Abstract
Poor preoperative nutritional status for individuals with Crohn's disease (CD) is associated with intra-abdominal septic complications (IASCs). The present study aimed to investigate the association of the common nutrition indices serum albumin and body mass index (BMI) with IASCs. Sixty-four CD patients who had received elective intestinal operations were retrospectively investigated. Among these patients, 32 had received individualized fortified nutrition support. IASCs occurred in 7 patients (10.9%). Compared with non-IASC patients, IASC patients had a lower BMI (17.6 ± 2.7 vs 15.6 ± 1.3 kg/m2, P = 0.048). The area under the receiver operating characteristic curve according to the BMI-based IASC prediction was 0.772 (95% confidence interval [CI], 0.601–0.944; P = 0.020) with an optimum diagnostic cutoff value of 16.2 kg/m2. A BMI < 16.2 kg/m2 significantly increased the risk of developing an IASC (odds ratio [OR], 10.286; 95% CI, 1.158–91.386). Even after correction with the simplified CD activity index (CDAI), a low BMI level remained associated with IASCs (OR, 7.650; 95% CI, 0.808–72.427; P = 0.076). Serum albumin was not associated with IASCs. Although the fortified nutrition support group had an albumin level comparable to the control group, this group had a higher simplified CDAI score, a lower BMI level, and a comparable incidence rate of IASCs. Thus, BMI more accurately reflects the basic preoperative nutritional status of CD patients than serum albumin. BMI can aid in guiding preoperative nutrition support and judging the appropriate operation time for CD.
INTRODUCTION
Crohn's disease (CD) is a nonspecific chronic inflammation of the intestine. Approximately 70% of CD patients must undergo operations even after active pharmacological treatment.1 However, intestinal operations in patients with CD have a high complication incidence, among which intra-abdominal septic complications (IASCs) are one of the most challenging conditions in clinical practice. An IASC prolongs the hospital stay, significantly increases the postoperative relapse rate, and decreases the patient's quality of life.2 IASCs include anastomotic fistulas, external intestinal fistulas, and intra-abdominal abscesses and have an incidence as high as 5% to 20%.3–8 To date, no effective intervention strategies have been found for IASCs.
Nutrition disorder complications occur in 20% to 85% of CD patients. This specific condition becomes more serious during the active phase of CD,9–11 which is associated with decreased food intake, intestinal absorption dysfunction, drug side effects, and active inflammation.12 Recent studies have shown that the nutritional status of CD patients is closely associated with complications after intestinal operations. Yamamoto et al4 retrospectively analyzed 343 patients who underwent 566 intestinal anastomosis operations and found that an albumin level < 30 g/L was an independent risk factor for IASCs. Alves et al13 investigated the risk factors for IASCs in 161 CD patients who had received an enterectomy for the first time and found that a 10% decrease in body weight within the 6 months before the operation increased the risk of an IASC by more than 5-fold. Because of the importance of nutrition, the Expert Consensus on Nutritional Support Therapy for Inflammatory Bowel Diseases (Consensus for short) was released in China in 2013. This report suggested that malnutrition increases the complication incidence and mortality after CD operations, whereas nutrition support decreases the risk of postoperative complications.14
Many indices have been used to evaluate the nutritional status of CD patients. Although body composition measurement is the most sensitive method, it entails special equipment,15 which, consequently, greatly restricts its clinical application. Evaluations based on nutritional risk screening (NRS-2002) and patient-generated subjective global assessment (PG-SGA) must be conducted by professional nutritionists. Therefore, simpler clinical examination indices, such as serum albumin, body mass index (BMI), triceps skinfold thickness, hemoglobin, and cholesterol, have been used more frequently to evaluate the nutritional status of CD patients in clinical practice.4,7,16 However, the most appropriate indices to accurately and easily evaluate nutrition preparation sufficiency before CD operations remain unknown.
This study primarily analyzed the associations of nutrition indices with the occurrence of IASCs in postoperative CD patients. The results may provide useful data for the selection of appropriate operation times for CD patients.
METHODS
Patients
Clinical data from patients who underwent intestinal operations for CD at the Sixth Affiliated Hospital of Sun Yat-Sen University between March 2008 and May 2014 were retrospectively analyzed. This institution is a center for inflammatory bowel diseases that was established in July 2012 to conduct comprehensive treatment of inflammatory bowel diseases based on multi-disciplinary cooperation (including the gastrointestinal department, the gastrointestinal surgery department, the nutritional department, the type-B ultrasonic department, and the pathology department). Three CD patients who underwent emergency operations were excluded, and 64 CD patients who underwent elective intestinal operations were included.
This study was performed in accordance with the Helsinki Declaration and was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-Sen University. All participants signed an informed consent form.
Evaluation of the Patients’ Condition
CD was typed according to the Montreal classification in combination with imaging, enteroscopy, and operation research.17 CD activity was evaluated according to the simplified Crohn's disease activity index (CDAI).18
Nutrition Support Schemes
After the Center for Inflammatory Bowel Diseases was established, a protocol was developed to provide individualized nutritional support for CD intestinal operations via the gastrointestinal nutritionists at a rate of no <30 kcal/kg/day. Thirty-two patients receiving this support were grouped into the fortified nutritional support group. In this group, 4 patients (12.5%) received total parenteral nutrition, 15 patients (46.9%) received parenteral nutrition combined with (or in sequence with) enteral nutrition, and 13 patients (40.6%) received enteral nutrition, with a nutritional support treatment time of 23 ± 16 days (ranging from 5 to 69 days). Before the center was established, patients who underwent CD intestinal operations were given short-term (5–10 days) preoperative parenteral nutritional support by the surgeon who was responsible for the operation, according to conventions. These patients were grouped into the conventional group, which served as the control.
Medication
According to clinical conditions, the patients were continually given mesalazine (2–4 g/day) and immunosuppressive agents (azathioprine 1–2 mg/kg/day or 6-mercaptopurine 0.5–1 mg/kg/day). Biological agents were withdrawn, and the amount of hormone was gradually decreased. For cases that were complicated with abdominal inflammation, the decision to administer antibiotics, including ciprofloxacin, imidazoles, or cephalosporin, was made based on clinical experience or according to the results of a bacterial culture of B ultrasound-guided abscess puncture fluid.
Operational Approach
All operations were performed by the surgeons in the gastrointestinal surgery department, and each surgeon had at least 8 years of experience in CD surgery.
Forty-seven patients (73%) underwent a partial intestinal resection; 13 patients (20%) underwent a partial intestinal resection plus an ostomy; 1 patient (2%) underwent an ostomy alone; and the remaining 3 patients (5%) underwent other operations, such as intestinal perforation repair. Of the study patients, 46 (72%) were subjected to end-to-end intestinal anastomoses, and 7 (11%) received side-to-side intestinal anastomoses.
Observational Indices
Postoperative short-term abdominal complications were observed, including hemorrhage, complications at the abdominal incision/stoma (inflammation, splitting, and fat liquefaction), and IASCs (anastomotic fistulas, external intestinal fistulas, and intra-abdominal abscesses). Short term was defined as a hospital stay duration for the time of the operation (normally within 3 months).
Statistical Analysis
The data were analyzed with SPSS 13.0 software. The measurement data are presented as means ± standard deviation of the mean (x ± s) or medians (interquartile range) (M [Q25, Q75]. The t test or the Mann–Whitney U test was used to compare between groups. Numeration data are presented as cases and percentages (%), and the χ2 test or Fisher's exact probability test was used to compare between groups. The receiver operating characteristic (ROC) curve was drawn to explore the differentiation value of the nutrition index for IASCs. The maximum point of the correct diagnosis index (sensitivity + specificity − 1) was selected as the cutoff value of the variable. The odds ratio (OR) of the occurrence of an IASC and the 95% confidence interval (CI) were calculated using the logistic regression model. A bilateral P < 0.05 was considered significant.
RESULTS
Basic Clinical Characteristics
Of the 64 patients, 48 (75%) received an elective operation due to an abdominal fistula/abscess, and 16 (25%) underwent an operation for intestinal obstruction. Thirty patients (46.9%) underwent an intestinal operation for the first time, and 19 (29.7%), 11 (17.2%), and 4 (6.3%) patients received their second, third, and fourth intestinal operations, respectively. The diseased regions included the ileocolon (55 cases, 85.9%), the terminal ileum (8 cases, 12.5%), and the colon (1 case, 1.6%). Illness behaviors were manifested as penetration in 48 patients (75%) and stenosis in 16 patients (25%).
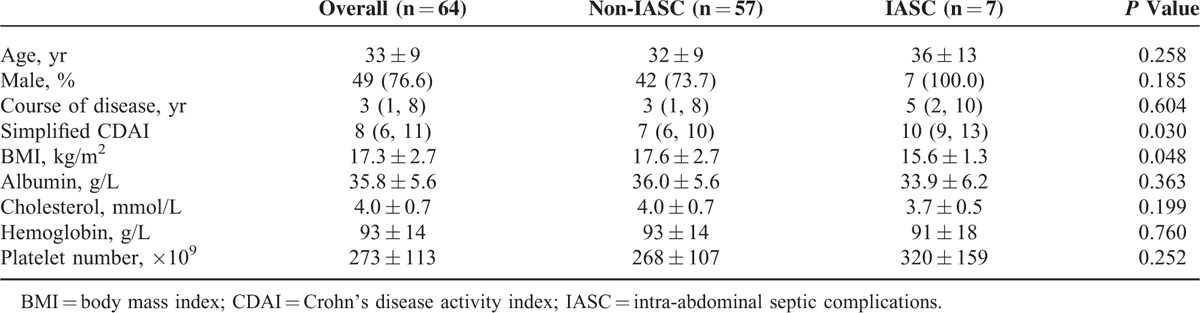
After operation, abdominal complications occurred in 17 patients (26.6%), including incision/stoma complications in 8 patients (12.5%), IASCs in 7 patients (10.9%), and alimentary tract hemorrhage in 2 patients (3.1%). Compared with non-IASC patients, lower preoperative BMI and higher simplified CDAI were found in IASC patients. There was no significant difference in the other indices between the 2 groups (Table 1).
TABLE 1.
Comparison of the Basic Clinical Indices Between the IASC and Non-IASC Groups
Clinical Values of Nutrition Indices for IASC Prediction
The analysis of the ROC curve showed that BMI had clinical predictive value for IASCs, whereas the albumin index did not. The maximum BMI value (16.2 kg/m2) of the correct diagnosis index was selected as the cutoff value for IASC prediction and had a sensitivity of 85.7% and a specificity of 68.4% (Figure 1). The incidence of IASCs was 10.9%. The areas under the ROC curves of BMI and serum albumin for IASCs were 0.722 (95% CI, 0.601–0.944; P = 0.020) and 0.594 (95% CI, 0.358–0.829; P = 0.594), respectively.
FIGURE 1.
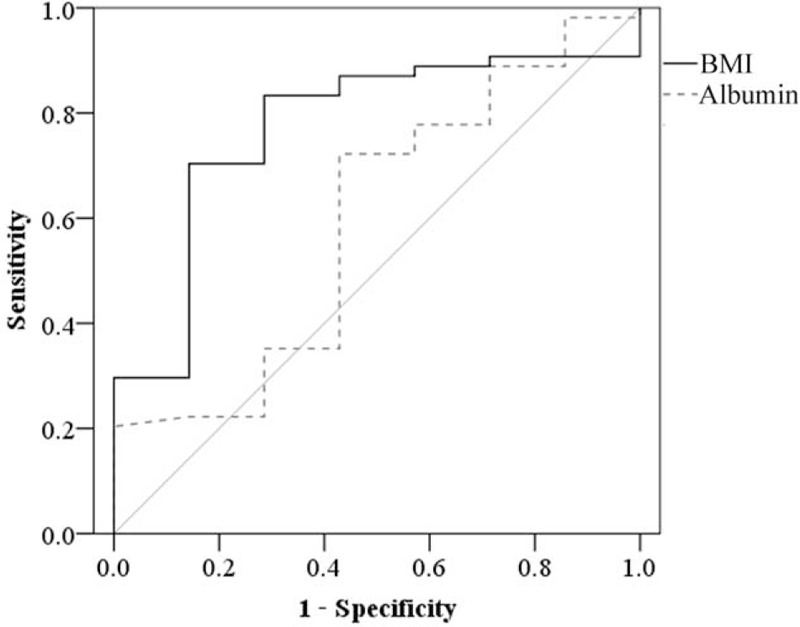
Receiver operating characteristic analysis of the clinical values of body mass index and serum albumin for predicting intra-abdominal septic complications.
Association of Nutrition Indices With IASCs
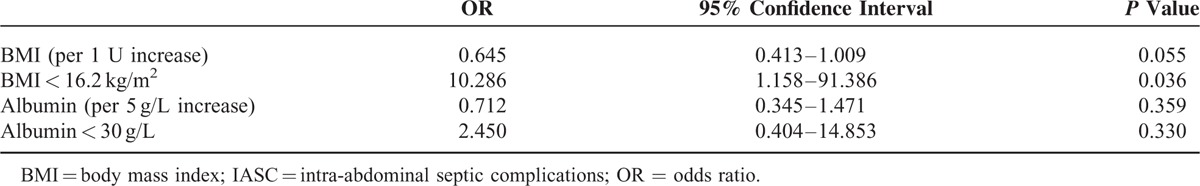
According to the analytical results of the ROC curves, a BMI < 16.2 kg/m2 was defined as a low BMI (a comparative value in this study). Because the albumin index had no statistical significance based on the ROC analysis, a level < 30 g/L was defined as hypoalbuminemia according to the literature.4,8 BMI and albumin were introduced into the univariate logistic regression model for calculating the risk of developing an IASC. The results showed that the patients with a low BMI had a 9 times greater risk of IASCs, whereas the albumin index had no association with IASCs (Table 2). After correction by the simplified CDAI, a low BMI remained associated with the risk of IASC (OR, 7.650; 95% CI, 0.808–72.427; P = 0.076).
TABLE 2.
Associations of Nutrition Indices With IASC
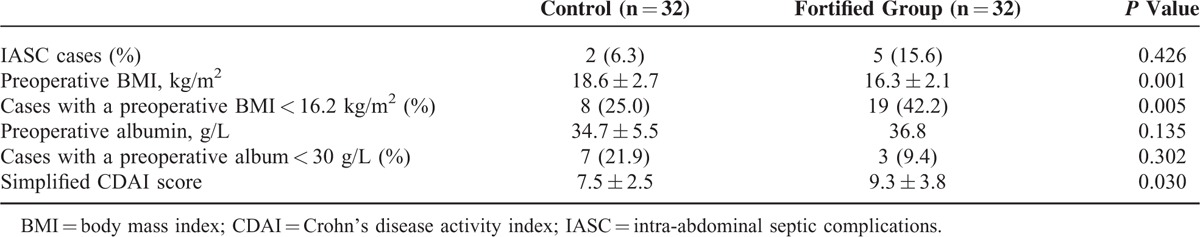
Effect of Nutrition Support on IASCs
The incidence rates of IASC were compared between the fortified nutrition support group and the control group. However, the result was not consistent with what has been hypothesized: the incidence of IASCs in the fortified nutrition support group was not lower than that in the control group (Table 3). A further analysis of the preoperative nutritional status showed that the former group had a lower BMI and a higher simplified CDAI score compared with the control group; however, the 2 groups had comparable serum albumin levels (Table 3). Therefore, the patients in the fortified nutrition support group had worse pathogenetic conditions. Moreover, their BMIs did not reach a satisfactory level before the operation, which may explain why the postoperative incidence of IASCs did not decrease after the fortified nutrition support.
Table 3.
Effect of Fortified Nutrition Support on the Incidence of Postoperative IASC
DISCUSSION
In this study, fortified nutrition support was given to the patients who underwent elective intestinal operations for 5 to 69 days. The serum albumin levels of the patients after nutrition support reached an approximately normal level. However, their preoperative low BMI was not sufficiently improved, and their postoperative incidence of IASCs did not decrease. These findings prompted us to reconsider and explore the standard for sufficient nutrition preparation before CD operations and how to implement those preparations. We found that BMI is a significant clinical index for patients experiencing IASC and that it better predicted the risk of postoperative IASCs in CD patients than serum albumin.
In recent years, researchers have investigated the effect of preoperative nutrition support on complications after CD operations. Parenteral nutrition support for 3 weeks noticeably improves CD patients’ immunoglobulin levels and BMIs.19 In another study, preoperative enteral nutrition support significantly decreased the incidence of IASCs after operation.20 Jacobson16 found that no IASCs occurred in 15 patients who received preoperative nutrition support, whereas 27.7% of the matched 105 patients suffered complications. These results indicate that preoperative nutrition support treatment can reduce the incidence of postoperative complications.
BMI and serum albumin are the nutrition markers of CD pathogenetic conditions.21 These measures are used extensively in clinical practice due to their advantages of being easy to manipulate and providing a dynamic evaluation of nutritional status. Although these indices may be influenced by infusions, obesity, edema, and fluid retention,14 no such influence was significant in the 64 patients in this study. Albumin, which is synthesized in the liver, is an acute phase protein with a half-life of approximately 20 days. In contrast, BMI reflects the basic nutrition storage of the organism, which changes slowly. Although the preoperative nutrition support for 6 to 71 days (20 ± 15 days) significantly corrected hypoalbuminemia in the CD patients, BMI can only be improved after 3 months of postoperative home nutrition support.22
In 2013, the Chinese Consensus recommended a duration of 4 to 6 weeks for adult parenteral nutrition.14 In the institution involved in this study, nutrition support was implemented before the publication of the Consensus, and the duration of nutrition support given to the patients was shorter than that recommended by the Consensus. The present findings showed that the fortified nutrition support did not decrease the incidence rate of IASCs after operation, even though the patients’ blood albumin levels approached a normal level. This unexpected result was ascribed to insufficient preoperative nutrition preparation, which did not reach standards, that is, the patients’ preoperative baseline BMI remained low. In a nonrandomized controlled study conducted at another center, 3 weeks of parenteral nutrition support was given to 16 CD patients; although the patients’ BMIs were significantly improved, the average BMI was only 15.3 ± 0.7 kg/m2 before the operation, and the postoperative complication incidence was comparable to that of the control group.19 Thus, our results are partially consistent with those reported in the literature.19
Another explanation for the failure of fortified nutrition support in reducing IASCs is patient selection bias. The simplified CDAI in the IASC group was higher than that in the non-IASC group (Table 1), and this observation is consistent with the understanding of CD activity and IASCs. Theoretically, fortified nutrition support will improve nutrition while also reducing CD activity.23 However, the preoperative simplified CDAI in the fortified group was significantly higher than that in control in this study, which may be largely attributed to differences between the patients’ conditions in the 2 groups. The patients in the control group were mild cases before 2012, but since the Inflammatory Bowel Disease Center was established and intensive nutrition therapy was started in 2012, the patients we treated have shifted to mainly critical cases. This bias partially offsets the benefits of fortified nutrition support. Therefore, we cannot conclude that fortified nutrition support does not contribute to IASC reduction.
This study has some limitations. First, this study was a retrospective study, which could have suffered from historical bias. To overcome this shortcoming, prospective studies are required. Second, the use of a single center and a small sample size constitute other limitations of this study. In addition, we failed to subgroup the participants to further explore the ideal BMI. Therefore, the target BMI for preoperative preparation for CD patients remains to be explored.
In conclusion, BMI better reflects the basic nutritional status of CD patients before operation compared with serum albumin. BMI can aid in guiding preoperative nutrition support and judging the appropriate operation time for CD. Therefore, much attention should be directed to this index during preoperative nutrition preparation.
ACKNOWLEDGMENTS
The authors thank their colleagues in the Sixth Affiliated Hospital of Sun Yat-Sen University for their assistance during this study.
Footnotes
Abbreviations: BMI = body mass index, CD = Crohn's disease, CDAI = Crohn's disease activity index, IASCs = intra-abdominal septic complications, PG-SGA = patient-generated subjective global assessment.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Nandivada P, Poylin V, Nagle D. Advances in the surgical management of inflammatory bowel disease. Curr Opin Gastroenterol 2012; 28:47–51. [DOI] [PubMed] [Google Scholar]
- 2.Kanazawa A, Yamana T, Okamoto K, et al. Risk factors for postoperative intra-abdominal septic complications after bowel resection in patients with Crohn's disease. Dis Colon Rectum 2012; 55:957–962. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Keighley MR. Factors affecting the incidence of postoperative septic complications and recurrence after strictureplasty for jejunoileal Crohn's disease. Am J Surg 1999; 178:240–245. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn's disease. Dis Colon Rectum 2000; 43:1141–1145. [DOI] [PubMed] [Google Scholar]
- 5.Dietz DW, Laureti S, Strong SA, et al. Safety and longterm efficacy of strictureplasty in 314 patients with obstructing small bowel Crohn's disease. J Am Coll Surg 2001; 192:330–337. [DOI] [PubMed] [Google Scholar]
- 6.Tay GS, Binion DG, Eastwood D, et al. Multivariate analysis suggests improved perioperative outcome in Crohn's disease patients receiving immunomodulator therapy after segmental resection and/or strictureplasty. Surgery 2003; 134:565–572.discussion 572–573. [DOI] [PubMed] [Google Scholar]
- 7.Iesalnieks I, Kilger A, Glass H, et al. Intraabdominal septic complications following bowel resection for Crohn's disease: detrimental influence on long-term outcome. Int J Colorectal Dis 2008; 23:1167–1174. [DOI] [PubMed] [Google Scholar]
- 8.Yang SS, Yu CS, Yoon YS. Risk factors for complications after bowel surgery in Korean patients with Crohn's disease. J Korean Surg Soc 2012; 83:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh J, O’Morain CA. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther 2003; 17:307–320. [DOI] [PubMed] [Google Scholar]
- 10.Valentini L, Schulzke JD. Mundane, yet challenging: the assessment of malnutrition in inflammatory bowel disease. Eur J Intern Med 2011; 22:13–15. [DOI] [PubMed] [Google Scholar]
- 11.Massironi S, Rossi RE, Cavalcoli FA, et al. Nutritional deficiencies in inflammatory bowel disease: therapeutic approaches. Clin Nutr 2013; 32:904–910. [DOI] [PubMed] [Google Scholar]
- 12.Donnellan CF, Yann LH, Lal S. Nutritional management of Crohn's disease. Therap Adv Gastroenterol 2013; 6:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alves A, Panis Y, Bouhnik Y, et al. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn's disease: a multivariate analysis in 161 consecutive patients. Dis Colon Rectum 2007; 50:331–336. [DOI] [PubMed] [Google Scholar]
- 14.Inflammatory Bowel Disease Group of Chinese Society of Gastroenterology. Expert consensus on nutritional support therapy for inflammatory bowel diseases. Chinese J Intern Med 2013; 52:1082–1087.(in Chinese). [Google Scholar]
- 15.Valentini L, Schaper L, Buning C, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition 2008; 24:694–702. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson S. Early postoperative complications in patients with Crohn's disease given and not given preoperative total parenteral nutrition. Scand J Gastroenterol 2012; 47:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980; 1:514. [DOI] [PubMed] [Google Scholar]
- 19.Yao GX, Wang XR, Jiang ZM, et al. Role of perioperative parenteral nutrition in severely malnourished patients with Crohn's disease. World J Gastroenterol 2005; 11:5732–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Ren J, Wang G, et al. Preoperative exclusive enteral nutrition reduces the postoperative septic complications of fistulizing Crohn's disease. Eur J Clin Nutr 2014; 68:441–446. [DOI] [PubMed] [Google Scholar]
- 21.Mijac DD, Jankovic GL, Jorga J, et al. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med 2010; 21:315–319. [DOI] [PubMed] [Google Scholar]
- 22.Gong JF, Niu LY, Yu WK, et al. Perioperative nutrition support in patients with Crohn's disease. Parenter Enteral Nutr 2009; 16:201–204.208. [Google Scholar]
- 23.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2007; 1:CD000542. [DOI] [PubMed] [Google Scholar]


