Abstract
Neural tube defects (NTDs) are the most common congenital defects of the central nervous system among neonates and the folate status during pregnancy was considered as the most important etiopathogenesis of NTDs. Besides, methionine synthase (MTR) gene and methionine synthase reductase (MTRR) gene were folate metabolism involved genes and had been investigated in several previous studies with inconsistent results. Hence, we aimed to explore the association of 4 selected single-nucleotide polymorphisms (SNPs) on MTRR/MTR gene and the susceptibility of NTDs in a Chinese population.
Seven SNPs were selected from HapMap databases with Haploview 4.2 software. A polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was performed to genotype the polymorphisms from blood samples of 165 NTDs patients and 280 healthy controls. The correlation between these SNPs and NTDs risk was tested by Student t test and Chi-square test by STATA 11.0 software. Furthermore, we performed a meta-analysis of relevant studies to investigate the association between the SNPs MTRR 66A>G and MTR 2756A>G and the susceptibility of NTDs.
An increased risk of NTDs was verified to be significantly associated with MTRR 66A>G (G allele vs. A allele: OR = 1.36 (1.03–1.80), P = 0.028; GG + AG vs. AA: OR = 1.60 (1.05–2.43), P = 0.027) and MTR 2756A>G (G allele vs. A allele: OR = 1.45 (1.06–1.98), P = 0.021; GG + AG vs. AA: OR = 1.51 (1.02–2.23), P = 0.038) in our study. However, the other SNPs in our analysis showed no significant association with NTDs risk (all P > 0.05). Furthermore, the result of the meta-analysis supported the association between MTRR 66A>G and NTDs risk (G allele vs. A allele: OR = 1.32, 95% CI = 1.09–1.61, GG + GA vs. AA: OR = 1.49, 95% CI = 1.06–2.09, GG vs. AA: OR = 1.61, 95% CI = 1.04–2.49).
Our study confirmed that the MTRR 66A>G and MTR 2756A>G were significantly associated with the increased NTDs risk in a Chinese population. The further meta-analysis enhance that MTRR 66A>G was connected with the susceptibility of NTDs widely. Further investigations based on more detailed stratification were recommended.
INTRODUCTION
Neural tube defects (NTDs) are the most common and complicated congenital defects of the central nervous system at birth.1,2 The representations of NTDs range among various phenotypes including spina bifida, anencephaly, and encephalocele. Moreover, NTDs occurred around 1 to 28 days after conception as reported in previous studies.3,4 According to the epidemiology, the incidence of NTD's affection is about 0.2% worldwide. Merely, the rate of NTDs ranges among races and areas in previous studies. It was also observed that NTDs reach up to 25% of all the birth defects.5 Furthermore, NTDs were acquired or inherited with multifactorial pattern under both environmental and genetic influential factors during pregnancy. Low level of folate, vitamin B12 and high level of homocysteine in the serum during pregnancy were all established to increase the risk of NTDs.6 And it was confirmed in earlier researches that proper ingestion of folate acid could decrease the occurrence and recurrence of NTDs notably.7–9 Besides, various investigations paid attention to the association between the single nucleotide polymorphisms (SNPs) on folate-related genes and risk of NTDs. Consequently, elucidation of the NTDs mechanisms and focusing on candidate genes that are involved in folate-related pathways could be informative for developing improved prevention strategies and reducing the global burden of NTDs.10
The gene of methionine synthase (MTR) and methionine synthase reductase (MTRR) were 2 genes involved in the folate metabolism and had been investigated in several previous studies. It had been reported that the polymorphisms on the gene of MTR and MTRR acted as risk factors for NTDs. MTR gene could encodes the enzyme of vitamin B12-dependent and further converts 5-methyltetrahydrofolate (5-methyl THF) and homocysteine to tetrahydrofolate (THF) and methionine, respectively.11 This process is essential for maintaining ample tetrahydrofolate and methionine pools in the cell. On the other hand, MTRR gene is one of the most important genes and has been studied deeply among all the folate metabolism related genes. MTRR functions as an activation partner of MTR which could contribute to the demethylation of homocysteine (Hcy) to methionine through cobalamin and subsequently regenerates MTR.12 At the 66 bp of MTRR gene, an A to G switch (66A>G) has been widely studied and verified to result in the deficiency of MTRR in patients via the conversion of isoleucine to methionine (I22M).13,14 On the contrary, the defect of MTRR could subsequently affect the function of MTR and disrupt the methionine/homocysteine cycle.
In our study, we aim at 4 nonsynonymous polymorphisms (2756A>G (rs1805087), −1003A>G (rs10925250), +370G>C (rs12060570) and −1059T>C (rs10925235)) within the gene of MTR and 3 potential SNPs (66A>G (rs1801394), 1049A>G (rs162036), and 524C>T (rs1532268)) on the MTRR gene to investigate their association with the NTDs risk. All the SNPs were identified from public databases and tended to be representatives of the SNPs. However, considering the limitation of individual study with small sample size and the significant controversy of relevant studies, a further meta-analysis with public data for the purpose of conclude an exhaustive conclusion of empirical evidence to prove the association between the NTDs risk and the SNPs of MTRR 66A>G and MTR 2756A>G was also performed.
MATERIALS AND METHODS
Ethnic Statement
Our study was conducted in strict accordance with the protocol approved by the Ethics Committee and parental informed consent of the 1st Affiliated Hospital of Xinjiang Medical University. An informed consent granted by this ethics committee must be signed by each volunteer (as for the neonates, the informed consent must be signed instead by their parents or guardians).
Study Population
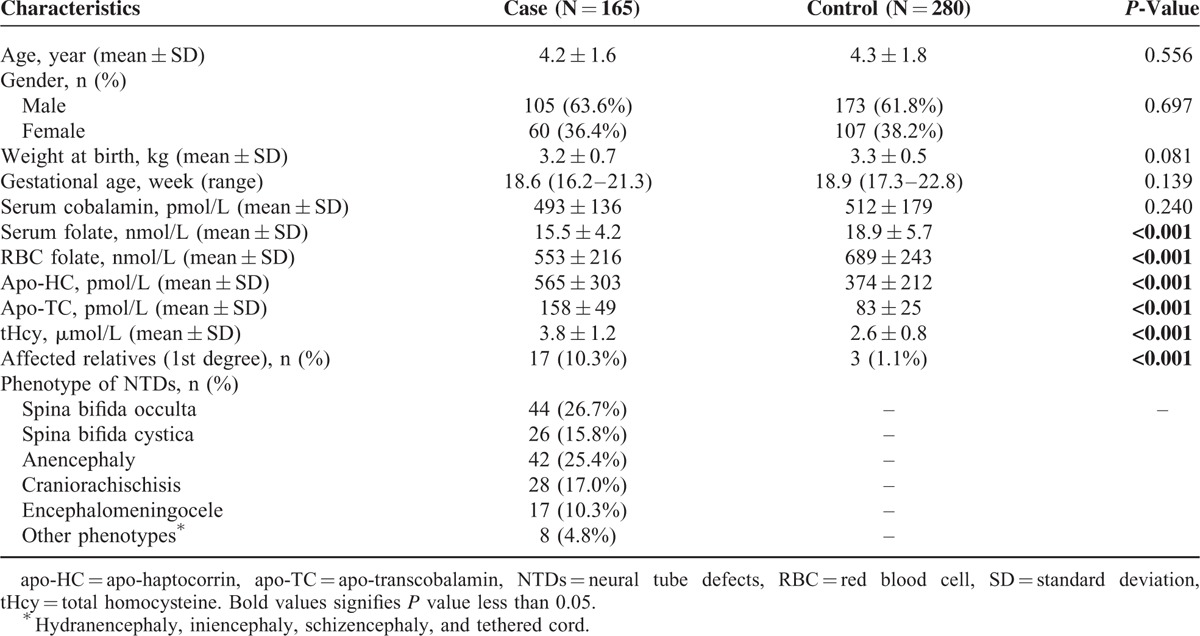
A total of 445 neonates in Chinese Han population were enrolled from August 2013 to December 2014. The subjects were consisted of 165 confirmatory NTDs patients from the Department of Pediatric Neurology who were identified using routine ultrasound scanning and 280 healthy controls who were randomly selected from the unrelated healthy neonates from inpatients of the gynecology and obstetrics department as outlined in Table 1. We collected 5 mL venous blood sample with EDTA vacutainer from each subject after their guardians provided a written informed consent. The clinical characteristics of all the cases and controls were collected as well. Further, regarding to the influencing factors which may related to NTDs of the neonates. The levels of serum cobalamin, serum folate, RBC (red blood cell) folate, apo-haptocorrin, and apo-transcobalamin were all quantified with the reagents and system of Ciba (Ciba Corning Diagnostics Corp., Medfield, MA). Similarly, the level of total homocysteine (tHcy) in plasma was detected with high-pressure liquid chromatography. All the samples and complete follow-up data were available for each specimen (more clinical characteristics are present in Table 1).
TABLE 1.
Characteristics of Cases and Control Children
SNPs Selection
The polymorphisms in our study were retrieved from online HapMap database (HapMap Data Rel 24/phaseII Nov08, on NCBI B36 assembly, dbSNP b126) of Chinese HapMap Consortium. And critical polymorphisms were identified with Haploview 4.2 (Broad Institute, Cambridge, MA) software, with the linkage disequilibrium (LD) pattern under HapMap genotype data and r2 of 0.8 was selected as a threshold for the analyses.15 As a result, we select 3 SNPs as representatives on MTRR gene (66A>G, 1049A>G, and 524C>T) and 4 SNPs (2756A>G, −1003A>G, +370G>C and −1059T>C) on MTR gene via our filter. Additionally, the SNP 66A>G on MTRR gene and SNP 2756A>G on MTR gene had been reported with high incidence and were supposed to result in amino acid change which would further vary the function of folate relevant enzymes.16 The accurate locations of the selected SNPs were as following. On the gene of MTR, SNP 2756A>G was located at exon 25, −1003A>G was located at intron 18, +370G>C was located at intron 10 and 1059T>C was located at intron 1. On the gene of MTRR, SNP 66A>G, 1049A>G, and 524C>T were located on exon 1, intron 5, and intron 8 regions, respectively, as demonstrated in Figure 1.
FIGURE 1.
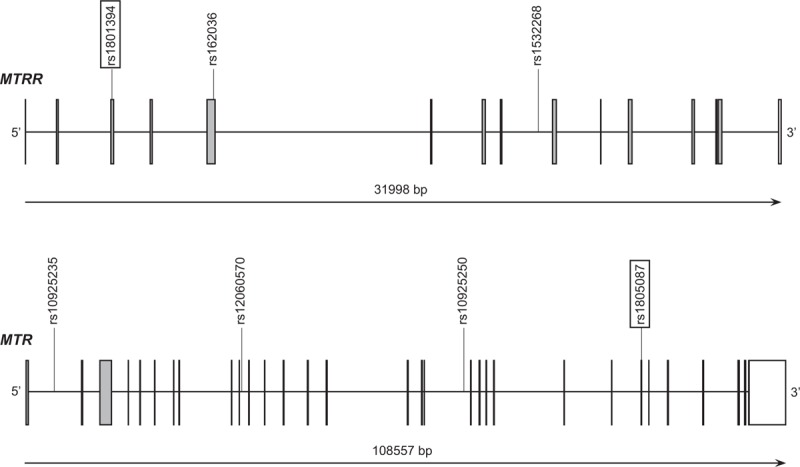
Locations of the selected SNPs. On the gene of MTR, SNP 2756A>G was located at exon 25, −1003A>G was located at intron 18, +370G>C was located at intron 10 and −1059T>C was located at intron 1. On the gene of MTRR, SNP 66A>G, 1049A>G, and 524C>T were located on exon 1, intron 5, and intron 8 regions, respectively.
Genotyping Method
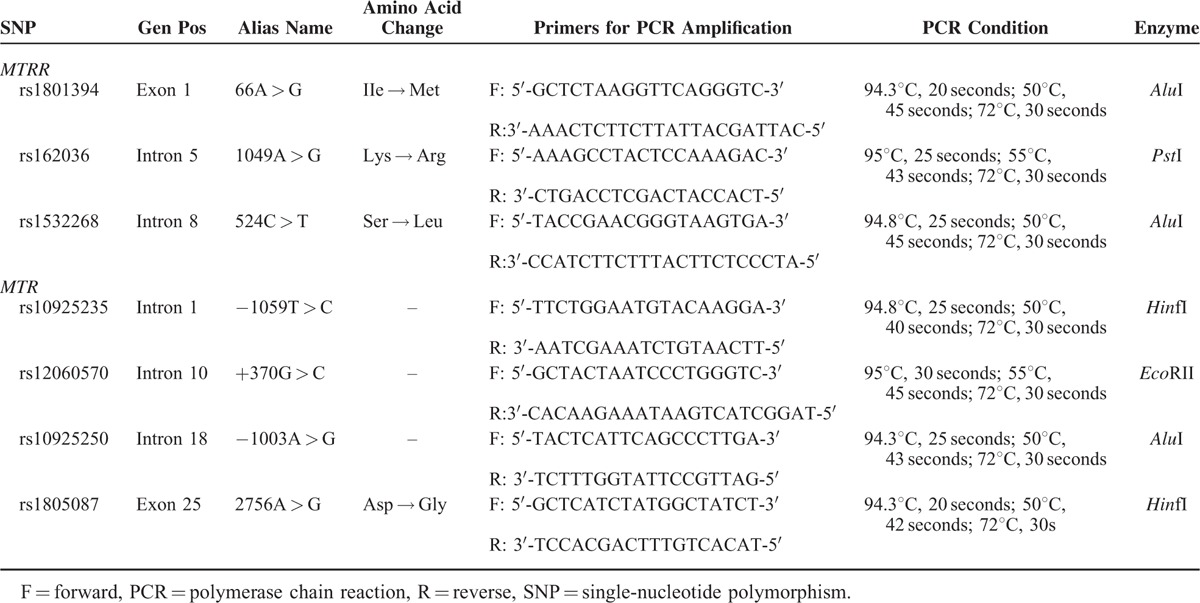
DNA was extracted from the whole blood of both NTD cases and controls by QIAamp DNA Blood Mini Kit (Qiagen, UK) using proteinase K and RNase A, isopropanol, cold acetic acid, and stored at −20°C. Further, the genotyping of selected SNPs were done by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis. And the quality of genotyping was tested by direct sequencing with >99% agreement for the 5 polymorphisms or repeat analysis genotyping of at least 10% of the samples by the initial genotyping method.17,18 The SNPs primers we used for PCR amplification were designed with Primer Premier 5 software as shown in Table 2 with their related restriction enzymes and reaction condition. The primers and conditions of all assays are available upon request.
TABLE 2.
Primers of MTR and MTRR Genetic Polymorphisms for PCR Amplification
Statistical Analysis
We performed all the statistical analysis in our case–control study with the STATA version 11.0 software (StataCorp LP, Texas 77845-4512 USA). During our analysis, the difference of clinical characteristics and frequency of genotypes between NTDs patients and controls was compared by Student t test and Pearson Chi-square (χ2) test. We also performed a chi-square goodness-of-fit test by comparing the observed and expected genotype frequencies to verify the data quality in each subject class, exploring significant departure from Hardy–Weinberg equilibrium (HWE) and investigate the representativeness of sample population. And the genotypes related NTDs risk was evaluated by odds ratios (ORs) with their corresponding respective confidence intervals 95% (CIs) value, for both combined and respective genotype. A 2-sided P-value less than 0.05 was considered to indicate statistical significance for all analyses. Further, we conducted a meta-analysis of all relevant studies on the interaction between folate relevant SNP MTRR 66A>G and MTR 2756A>G and the NTDs risk.
RESULTS
Study Characteristics
We enrolled 445 neonates as blood sample donate subjects in present study. The subjects were comprised of 165 NTDs (male = 105, female = 60) patients with the average age of 4.2 (SD = 1.6) and 280 healthy controls (male = 173, female = 107) with the average age of 1.3 (SD = 1.8). Despite the difference among the gender and ages, no significant difference was observed (P = 0.697 and P = 0.556, respectively). Similarly, the weight at birth of the neonates was also matched between the 2 groups (P = 0.081). Further, the tHcy and nutrient levels of each subject were tested. Cobalamin and folate levels were significantly lower and apo-haptocorrin (apo-HC), apo-transcobalamin (apo-TC), and total homocysteine (tHcy) significantly higher in cases as compared with controls (all P < 0.001). However, the difference on the level of serum cobalamin between cases and controls was not significant. Statistically significant difference of first-degree relatives affected by NTDs was also observed between the case and control groups Seventeen individuals in the case group, but only 3 in the control group, had a first-degree relative with NTDs (P < 0.001). Phenotypes of NTDs patients were classified intro 6 groups, with spina bifida occulta (26.7%) and anencephaly (17.0%) accounting for the most cases as shown in Table 1.
Association Between MTRR, MTR Polymorphisms, and NTDs Risk
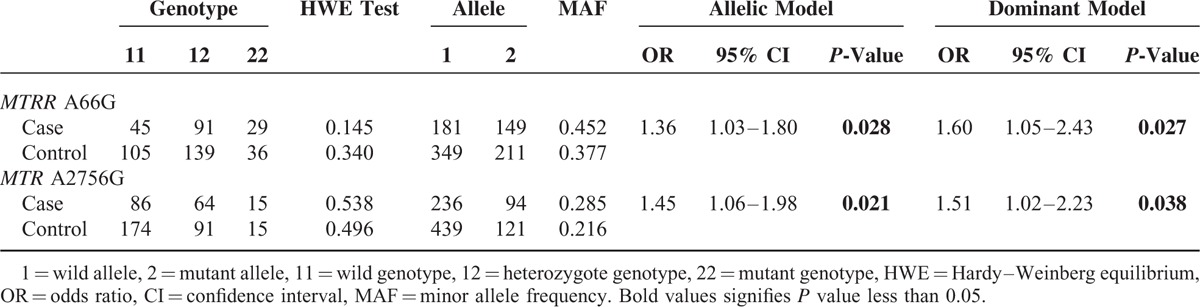
As present in Table 3, among 3 genotyped SNPs in MTRR gene in the present study, only MTRR 66A>G was found to be significantly associated with an increased risk of NTDs (G allele vs. A allele: OR = 1.36 (1.03–1.80), P = 0.028; GG + AG vs. AA: OR = 1.60 (1.05–2.43), P = 0.027). Similarly, for MTR gene, only MTR 2756A>G was suggested to be linked with the susceptibility of NTDs (G allele vs. A allele: OR = 1.45 (1.06–1.98), P = 0.021; GG + AG vs. AA: OR = 1.51 (1.02–2.23), P = 0.038). No statistical significant difference was observed between NTDs and other polymorphisms in the present study with all their P-value above 0.05, respectively. All these data of result are revealed in Table 3.
TABLE 3.
Association Between the MTR and MTRR Polymorphisms and NTDs Risk
Meta-Analysis
We further performed a meta-analysis to assess the association between the SNPs of MTRR 66A>G and MTR 2756A>G and the NTDs risk. Fifteen articles were collected with our search strategies in this meta-analysis (including the present study) in total. Among these researches, the data of 5306 specimens were included. Among all the specimens, 3823 were in case of MTRR 66A>G (consisted of 1407 cases and 2416 controls) and 3809 were MTR 2756A>G (consisted of 1492 cases and 2317 controls). The detailed result of the meta-analysis is revealed in Table 4. The results supported the conclusion of MTRR 66A>G as a risk factor for NTDs with P-value below 0.05 in allelic model (as shown in Figure 2), dominant model and homozygous model in the meta-analysis with present study (G allele vs. A allele: OR = 1.32, 95% CI = 1.09–1.61, GG + GA vs. AA: OR = 1.49, 95% CI = 1.06–2.09, GG vs. AA + AG: OR = 1.58, 95% CI = 0.85–2.61, GG vs. AA: OR = 1.61, 95% CI = 1.04–2.49). However, overall analysis, regardless with or without the present study, did not give support to the inference that MTR 2756A>G might be associated with susceptibility of NTDs (G allele vs. A allele: OR = 1.08, 95% CI = 0.89–1.30, GG + GA vs. AA: OR = 1.15, 95% CI = 0.91–1.45, GG vs. AA + AG: OR = 0.97, 95%CI = 0.67–1.40, GG vs. AA: OR = 1.05, 95% CI = 0.58–1.35).
TABLE 4.
Meta-Analysis of the Association Between the MTRR and MTR Polymorphisms and NTDs Risk
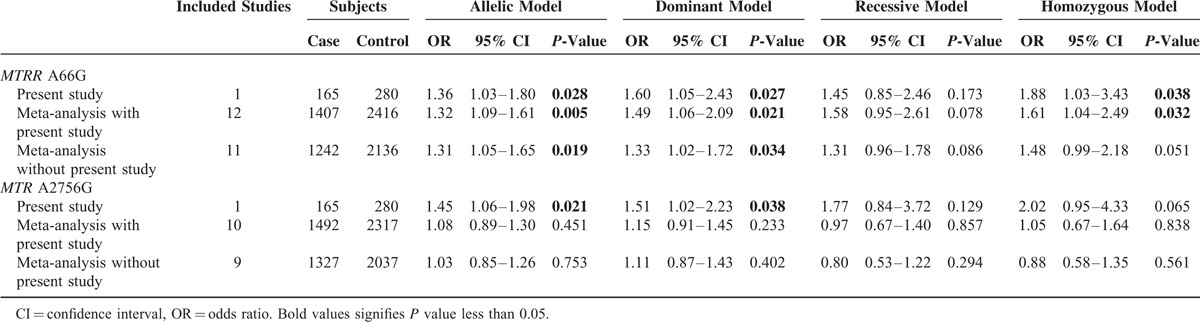
FIGURE 2.
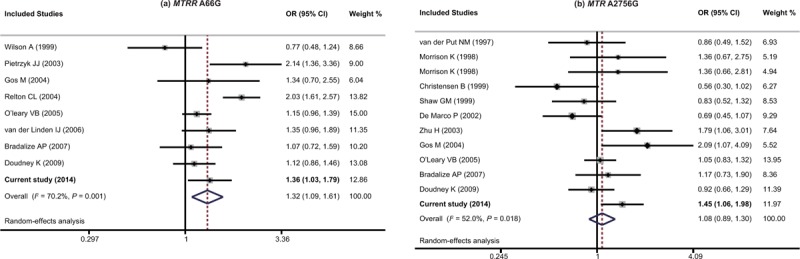
The forest plots of MTRR 66A>G and MTR 2756A>G in allelic model. (A) MTRR 66A>G, (B) MTR 2756A>G.
DISCUSSION
In the present study, we aim to investigate the association between the risk of NTDs and polymorphisms on the genes of MTR and MTRR. We revealed that the MTRR 66A>G and MTR 2756A>G were both significantly associate with an increased NTDs susceptibility. However, the other SNPs we tested in the present study showed no significant associations with the risk of NTDs. This result was subsequently confirmed by a meta-analysis of MTRR 66A>G and MTR 2756A>G which clarified the association of MTRR 66A>G with an increased risk of NTDs.
NTDs were among the most common birth defects widely which could be influenced by the metabolism of folic acid and the level of homocysteine.19,20 Various genetic and environmental factors could affect the metabolism of folic acid and further lead to NTDs. Various stratified environmental factors which were related to NTDs have been studied currently.21 Accumulating data implicated that the pregnant women without adequate intake of folate from dietary supplements and food were more likely to have a neonate with NTDs.22 Consequently, the susceptibility of NTDs could be remedied by proper ingestion of folate supplementation. Additionally, the ingestion of folic acid by 0.4 to 0.6 mg/day was considered as the most favorable quantum.23 Furthermore, the persistent low level of folate was also observed in neonates. Besides, according to the latest researches, methionine cycle and folate cycle both have influence on the pathopoiesis of NTDs genetically. In the present study, the folate status was significantly lower in the NTDs neonates. On the contrary, their levels of apo-HC, apo-TC, and tHcy were higher than the healthy controls notably. These implied that derangement in folate metabolism and the relevant status of apo-HC, apo-TC, and tHcy might be involved in the complex pathogenesis of NTDs.
Several previous studies had focused on the polymorphisms on the gene of MTR and MTRR in different populations. The abnormal activity of MTR could lead to accumulation of methyl THF which could not be used in folate-dependent reactions, such as thymidylate biosynthesis which is involved in NTD pathogenesis.24 It was corroborated that the polymorphism 2756A>G on MTR gene could convert asparthione to glycine.25,26 The subjects with the genotype GG were reported to have lower level of homocysteine in plasma compared with AA and AG in previous researches.27–31
The activation of MTR is maintained by MTRR which could demethylate cobalamin (II) to cobalamin (I) using S-adenosylmethionine (SAM). In has been proved that the SNP 66A>G on MTRR gene result in a conversion in the MTRR enzyme at position 22 by replacing isoleucine with methionine (I22M) and further deviate the ordinary function of MTRR which would express as decreasing the efficient repair of MTR.16,32 Most of the previous studies revealed that subjects with the GG genotype had degraded level of homocysteine compared with subjects with other genotypes.33
The SNPs we studied in the present study were not the only genetic factors of NTDs. The carbon atoms’ reduction at the oxidation levels of methyl, formyl, or methylene and the link to nitrogen were both involved in folate metabolism which would processes under a complicated pathway of many enzymes. To data, many folate pathway relevant SNPs have been investigated to the NTDs’ etiology, such as the SNPs on the gene of 5,10-methylenetetrahydrofolate reductase (MTHFR), serine hydroxymethyltranferase 1 (SHMT1), cystathionine-beta-synthase (CBS), and betaine–homocysteine methyltransferase (BHMT). The simplified version of the mechanisms of these SNPs is revealed in Figure 1. As we stated before, MTR gene could encodes the enzyme of vitamin B12-dependent and further convert 5-methyl THF and homocysteine to THF and methionine, respectively. Further, the genes above combine with the MTR and MTRR gene to finish the circle by the transfer from methyl group to homocysteine.34,35 As in the folate cycle, folate would convert in the form of tetrahydrofolate (THF) as the bioactive form firstly and further implement its function in folate metabolism (Figure 3). The conversion could be done under the function of the SHMT gene. Besides, MTHFR also performs a central role in the metabolism of folate by catalyzing the transfer of 5,10-methyleneTHF to 5-MTHF irreversibly and catalyzing the interconversion of serine and glycine. Likewise, CBS could catalyze the conversion of homocysteine to cysteine irreversibly. Consequently, the polymorphisms on these genes were supposed to disturb the regular function of relevant enzymes and lead to the suffering of NTDs. Similarly, the SNPs on BHMT gene could lead to the hyper of homocysteine and further result in NTDs.
FIGURE 3.
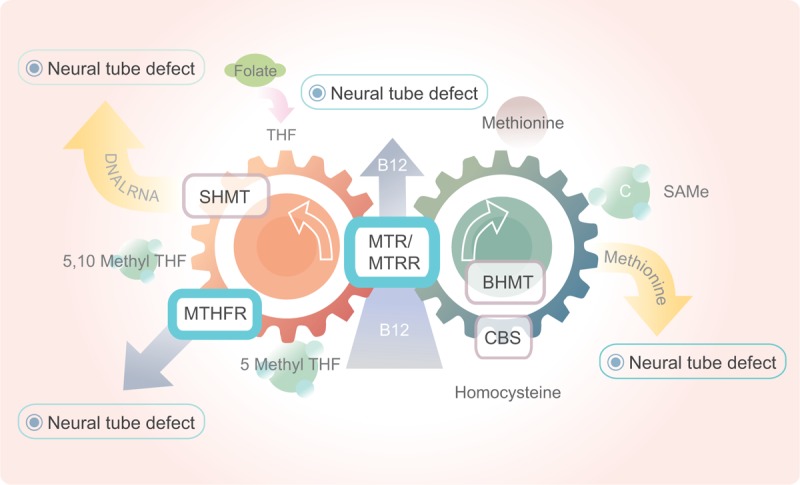
The process of folate cycle related to neural tube defect. BHM, betaine–homocysteine methyltransferase; CBS, cystathionine-β-synthase; MTR, methionine synthase; MTRR, methionine synthase reductase; MTHFR, methylene tetrahydrofolate reductase; RFC, the reduced folate carrier; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltranferase; THF, tetrahydrofolate.
Several limitations which could lead to inaccurate results have to be considered in our study. Firstly, the sample size of present study was relative small. Secondly, the outcome of NTDs varies among several symptoms which might not be considered severely and we did not restrict the NTDs type. Thirdly, environmental bias, like diet, smoking, and alcohol consumption, were not considered in the present study will also be a limitation. Certainly, gene–environment and gene–gene interaction also have influence on the present study.
In order to minimize the limitation of individual study with small sample size and the controversy of relevant studies, we performed a meta-analysis. According to our meta-analysis, MTRR 66A>G was significantly associated with the risk of NTDs widely which also elucidated that the mutation of folate related gene could lead to the suffering of NTDs. All the analysis was performed with no publication bias. Some studies with relative small sample size were also included in our meta-analysis, which also could be the limitation of the present analysis. The diversity of each study might be resulted from the bias of population or environmental stratification bias.
In conclusion, our result provide evidence that MTRR 66A>G and MTR 2756A>G were correlated with the increased risk of NTDs significantly. Moreover, the conclusion was supported by the further meta-analysis that MTRR 66A>G was associated with an increased NTDs risk. However, the other SNPs were not able to support the association significantly. Further study based on more detailed stratification and large populations were recommended in the research about folate relevant polymorphisms of NTDs.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy–Weinberg equilibrium, MTR = methionine synthase, MTRR = methionine synthase reductase, NTD = neural tube defect, OR = odds ratios, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, SNP = single-nucleotide polymorphism.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ouyang S, Liu Z, Li Y, et al. Cystathionine beta-synthase 844ins68 polymorphism is unrelated to susceptibility to neural tube defects. Gene 2014; 535:119–123. [DOI] [PubMed] [Google Scholar]
- 2.Marini NJ, Hoffmann TJ, Lammer EJ, et al. A genetic signature of spina bifida risk from pathway-informed comprehensive gene-variant analysis. PLoS One 2011; 6:e28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Ren A, Zhang L, et al. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol 2006; 76:237–240. [DOI] [PubMed] [Google Scholar]
- 4.Detrait ER, George TM, Etchevers HC, et al. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol 2005; 27:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Lou J, Zhong R, et al. Genetic variants in the folate pathway and the risk of neural tube defects: a meta-analysis of the published literature. PLoS One 2013; 8:e59570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom HJ, Shaw GM, den Heijer M, et al. Neural tube defects and folate: case far from closed. Nat Rev Neurosci 2006; 7:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De-Regil LM, Fernandez-Gaxiola AC, Dowswell T, et al. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2010; CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kibar Z, Capra V, Gros P. Toward understanding the genetic basis of neural tube defects. Clin Genet 2007; 71:295–310. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary VB, Mills JL, Pangilinan F, et al. Analysis of methionine synthase reductase polymorphisms for neural tube defects risk association. Mol Genet Metab 2005; 85:220–227. [DOI] [PubMed] [Google Scholar]
- 10.Etheredge AJ, Finnell RH, Carmichael SL, et al. Maternal and infant gene-folate interactions and the risk of neural tube defects. Am J Med Genet A 2012; 158A:2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucock M, Daskalakis I, Briggs D, et al. Altered folate metabolism and disposition in mothers affected by a spina bifida pregnancy: influence of 677c → t methylenetetrahydrofolate reductase and 2756a → g methionine synthase genotypes. Mol Genet Metab 2000; 70:27–44. [DOI] [PubMed] [Google Scholar]
- 12.Yadav U, Kumar P, Yadav SK, et al. Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: an updated meta-analysis. Metab Brain Dis 2014; 30:7–24. [DOI] [PubMed] [Google Scholar]
- 13.Olteanu H, Wolthers KR, Munro AW, et al. Kinetic and thermodynamic characterization of the common polymorphic variants of human methionine synthase reductase. Biochemistry 2004; 43:1988–1997. [DOI] [PubMed] [Google Scholar]
- 14.Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry 2002; 41:13378–13385. [DOI] [PubMed] [Google Scholar]
- 15.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21:263–265. [DOI] [PubMed] [Google Scholar]
- 16.Candito M, Rivet R, Herbeth B, et al. Nutritional and genetic determinants of vitamin B and homocysteine metabolisms in neural tube defects: a multicenter case-control study. Am J Med Genet A 2008; 146A:1128–1133. [DOI] [PubMed] [Google Scholar]
- 17.Naushad SM, Devi AR. Role of parental folate pathway single nucleotide polymorphisms in altering the susceptibility to neural tube defects in South India. J Perinat Med 2010; 38:63–69. [DOI] [PubMed] [Google Scholar]
- 18.Relton CL, Wilding CS, Laffling AJ, et al. Low erythrocyte folate status and polymorphic variation in folate-related genes are associated with risk of neural tube defect pregnancy. Mol Genet Metab 2004; 81:273–281. [DOI] [PubMed] [Google Scholar]
- 19.Ratan SK, Rattan KN, Pandey RM, et al. Evaluation of the levels of folate, vitamin B12, homocysteine and fluoride in the parents and the affected neonates with neural tube defect and their matched controls. Pediatr Surg Int 2008; 24:803–808. [DOI] [PubMed] [Google Scholar]
- 20.Wenstrom KD, Johanning GL, Owen J, et al. Amniotic fluid homocysteine levels, 5,10-methylenetetrahydrafolate reductase genotypes, and neural tube closure sites. Am J Med Genet 2000; 90:6–11. [DOI] [PubMed] [Google Scholar]
- 21.Lacasana M, Blanco-Munoz J, Borja-Aburto VH, et al. Effect on risk of anencephaly of gene-nutrient interactions between methylenetetrahydrofolate reductase C677T polymorphism and maternal folate, vitamin B12 and homocysteine profile. Public Health Nutr 2012; 15:1419–1428. [DOI] [PubMed] [Google Scholar]
- 22.Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr 2010; 91:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr 2000; 71:1295S–1303S. [DOI] [PubMed] [Google Scholar]
- 24.Dunlevy LP, Chitty LS, Burren KA, et al. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain 2007; 130:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon DL, Shields DC, Woodside JV, et al. Methionine synthase D919G polymorphism is a significant but modest determinant of circulating homocysteine concentrations. Genet Epidemiol 1999; 17:298–309. [DOI] [PubMed] [Google Scholar]
- 26.Leclerc D, Wilson A, Dumas R, et al. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci U S A 1998; 95:3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anwar W, Gueant JL, Abdelmouttaleb I, et al. Hyperhomocysteinemia is related to residual glomerular filtration and folate, but not to methylenetetrahydrofolate-reductase and methionine synthase polymorphisms, in supplemented end-stage renal disease patients undergoing hemodialysis. Clin Chem Lab Med 2001; 39:747–752. [DOI] [PubMed] [Google Scholar]
- 28.Silaste ML, Rantala M, Sampi M, et al. Polymorphisms of key enzymes in homocysteine metabolism affect diet responsiveness of plasma homocysteine in healthy women. J Nutr 2001; 131:2643–2647. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Stampfer MJ, Ma J, et al. Influence of a methionine synthase (D919G) polymorphism on plasma homocysteine and folate levels and relation to risk of myocardial infarction. Atherosclerosis 2001; 154:667–672. [DOI] [PubMed] [Google Scholar]
- 30.Dekou V, Gudnason V, Hawe E, et al. Gene-environment and gene-gene interaction in the determination of plasma homocysteine levels in healthy middle-aged men. Thromb Haemost 2001; 85:67–74. [PubMed] [Google Scholar]
- 31.Ma J, Stampfer MJ, Christensen B, et al. A polymorphism of the methionine synthase gene: association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 1999; 8:825–829. [PubMed] [Google Scholar]
- 32.Gos M, Sliwerska E, Szpecht-Potocka A. Mutation incidence in folate metabolism genes and regulatory genes in Polish families with neural tube defects. J Appl Genet 2004; 45:363–368. [PubMed] [Google Scholar]
- 33.Geisel J, Zimbelmann I, Schorr H, et al. Genetic defects as important factors for moderate hyperhomocysteinemia. Clin Chem Lab Med 2001; 39:698–704. [DOI] [PubMed] [Google Scholar]
- 34.Bassuk AG, Kibar Z. Genetic basis of neural tube defects. Semin Pediatr Neurol 2009; 16:101–110. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich CM, Robien K, McLeod HL. Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer 2003; 3:912–920. [DOI] [PubMed] [Google Scholar]


