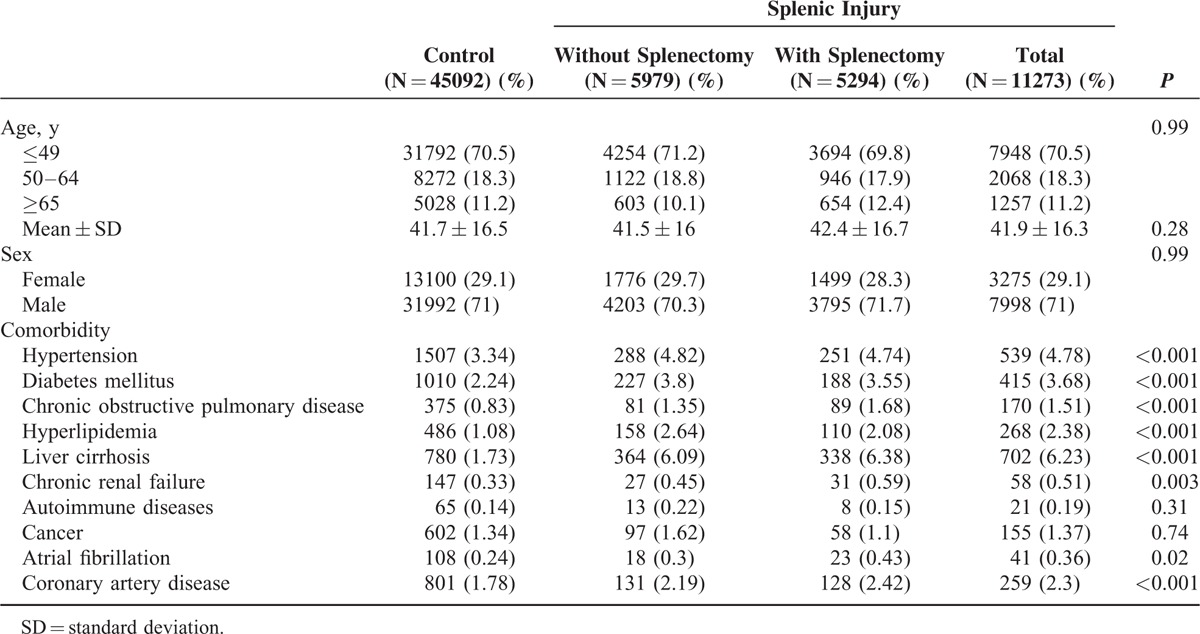
A total of 11,273 patients with splenic injury and 45,092 control patients were included in this study (Table 1). In the splenic injury cohort, 5294 patients received splenectomy, and 5979 patients did not receive splenectomy. The age of the patients with splenic injury was 41.9 ± 16.3 years (mean ± standard deviation). Most patients were aged 49 years or younger. Males accounted for approximately 71% of the patients in each cohort. Compared with the control cohort, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, hyperlipidemia, chronic renal failure, atrial fibrillation, and coronary artery disease were more prevalent in the splenic injury cohort. The average follow-up durations were 6.08, 6.26, and 6.71 years in splenic injury patients without splenectomy, those with splenectomy, and the control cohort, respectively.
TABLE 1.
Comparison of Demographics and Comorbidity Between the Control Cohort and Splenic Injury Patients With and Without Splenectomy
The cumulative incidences of stroke were the highest in splenic injury patients with splenectomy, followed by those without splenectomy and the control cohort. Significant differences in the cumulative incidences of stroke were observed among these 3 cohorts (Figure 1). The incidence rates of stroke were 8.05 per 1000 person-years in splenic injury patients with splenectomy and 6.53 per 1000 person-years in those without splenectomy; both were significantly higher than the incidence rate of the control cohort (4.25 per 1000 person-years; Table 2). Compared with the control cohort, splenic injury patients with splenectomy exhibited a 2.05-fold increased risk of stroke (95% CI 1.8–2.34), whereas those without splenectomy exhibited a 1.74-fold increased risk of stroke (95% CI 1.51–2).
FIGURE 1.

Cumulative incidences of stroke in the control cohort and splenic injury patients with and without splenectomy. The cumulative incidence of stroke was the highest in splenic injury patients with splenectomy, followed by those without splenectomy and the control cohort. Log-rank tests: splenic injury patients with splenectomy vs those without splenectomy, P = 0.02; splenic injury patients with splenectomy vs control cohort, P < 0.001; splenic injury patients with splenectomy vs control cohort, P < 0.001.
TABLE 2.
HRs of Hemorrhagic and Ischemic Strokes Among the Control Cohort and Splenic Injury Patients With and Without Splenectomy Stratified by Demographics, Comorbidity, and Follow-Up Duration
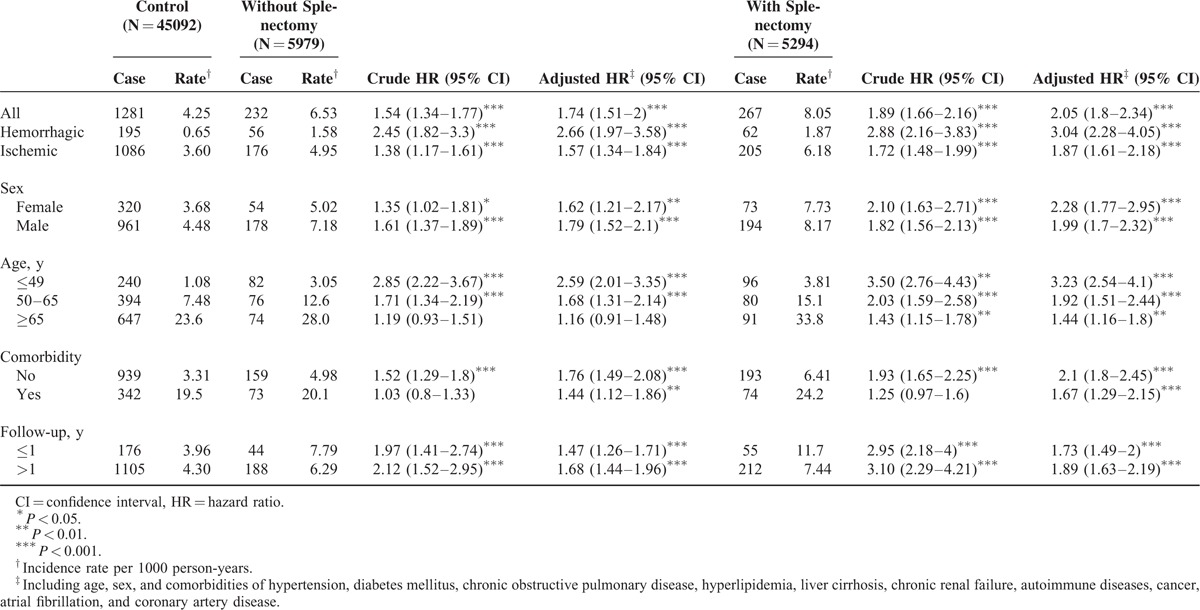
Among the splenic injury patients without splenectomy, the adjusted HRs for hemorrhagic and ischemic strokes were 2.66 (95% CI 1.97–3.58) and 1.57 (95% CI 1.34–1.84), respectively. Furthermore, splenic injury patients receiving splenectomy were 3.04-fold more likely to develop hemorrhagic stroke (95% CI 2.28–4.05) and 1.87-fold more likely to develop ischemic stroke (95% CI 1.61–2.18) than the control cohort after adjustment for age, sex, and comorbidities.
Stroke occurred more frequently in males in all 3 cohorts (Table 2). Although male patients exhibited a higher incidence rate of stroke in the nonsplenectomized cohort (male vs female, 1.79 vs 1.62), females exhibited a higher adjusted HR for stroke in the splenectomized cohort (male vs female, 1.99 vs 2.28). The incidence of stroke increased with age in the control, splenectomized, and nonsplenectomized patients. The increased risk of stroke relative to that of the control cohort was the highest in the younger age group of ≤49 years (adjusted HR in the nonsplenectomized cohort, 2.59; in the splenectomized cohort, 3.23). The comorbidity-stratified risk of stroke was increased in the patients with splenic injury, irrespective of whether they underwent splenectomy. We further analyzed the incidence of stroke associated with the follow-up duration and demonstrated that the incidence and relative risk of stroke increased in both the splenectomized and nonsplenectomized cohorts when the follow-up duration was >1 year.
Table 3 shows a comparison of the risk of stroke between splenectomized and nonsplenectomized patients who had splenic trauma, revealing a 1.21-fold higher risk of stroke for patients who received splenectomy (95% CI 1.01–1.44). Females exhibited a significantly higher risk of stroke after splenectomy (adjusted HR 1.5; 95% CI 1.05–2.16). However, the increased risk of stroke in male patients receiving splenectomy compared with nonsplenectomy was nonsignificant (adjusted HR 1.13; 95% CI 0.92–1.39).
TABLE 3.
HRs of Hemorrhagic and Ischemic Strokes Among Splenic Injury Patients With and Without Splenectomy Stratified by Demographics and Comorbidity

DISCUSSION
This large nationwide cohort study confirmed the increased risk of hemorrhagic and ischemic strokes among patients with splenic injury. We observed that splenectomy increased the risk of stroke compared with nonsplenectomy in splenic injury patients.
The spleen is a reticuloendothelial organ that constitutes a vital part of the hematologic and immunologic systems. Multiple factors are likely to contribute to vascular events in patients with splenic injury and splenectomy, including platelet activation, hypercoagulability, activation of the endothelium, and altered lipid profiles.3 Several studies have reported that splenectomy resulted in thrombocytosis, leukocytosis, concentrated hemoglobin, hyperlipidemia, and increased C-reactive protein levels.18–23 All these alterations are independently associated with an increased risk of vascular complications in splenectomized patients.
In addition to the aforementioned factors, bidirectional interaction between brain injury and spleen is proposed.24,25 Brain injury, including trauma, hemorrhage, and ischemia, induces an immunoinflammatory response and release of inflammatory cytokines by macrophages in the spleen, the so-called “brain-spleen inflammatory coupling.”24,25 The brain is susceptible to inflammatory substances activated by the immune-inflammatory processes.26 For example, tumor necrosis factor (TNF)-α has been shown to result in exacerbation of brain ischemia. TNF-α inhibitors could improve the outcome of ischemic brain in animal models.25,26 Immune alterations, including increased ratio of interferon (IFN)-γ to interleukin-10 and significant elevation of absolute lymphocyte, CD4+ T cell, and CD8+ T cell counts, have been described after splenectomy.27,28 T lymphocytes are associated with the production of IFN-γ and TNF-α, which have shown a direct pathogenetic role in neuronal ischemic damage.29 Tuttolomondo et al30 reported a significant elevation in peripheral frequency of CD4+ cells and CD28 null cells (CD4+CD28- T cells) in patients with acute ischemic stroke, particularly in those with cardioembolic stroke. These complex multifactorial interactions could possibly play a critical role in the pathogenesis of stroke in splenectomized patients.
Our study revealed that the cumulative incidence of stroke was the highest in splenic trauma patients who underwent splenectomy, followed by those who did not receive splenectomy. Cox proportional hazard modeling showed that splenectomy and splenic injury were independently associated with an increased risk of stroke. Patients with splenic injury are typically young (approximately 70% were younger than 50 years in this study) and have few medical comorbidities. These young patients are at a lower risk of stroke. Recently, surgery and invasive procedures were shown to be associated with an increased risk of stroke within 30 days after the procedures and operations.31 However, the risk of stroke returned to the baseline if the surgery and invasive procedures were performed >30 days previously.31 The increased risk of stroke after splenic injury and splenectomy may be a postprocedural or postoperative phenomenon. But our study showed that splenic trauma patients, both those who underwent splenectomy and those who did not, exhibited a higher HR of stroke during a follow-up period of >1 year than those who were observed during a follow-up duration of ≤1 year. Therefore, merely the postprocedural or postoperative attribution of increased risk of stroke cannot explain the results of our study. In other words, the increased risk is likely attributable to the splenic injury and splenectomy.
Kristinsson et al8 reported the long-term complications of 8149 veterans who underwent splenectomy irrespective of indications. During a 27-year follow-up, they determined that splenectomized patients exhibited an increased risk of pulmonary embolism and deep vein thrombosis, but not ischemic stroke, coronary artery disease, or acute myocardial infarction.8 By contrast, our study revealed that splenic trauma patients exhibited a 1.74-fold greater risk of stroke if they did not receive splenectomy and a 2.05-fold increased risk of stroke if they received splenectomy. The risk of thromboembolic events after splenectomy varies considerably in previous studies.8,10,12,13 The conditions under which splenectomy is indicated and the presence or absence of ongoing intravascular hemolysis are likely to determine the risk of thromboembolic events after splenectomy.3 Patients with thalassemia intermedia, Hgb E/β-thalassemia, sickle cell anemia, and hereditary stomatocytosis exhibit a greatly increased risk of vascular complications after splenectomy. Moreover, these hematologic disorders themselves could be associated with vascular complication.3 Trauma patients with splenectomy are thought to have a baseline or increased risk.3 In our study, all patients underwent splenectomy because of splenic injury. The differences in indications for splenectomy may explain the conflicting results regarding the risk of thromboembolic events after splenectomy.
Our study revealed that both patients with splenic injury who underwent splenectomy and those who did not exhibited an increased risk of hemorrhagic and ischemic strokes. The increased HRs of hemorrhagic stroke were higher than those of ischemic stroke after adjustment for age, sex, and comorbidities. Based on our research, there has been no comprehensive study that evaluated the risk of hemorrhagic stroke after splenic injury and splenectomy.32 Intracranial hemorrhage may be associated with cerebral venous thrombosis.33 Ischemic stroke and hemorrhagic stroke also share several similar risk factors, including hypertension, atherosclerosis, coronary artery disease, heart failure, atrial fibrillation, hematologic disorders, hyperlipidemia, diabetes mellitus, obesity, and alcohol use.34 Some factors, such as diabetic foot, increased CD4+CD28- T cells (CD28 null cells), arterial stiffness, and elevation of immuno-inflammatory markers, are found commonly in patients with stroke and coronary artery disease.30,35–39 The mechanism of ischemic stroke in splenectomized patients could be also responsible for the increased incidence of hemorrhagic stroke in patients after splenectomy.
Although this study was a large nationwide study, several limitations remain. First, the lack of data on risk factors for stroke, such as smoking, obesity, and socioeconomic status, represents a major limitation. Second, the severity of stroke is not specified in the dataset. Therefore, we could not evaluate the association of the severity of stroke with splenic injury and splenectomy. Third, laboratory data are not included in the database. Fourth, information on the etiology and subtypes of ischemic stroke was not available in the NHIRD. We cannot discriminate the subtypes of ischemic stroke according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.40 Finally, the diagnoses of stroke, splenic injury, and splenectomy could not be validated using the NHIRD. Misclassification of diseases may cause bias in our study, although several studies have proven the accuracy of the NHIRD.41,42
In conclusion, our study results show that splenic injury and splenectomy are significantly associated with an increased risk of hemorrhagic and ischemic strokes. In addition, splenectomy additionally increased the risk of stroke in splenic injury patients. Higher HRs of hemorrhagic stroke than of ischemic stroke were observed among patients with splenic injury and splenectomy. The results of this study may alert physicians and patients to the severe complications of splenic injury and splenectomy. Additional trials may be required to investigate prophylactic management of these complications.
Acknowledgments
None.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IFN = interferon, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, TNF = tumor necrosis factor, TOAST = Trial of Org 10172 in Acute Stroke Treatment.
All authors have contributed significantly, and all authors are in agreement with the content of the manuscript: Conception/Design: J-NL, C-HK; Provision of study materials: C-HK; Collection and/or assembly of data: J-NL, C-LL, C-HK; Data analysis and interpretation: all authors; Manuscript writing: all authors; Final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
All authors report no conflicts of interest.
REFERENCES
- 1.Zonies D, Eastridge B. Combat management of splenic injury: trends during a decade of conflict. J Trauma Acute Care Surg 2012; 73:S71–74. [DOI] [PubMed] [Google Scholar]
- 2.Cochran A, Mann NC, Dean JM, et al. Resource utilization and its management in splenic trauma. Am J Surg 2004; 187:713–719. [DOI] [PubMed] [Google Scholar]
- 3.Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood 2009; 114:2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13 2007; 165:1–209. [PubMed] [Google Scholar]
- 5.Rose AT, Newman MI, Debelak J, et al. The incidence of splenectomy is decreasing: lessons learned from trauma experience. Am Surg 2000; 66:481–486. [PubMed] [Google Scholar]
- 6.Rosati C, Ata A, Siskin GP, et al. Management of splenic trauma: a single institution's 8-year experience. Am J Surg 2015; 209:308–314. [DOI] [PubMed] [Google Scholar]
- 7.Hommes M, Navsaria PH, Schipper IB, et al. Management of blunt liver trauma in 134 severely injured patients. Injury 2015; 46:837–842. [DOI] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Gridley G, Hoover RN, et al. Long-term risks after splenectomy among 8149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica 2014; 99:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisharat N, Omari H, Lavi I, et al. Risk of infection and death among post-splenectomy patients. J Infect 2001; 43:182–186. [DOI] [PubMed] [Google Scholar]
- 10.Stamou KM, Toutouzas KG, Kekis PB, et al. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg 2006; 141:663–669. [DOI] [PubMed] [Google Scholar]
- 11.Jaïs X, Ioos V, Jardim C, et al. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax 2005; 60:1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson AA, Bennett B, Jones PF, et al. Thrombotic risks of staging laparotomy with splenectomy in Hodgkin's disease. Br J Surg 1981; 68:842–845. [DOI] [PubMed] [Google Scholar]
- 13.Cappellini MD, Robbiolo L, Bottasso BM, et al. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol 2000; 111:467–473. [DOI] [PubMed] [Google Scholar]
- 14.Skattum J, Naess PA, Gaarder C. Non-operative management and immune function after splenic injury. Br J Surg 2012; 99:59–65. [DOI] [PubMed] [Google Scholar]
- 15.Borgna Pignatti C, Carnelli V, Caruso V, et al. Thromboembolic events in beta thalassemia major: an Italian multicenter study. Acta Haematol 1998; 99:76–79. [DOI] [PubMed] [Google Scholar]
- 16.Schilling RF, Gangnon RE, Traver MI. Delayed adverse vascular events after splenectomy in hereditary spherocytosis. J Thromb Haemost 2008; 6:1289–1295. [DOI] [PubMed] [Google Scholar]
- 17.Schilling RF. Spherocytosis, splenectomy, strokes, and heat attacks. Lancet 1997; 350:1677–1678. [DOI] [PubMed] [Google Scholar]
- 18.Hathirat P, Mahaphan W, Chuansumrit A, et al. Platelet counts in thalassemic children before and after splenectomy. Southeast Asian J Trop Med Public Health 1993; 24 Suppl 1:213–215. [PubMed] [Google Scholar]
- 19.Boxer MA, Braun J, Ellman L. Thromboembolic risk of postsplenectomy thrombocytosis. Arch Surg 1978; 113:808–809. [DOI] [PubMed] [Google Scholar]
- 20.Visudhiphan S, Ketsa-Ard K, Piankijagum A, et al. Blood coagulation and platelet profiles in persistent post-splenectomy thrombocytosis. The relationship to thromboembolism. Biomed Pharmacother 1985; 39:264–271. [PubMed] [Google Scholar]
- 21.Troendle SB, Adix L, Crary SE, et al. Laboratory markers of thrombosis risk in children with hereditary spherocytosis. Pediatr Blood Cancer 2007; 49:781–785. [DOI] [PubMed] [Google Scholar]
- 22.Goldfarb AW, Rachmilewitz EA, Eisenberg S. Abnormal low and high density lipoproteins in homozygous beta-thalassaemia. Br J Haematol 1991; 79:481–486. [DOI] [PubMed] [Google Scholar]
- 23.Aviram M, Brook JG, Tatarsky I, et al. Increased low-density lipoprotein levels after splenectomy: a role for the spleen in cholesterol metabolism in myeloproliferative disorders. Am J Med Sci 1986; 291:25–28. [DOI] [PubMed] [Google Scholar]
- 24.Rasouli J, Lekhraj R, Ozbalik M, et al. Brain-spleen inflammatory coupling: a literature review. Einstein J Biol Med 2011; 27:74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuttolomondo A, Maida C, Pinto A. Inflammation and inflammatory cell recruitment in acute cerebrovascular diseases. Curr Immunol Rev 2015; 11:24–32. [Google Scholar]
- 26.Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des Devel Ther 2014; 8:2221–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto N, Shimoda S, Kawanaka H, et al. Modulation of CD4+ T cell responses following splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp Immunol 2011; 165:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sari TT, Gatot D, Akib AAP, et al. Immune response of thalassemia major patients in Indonesia with and without splenectomy. Acta Medica Indones 2014; 46:217–225. [PubMed] [Google Scholar]
- 29.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuttolomondo A, Pecoraro R, Casuccio A, et al. Peripheral frequency of CD4+ CD28- cells in acute ischemic stroke: relationship with stroke subtype and severity markers. Medicine 2015; 94:e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbanek C, Palm F, Buggle F, et al. Recent surgery or invasive procedures and the risk of stroke. Cerebrovasc Dis Basel Switz 2014; 38:370–376. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Xu M, Ji L, et al. Splenectomy for chronic idiopathic thrombocytopenic purpura in children: a single center study in China. Acta Haematol 2006; 115:39–45. [DOI] [PubMed] [Google Scholar]
- 33.Korathanakhun P, Sathirapanya P, Geater SL, et al. Predictors of hospital outcome in patients with cerebral venous thrombosis. J Stroke Cerebrovasc Dis 2014; 23:2725–2729. [DOI] [PubMed] [Google Scholar]
- 34.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin 2008; 26:871–895.vii. [DOI] [PubMed] [Google Scholar]
- 35.Pinto A, Tuttolomondo A, Di Raimondo D, et al. Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metabolism 2008; 57:676–682. [DOI] [PubMed] [Google Scholar]
- 36.Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Arterial stiffness and ischemic stroke in subjects with and without metabolic syndrome. Atherosclerosis 2012; 225:216–219. [DOI] [PubMed] [Google Scholar]
- 37.Tuttolomondo A, Pecoraro R, Di Raimondo D, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome. Diabetol Metab Syndr 2014; 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011; 151:318–322. [DOI] [PubMed] [Google Scholar]
- 39.Strano A, Hoppensteadt D, Walenga JM, et al. Plasma levels of the molecular markers of coagulation and fibrinolysis in patients with peripheral arterial disease. Semin Thromb Hemost 1996; 22 Suppl 1:35–40. [PubMed] [Google Scholar]
- 40.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke J Cereb Circ 1993; 24:35–41. [DOI] [PubMed] [Google Scholar]
- 41.Cheng CL, Kao YHY, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 42.Yu YB, Gau JP, Liu CY, et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost 2012; 108:225–235. [DOI] [PubMed] [Google Scholar]


