Supplemental Digital Content is available in the text
Abstract
Several reports suggest that gemcitabine (GEM) plus S-1 combination (GS) is associated to prolong the survival in patients with unresectable pancreatic cancer (PC). We conducted a systemic review and meta-analysis of studies comparing the safety and efficacy of GS versus GEM.
Summary data from randomized trials and retrospective studies were searched in PubMed, EMBASE, Web of Science, and the Cochrane Library. Statistical analyses were conducted to calculate the hazard ratios (HRs) and relative risk (RR) with 95% confidence intervals (CIs) using random-effects models. Subgroup analyses based on the chemotherapy cycles were performed to explore the efficacy and toxicity for therapy. Sensitivity analyses were conducted by removing specific studies to assess the effects of study quality.
Between January 2004 and August 2012, 4 RCTs and 2 retrospective studies including a total of 1025 cases were identified. The overall survival (OS) (HR: 0.82; 95% CI, 0.70–0.96; P = 0.01) and progression-free survival (PFS) (HR: 0.65; 95% CI, 0.55–0.77; P < 0.001) for the GS arm were significantly longer than the GEM arm. The differences in objective response rate (ORR) (RR: 1.24; 95% CI, 1.17–1.33; P < 0.001) and disease control rate (DCR) were also better in the GS arm (RR: 1.37; 95% CI, 1.19–1.59; P < 0.001). Grades 3 to 4 toxicities in both the groups were similar except neutropenia and diarrhea, which were more frequent in the GS arm (P < 0.001). In the subgroup analysis, the cycle for chemotherapy every 4 weeks has equivalent efficacy and less toxicity than regimens every 3 weeks in the GS arm.
The current meta-analysis suggested that GEM significantly prolonged OS and PFS when added to S-1 combination in patients with unresectable PC. GS therapy also offers better ORR and DCR than GEM monotherapy and no unexpected toxicity was evident.
INTRODUCTION
Pancreatic cancer (PC) is one of the most lethal digestive system tumor with a 5-year survival rate of <5%. According to the latest cancer data released by the American Cancer Society, PC reached the fourth leading cause of cancer-related mortality worldwide.1 Although surgical removal is the only effective way to cure, almost 80% of new cases at the time of diagnosis for local development and metastasis, known as “advanced PC,” lost the opportunity to operate.2,3 The prognosis of those patients remains extremely poor with a median survival time of 2 to 4 months.4 Thus, it is urgent to explore the effective chemotherapy regimens to further improve the prognosis of advanced PC.
Since the 1990s, gemcitabine (GEM) was used as the standard treatment of advanced PC; compared with 5-fluorouracil (FU), GEM can significantly prolong the overall survival (OS) with a response rate of 5%. Nevertheless, progress advance in improving the role of long-term prognosis of PC is still limited, with a median survival of <6 months.5 Although some combination therapies including GEM have shown survival benefit, these are not considered as standard treatment.6
S-1 consists of a 5-FU prodrug (tegafur) and 2 modulators of 5-FU metabolism, gimeracil and oteracil, in a 1:0.4:1 molar concentration ratio, which was used as an oral anticancer agent.7 The efficacy of S-1 has already been demonstrated in the treatment of solid tumors in gastric, colorectal, and nonsmall lung cancers.8 Several phase II trials of S-1 monotherapy in treating PC showed objective response rate (ORR) between 21.1% and 37.5%, and OS of 5.6 to 9.2 months.9,10 Subsequently, GEM-combined S-1 (GS) therapy in several large-scale clinical trials showed that GS in the treatment of advanced PC and its ORR can be up to 44% to 48%, with the median OS of 10 to 12 months.11–16
Even though several studies comparing GEM and GS have been reported, most are small-scale studies with unclear results. It is still uncertain whether the benefits of GS are restricted to improved OS. Thus, we systemically reviewed and analyzed the available literatures to evaluate the efficiency, safety, and potential advantages of GS compared with GEM. We will focus on the feasibility and acceptable toxicity profile of GS therapy that the patients can tolerate.
MATERIALS AND METHODS
Search Strategy
A literature search was performed in June 2014 without restriction to regions and publication types. Five electronic databases (PubMed, MEDLINE, EMBASE, Web of Science, and the Cochrane Library) were searched to identify possible articles relevant to the topic of interest. The following MeSH terms and their combinations were searched in (Title/Abstract): ([GS/S-1 combination/gemcitabine plus S-1 combination/gemcitabine and S-1 combination] and [gemcitabine/GEM] and [pancreatic cancer/pancreatic carcinoma]). When multiple reports describing the same population were published, the most recent or complete report was used.
Selection Criteria
Search findings were screened for potentially eligible studies. Abstracts and full articles were obtained for detailed evaluation, the peer-reviewed publications of studies that met the following criteria were eligible for inclusion: first, randomized controlled trials (RCTs) and retrospective studies comparing GS with GEM in all age groups, and which had at least reported 1 of the outcomes mentioned in the next section of this article; second, patients must have had locally advanced or metastatic PC with histological or cytological confirmation; third, the patients must experience no prior treatment including surgery, chemotherapy, or radiation therapy; fourth, patients had an Eastern Cooperative Oncology Group performance status of 0 to 2 and had adequate organ function defined by the standard parameters; fifth, in addition, the articles must include response rate, hazard ratio (HR) for progression-free survival (PFS) and OS, along with their 95% confidence intervals (CIs) or relevant data. Studies meeting any 1 of the following criteria were excluded: laboratory studies; letters, review articles, or case reports; animal experimental studies; the outcomes of interest (as OS, ORR, etc.) that were impossible to calculate or the standard deviation and CI of the tested parameters that were not reported; and absence of key information such as sample size, HR, 95% CI, and P value.
Quality Assessments
Quality of each included study was rated independently by 2 reviewers (D.L. and C.C.) by assessing the methodology of studies using either Cochrane risk of bias tool (for RCTs) or the modified Newcastle–Ottawa (for retrospective studies).17,18 Any disagreement was resolved by the adjudicating senior author (Y.L.). For each included RCTs, the following criteria were evaluated: application of adequate eligibility criteria, adequate measurement of outcomes, adequate control of confounding factors, completeness of follow-up and adequacy of its duration, adequate reporting of outcomes, and absence of other sources of bias. In addition, retrospective studies were evaluated by using the modified Newcastle–Ottawa scale that consisted of 3 factors: patient selection, comparability of the study groups, and assessment of outcome with a total score of 0 to 9 (allocated as stars); studies achieving ≥6 score were considered to be of high quality.11
Data Extraction and Outcomes of Interest
Two investigators (Y.Z. and X.F.) searched the publications independently using standardized data-abstraction forms. When the 2 investigators discovered different results, an independent expert in oncology (Z.L.) made the final decision of study conclusions. Information collected from these publications included first author, year of publication, targeted treatment, chemotherapy regimens, number of centers, number of patients, patient characteristics, study design (blinded or not), and the outcomes.
The primary outcomes were OS. The secondary outcomes included PFS, 1-year survival rate, ORR, disease control rate (DCR), and treatment and toxicity. In this study, OS is defined as the time from random assignment to death, irrespective of the cause of death. PFS is defined as the duration of time from random assignment to documented disease progression or death, whichever occurs first. ORR is defined as the proportion of complete response (CR) along with partial response (PR) among evaluable patients. For patients with no event observed, the time to censor refers to the time to last follow-up. The treatment efficacy between GS and GEM was measured by HR for PFS and OS. Additionally, a manual search was performed using references from the relevant literature, including all of the identified studies, reviews, and editorials. When duplicate publications were found, the study with reported HRs or involving additional patients was used for meta-analysis.
Statistical Analysis
Meta-analyses were carried out using Review Manager Version 5.3 software (Version 5.3 for Windows, The Cochrane Collaboration, 2014). Relative risk (RR) was selected as effect measure dichotomous outcomes and a weighted mean difference was selected for continuous variables, which reported along with the corresponding 95% CI. For studies presenting continuous data as median and range, the estimation of mean and variance from the median, range, and the size of a sample were performed using the technique described by Hozo et al.19 The Cochran Q test and Higgins I2 statistic were used to examine heterogeneity across studies. A P value of <0.05 was considered statistically significant difference. The random-effects model was used if there was heterogeneity between the studies; otherwise, the fixed-effects model was used.19 The pooled RR for ORR, and HRs for PFS and OS were calculated. Subgroup analyses were performed to compare GS arm and GEM arm in the chemotherapy cycle every 3 weeks or every 4 weeks in ORR, DCR, nausea and vomiting, and neutropenia. Sensitivity analyses were performed for high-quality studies. Presence of publication bias was evaluated using Funnel plot analyses.
Ethics Approval
The study was reviewed and approved by the Institutional Review Board and the Ethics Committee of the Sun Yat-sen Memorial Hospital, Guangzhou, China.
RESULTS
Data Retrieval
The flow chart of our study is shown in Figure 1. Through initial searches of electronic databases and other sources, 392 studies were identified; 86 were excluded from this study because of duplications and 306 were excluded based on our inclusion/exclusion criteria. Among the 78 articles that were selected on the basis of the inclusion/exclusion criteria, 72 articles were editorials or incomplete data and therefore excluded. Our final sample from 4 randomized clinical trials (level of evidence: 2b)20–23 and 2 retrospective study (level of evidence: 3b)24,25 that included 1025 patients were collected.
FIGURE 1.
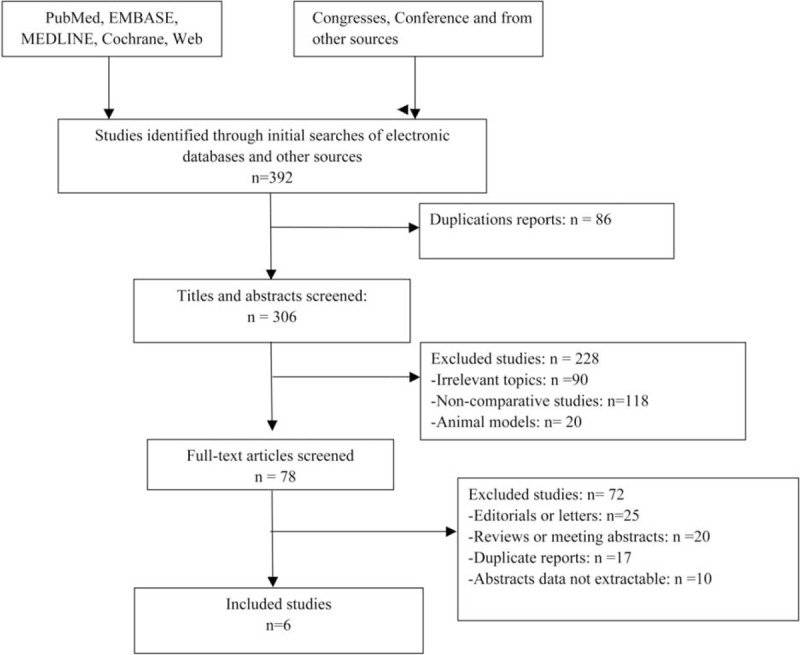
Flow diagram of studies identified, included, and excluded.
Study Characteristics
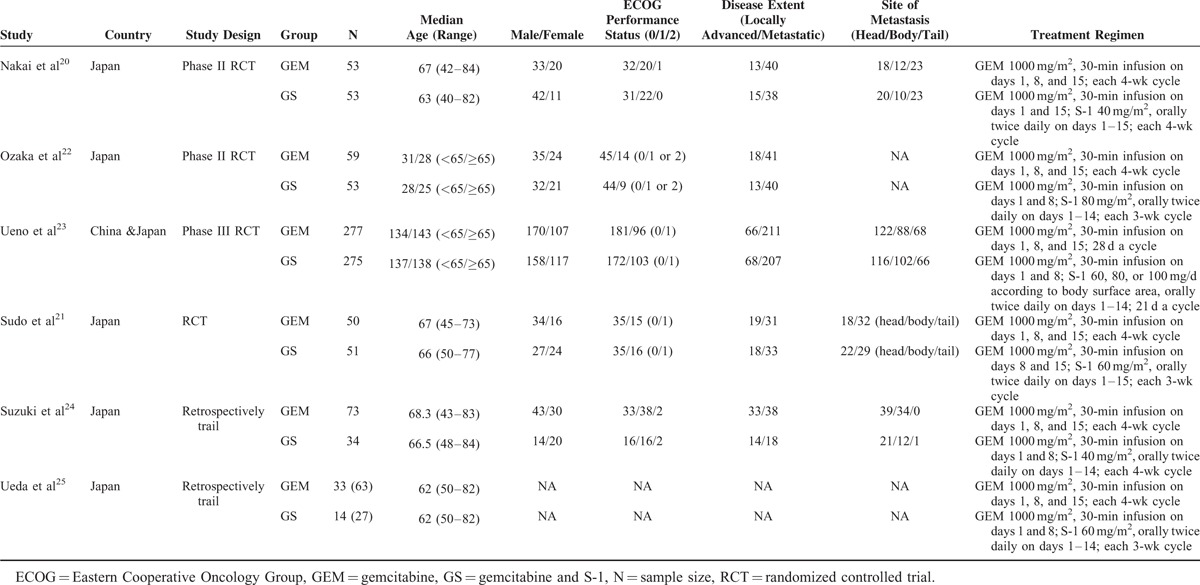
The characteristics of included studies are shown in Table 1. We collected data from 6 studies including a total of 1025 patients. All studies were conducted in Asian countries, including 5 in Japan,20–22,24,25 and one Japanese–Chinese collaboration article.23 The patient-level analyses showed that patient age varied from 40 to 70. All studies assigned unresectable PC patients to either the GS arm or the GEM arm. Patients allocated to the GEM arm received GEM intravenously at 1000 mg/m2 over 30 min. In the 6 studies, values for GEM arm were analyzed by different means in each study. In 4 studies, the GEM arm regimen was measured on days 1, 8, and 15, repeated every 4 weeks. In the other 2 studies, the GEM arm regimen consisted of intravenous 1000 mg/m2 GEM on days 1 and 8, repeated every 3 weeks. On the contrary, values for GS arm were analyzed by different means in each study. In 2 studies, patients randomly allocated to the GS arm received GEM intravenously at 1000 mg/m2 over 30 min on days 1 and 15 and S-1 orally twice daily for 2 weeks followed by a 2-week rest between each 4-week cycle. Three doses of S-1 were established according to the body surface area (BSA) as follows: BSA <1.25 m2, 80 mg/d; 1.25 m2 < BSA ≤ 1.5 m2, 100 mg/d; and BSA ≥ 1.5 m2, 120 mg/d. In other 4 studies, the GS arm regimen was measured at every 3-week cycle.
TABLE 1.
Basic Characteristic of Included Studies
According to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0, we evaluated all the adverse events at each cycle. Treatment was temporarily suspended in the case of grade 3/4 toxicity.
Qualities of Included Studies
The agreements of the reviewers for selection and validity assessment of the studies were scored by the κ-coefficient (a measure of agreement), which 0.83 with 91.2% were observed agreement and 0.85 with 91.6% observed agreement, respectively. True randomization was used in 4 RCTs that used adequate random sequence generation and assessment of each outcome. All RCTs20–23 applied allocation concealment, and 2 trials22,23 were double blind and avoided selective outcome and other bias (Appendix Table S2, http://links.lww.com/MD/A378). The risks of bias were evaluated by a modification of the Newcastle–Ottawa scale for retrospective studies (Appendix Table S3, http://links.lww.com/MD/A378). The quality of included retrospective studies was generally low.
Primary Outcomes
Overall Survival
Among the 6 clinical trials included in the meta-analysis, 4 reported HRs for OS and the corresponding 95% CIs. These studies assessed OS in 871 patients showed clearly significant difference between the GS arm and GEM arm (HR: 0.82; 95% CI, 0.70–0.96; p = 0.01) (Figure 2).
FIGURE 2.
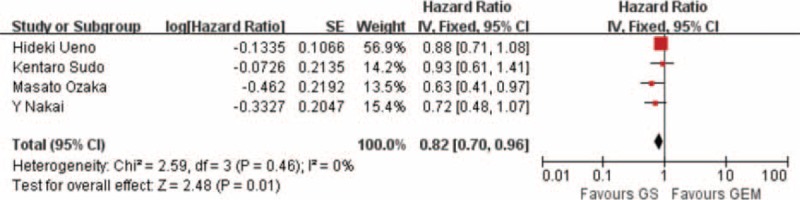
Forest plots of studies included between GS group versus GEM group in overall survival (OS).
Secondary Outcomes
PFS and 1-Year Survival Rate
Pooling the data of 3 studies including 759 patients that reported PFS indicated that the GS arm was significantly better than the GEM arm (HR: 0.65; 95% CI, 0.55–0.77; P < 0.001) (Figure 3). We collected data from 4 studies including a total of 877 patients that reported that 1-year survival rate showed a clear significance difference between the GS arm and the GEM arm (RR: 1.12; 95% CI, 1.09–1.35; P < 0.001) (Figure 4).
FIGURE 3.
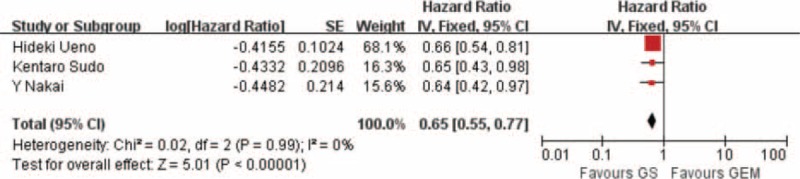
Forest plots of studies included between GS group versus GEM group in progression-free survival (PFS).
FIGURE 4.
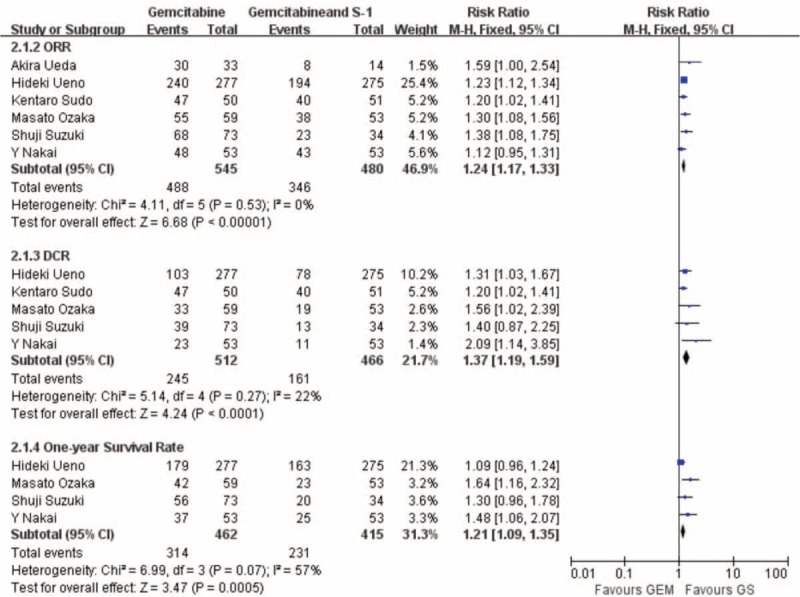
Forest plots of studies included between GS group versus GEM group in therapeutic effect (ORR, DCR, 1-y survival rate).
ORR and DCR
All the studies evaluating ORR presented a significant difference between the GS arm and the GEM arm (RR: 2.94; 95% CI, 2.16–4.01; P < 0.001) (Figure 4). Studies evaluating OS presented no evidence of significant heterogeneity between the GS arm and the GEM arm (P = 0.52). Pooling the data of 5 studies consisting of 978 patients that demonstrated DCR indicated that the GS arm was significant better than the GEM arm (RR: 1.37; 95% CI, 1.19–1.59; P < 0.001) (Figure 4).
Adverse Events
Treatment-related toxicity is reported in all the studies. Neutropenia was the most frequent grade ≥3 toxicity in both the arms. There were significant differences between the GS arm and the GEM arm (RR: 0.65; 95% CI, 0.56–0.75; P < 0.001) (Figure 5), and diarrhea that occurred in the GS arm was more significant than the GEM arm (RR: 0.36; 95% CI, 0.16–0.83; P = 0.02). However, another adverse effects, nausea and vomiting (RR: 0.64; 95% CI, 0.34–1.19; P = 0.16), anemia (RR: 0.83; 95% CI, 0.60–1.15; P = 0.26), stomatitis (RR: 0.19; 95% CI, 0.0.3–1.10; P = 0.06), and fatigue (RR: 0.92; 95% CI, 0.52–1.81; P = 0.92), show no statistically significant difference in all these studies (Figure 5).
FIGURE 5.
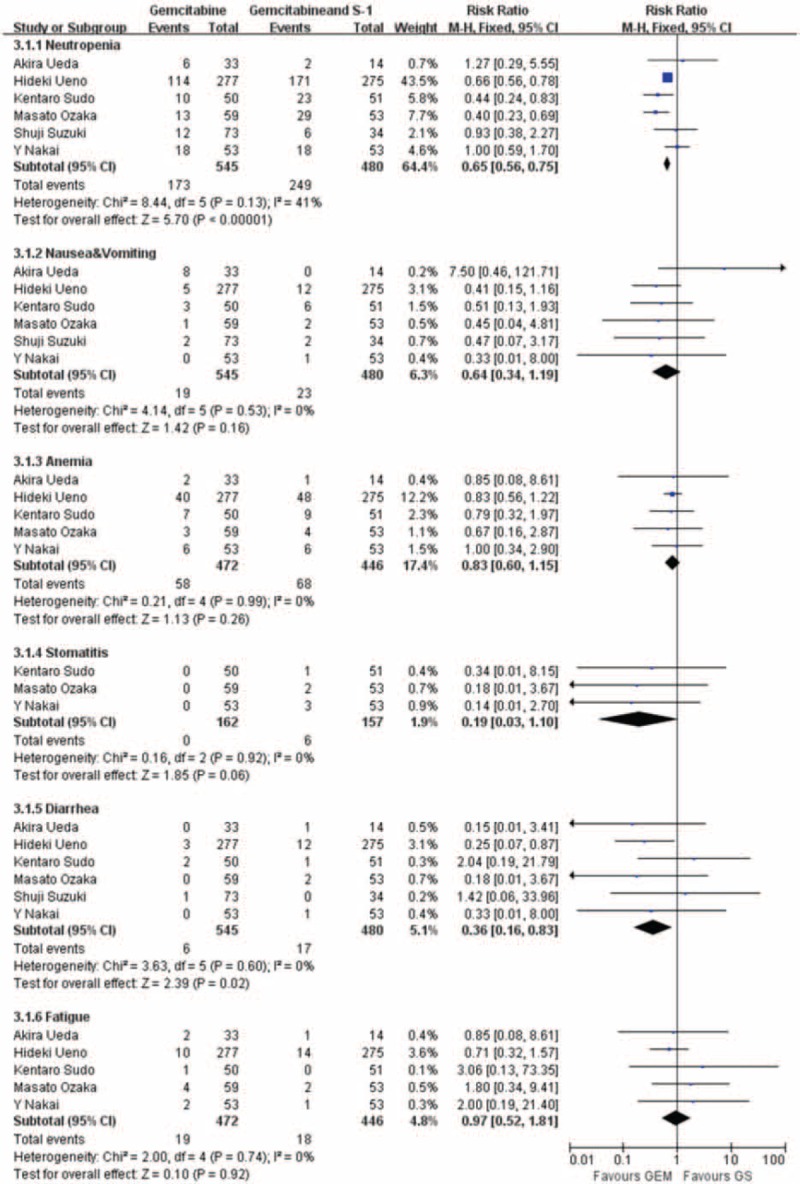
Forest plots of studies included between GS group versus GEM group in adverse events.
Subgroup Analysis
GS Arm Versus GEM Arm Chemotherapy Cycle Every 3 Weeks or Every 4 Weeks in ORR and DCR
In subgroup meta-analyses performed separately, there were no significant differences compared with the original analysis in ORR and DCR. The pooled studies showed the ORR (RR: 1.25; 95% CI, 1.16–1.34; P < 0.001) in the chemotherapy treatment every 3 weeks and the ORR (RR: 1.23; 95% CI, 1.07–1.41; P = 0.004) in the chemotherapy every 4 weeks (Appendix Figure S1, http://links.lww.com/MD/A378). For DCR, we found similar results for both chemotherapy every 3 weeks patients and chemotherapy every 4 weeks patients (RR: 1.66; 95% CI, 1.14–2.42; P = 0.008; and RR: 1.36; 95% CI, 1.10–1.68; P = 0.004) (Appendix Figure S2, http://links.lww.com/MD/A378).
GS Arm Versus GEM Arm Chemotherapy Cycle Every 3 Weeks or Every 4 Weeks in Neutropenia, Nausea, and Vomiting
There were no significant differences in this subgroup analysis compared with the original analysis in nausea and vomiting, except that no significant difference was found in the chemotherapy every 4 weeks about neutropenia (RR: 0.98; 95% CI, 0.62–1.55; P = 0.93), but in the GS group showed more toxicity in treatment every 3 weeks (RR: 0.61; 95% CI, 0.52–0.72; P < 0.001) (Figure 6).
FIGURE 6.
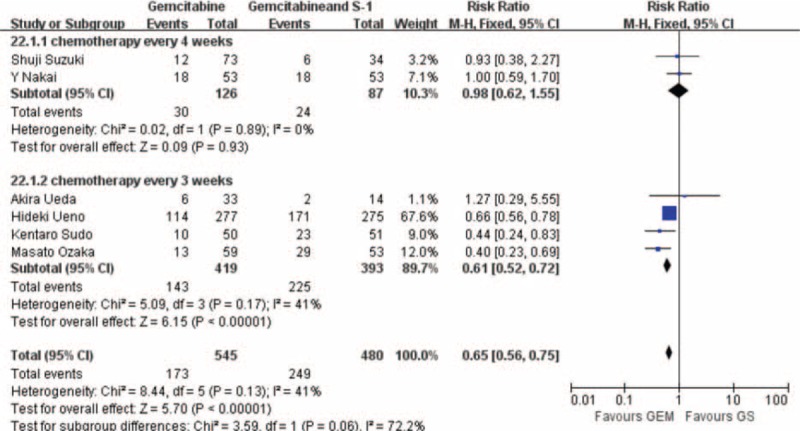
Forest plots of studies reported neutropenia included in subgroup: (A) GS arm versus GEM arm chemotherapy cycle every 4 wk; (B) GS arm versus GEM arm chemotherapy cycle every 3 wk.
Sensitivity Analysis and Publication Bias
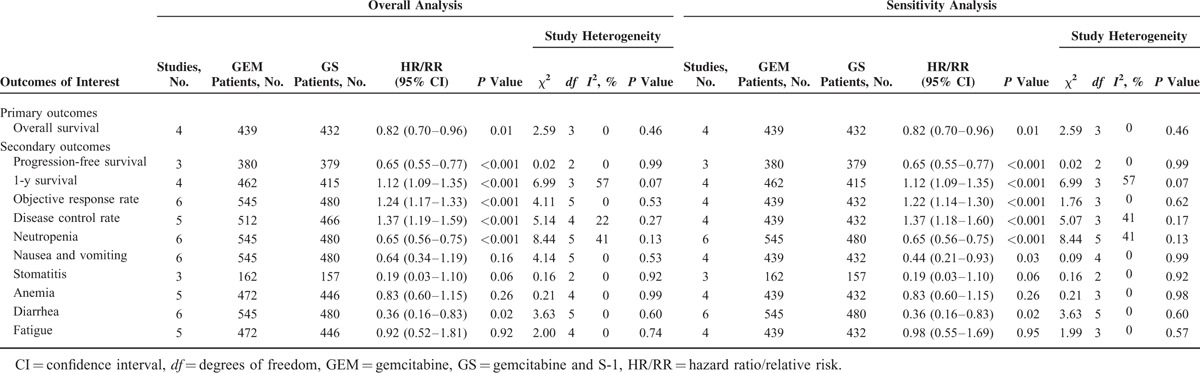
Four RCTs and 2 retrospective studies that scored ≥6 stars on the modified Newcastle–Ottawa scale were included in the sensitivity analysis (Table 2). There was no change in the significance of any of the outcomes except for nausea and vomiting, which was shown to be significantly lower in the GEM group than the GS group (RR: 0.44; 95% CI, 0.21–0.93; P = 0.03). According to the Cochrane Handbook for Systematic Reviews of Interventions,26 because of the number of the included studies that were <8, the funnel plots can be regarded as insignificant. We consider the funnel plots is unnecessary.
TABLE 2.
Results of Meta-Analysis Comparison of GEM and GS∗
DISCUSSION
PC is considered a high malignant degree with onset conceals and rapidly progress. Owing to the major hallmarks of PC, aggressive local invasion, early hematogenic and lymphogenic metastasis, and high risk of local recurrence, the prognosis of pancreatic carcinoma is still poor. A majority of new cases at the time of diagnosis lost the opportunity to operation because of local development and metastasis. Chemotherapy is considered as an option of treatment, but markedly resistant to chemotherapy contribute to modest effect. Therefore, it is urgent to explore novelty regimes to improve treatment effects.
GEM was recommended as a first-line chemotherapy drug for PC; the ORR of GEM single agent in the treatment of advanced PC has reached the bottleneck with limited survival benefit. According to the latest data from the American Society of Clinical Oncology research, S-1 possesses equal curative effect in treating advanced PC. In recent years, studies have identified that S-1 achieve favorable therapeutic effect in GEM-resistant PC.27,28 Thus, treatment comparison between GEM and GS had been launched in several large-scale clinical trials.
This meta-analysis of 4 RCTs and 2 retrospective studies including 1025 patients comparing the efficacy of GS arm and GEM arm showed that GS arm was effective, with significantly longer OS and PFS, higher ORR, better DCR, and longer 1-year survival. Toxicity profiles of these 2 drugs differed slightly: GS arm tended to show neutropenia and diarrhea toxicity. However, both GS and GEM were generally well tolerated. Furthermore, the cycle for chemotherapy every 4 weeks has equivalent efficacy and less toxicity than regimens every 3 weeks in the GS arm. Hence, our results suggest that GS therapy may be considered to be used as first-line therapy and as a convenient oral alternative for locally advanced and metastatic PC. To the best of our knowledge, this is the first meta-analysis to demonstrate that the GS regime has inferiority compare to a single anticancer agent of GEM alone for locally advanced and metastatic PC.
Combination therapy with GEM and other cytotoxic drugs or molecular-targeted agents has been thoroughly investigated in patients with PC, but no significant improvement was found in OS. Several other combination regimens (oxaliplatin, irinotecan, pemetrexed) have been tested but have shown disappointing results in PFS.29–31 Only GEM plus erlotinib compared to GEM monotherapy has a slight OS benefit of 6.24 versus 5.91 months.6 Two contrast GEM plus capecitabine phase III clinical trials with GEM monotherapy in the treatment of advanced PC showed that combination therapy can prolong PFS and OS to a nonsignificant level. After meta-analysis of these phase III clinical trials showed that combination therapy was beneficial for OS. Conroy et al32 reported a significantly longer OS with FOLFIRINOX than GEM alone in patients with metastatic PC in 2011. However, FOLFIRINOX programs have a greater toxicity and only used with patients of greater physical health.33 Therefore, an urgent need is to explore the well-tolerated palliative chemotherapy, prolong survival, reduce patient pain, and improve quality of life.
This meta-analysis suggests that improved OS and PFS is an apparent advantage of GS arm. The finding is encouraging for the use of GS chemotherapy that can significantly prolong the survival of patients with unresectable PC. Although several previous randomized clinical trials did not reach statistical significance in OS, we evaluated OS in 871 patients that showed clearly longer significantly in the GS arm (P < 0.05). In addition, ORR, which refers to the proportion of CR + PR, represents the percentage of patients whose cancer shrinks (termed PR) or disappears after treatment (termed CR). In our studies, GS chemotherapy occupies obvious advantage in ORR and is superior to GEM monotherapy. These results explain why GS group antagonistic activities have a strong effect in advanced PC.
In the application of new chemotherapy, the safety of the patients is always of paramount importance. The pooled data of the prognostic value indicate that the GS arm approach is safe and effective for chemotherapy in patients with unresectable PC. There was no significant difference in nausea and vomiting. The neutropenia was only slightly more. As for toxicity, grades 3 to 4 neutropenia and stomatitis were more frequent in the GS arm, but the incidence of gastrointestinal reactions or anemia or fatigue was similar in both the groups and showed no significant difference between the GS arm and the GEM arm. Moreover, no significant difference was revealed in the chemotherapy every 4 weeks about neutropenia in the subgroup analysis. S-1 is an oral anticancer agent that consists of a 5-FU prodrug (tegafur), gimeracil, and oteracil.7 The combined use of tegafur and gimeracil leads to prolonged maintenance of 5-FU concentrations in plasma and tumor tissues. Additionally, oteracil preferentially localizes in the gut and inhibits phosphorylation of 5-FU. Thus, administration of oteracil theoretically reduces the gastrointestinal toxicity of 5-FU.34,35 Several phase II clinical studies have shown that not only did most of advanced PC patients’ benefit from GS treatment, but also tolerated its mild toxicity.9,10,36 This is consistent with the results of our study. Therefore, GS chemotherapy would not increase side effects in the gastrointestinal tract and bone marrow suppression. In terms of GS therapy safety and security, GS treatment can be suggested for the majority of patients.
Yanagimoto et al37 have pooled analysis of 3 randomized studies for locally advanced PC. The results manifest that GS can improve ORR, PFS, and OS in patients with locally advanced PC over GEM alone. In addition, because of the differences in natural history and the potential impact of radiation therapy on survival in patients with localized disease, localized unresectable PDAC must be studied in trials that do not include patients with metastatic disease. However, advanced PC can be defined locally advanced unresectable and metastases PC. According to the NCCN guidelines, the recommended treatments of locally advanced unresectable and metastases PC are in common. Chemotherapy is the primary therapy, and clinical trial is preferred.38 Therefore, we collect patients with advanced PC to be target patients to assess the survival benefit between 2 regimes. The present meta-analysis has the following limitations that must be taken into account. The main limitation is that the studies were all conducted in Asian countries. S-1 has now emerged as a potential adjuvant alternative to GEM and is available in several Asian countries and most of Europe, although it is not yet approved in the United States. The application of S-1 has been delayed in Western countries because of the metabolic differences between Asian and Caucasian ethnic groups. Gastrointestinal side effects of S-1 are more severe among Caucasians, requiring use of lower doses of the drug for Caucasian patients.39,40 For these reasons, the findings of this study are not immediately applicable to non-Asian populations. Furthermore, subgroup analysis yielded some different results compared with the original analysis. Future systematic reviews should evaluate different treatment regimens separately when enough literature is available. Last, but not least, the need for more international institutions, particularly in Europe and the United States, further research with standardized, unbiased methods, and larger, worldwide sample sizes confirm safety and effectiveness of GS chemotherapy.
Nevertheless, this meta-analysis was conducted at an appropriate time, because enough data have been accumulated for inspection by meta-analytical methods, and we reach to the conclusions that reported OS and PFS indicated that the GS arm was significantly better than the GEM arm. We applied multiple strategies to identify studies, strict criteria to include and evaluate the methodological quality of the studies, and subgroup and sensitivity analysis to minimize the heterogeneity. Hence, we provide the most update information in this area.
CONCLUSIONS
This meta-analysis of randomized studies indicates that GEM significantly prolonged OS and PFS when added to S-1 combination in patients with advanced PC. GS therapy also offers better ORR and DCR than GEM monotherapy and no unexpected toxicity was evident.
Acknowledgments
The authors would like to thank Chengsan Sun and David L. Hill, Department of Psychology, University of Virginia, Charlottesville, VA, for modification and research comments; and Professor Y.T. Hao, Department of Medical Statistics, Sun Yat-sen University, Guangzhou, China, for statistical advice and research comments. None of these persons received compensation for the work performed.
Footnotes
Abbreviations: BSA = body surface area, CIs = confidence intervals, CR = complete response, DCR = disease control rate, GEM = gemcitabine, GS = gemcitabine and S-1, HRs = hazard ratios, ORR = objective response rate, OS = overall survival, PC = pancreatic carcinoma, PFS = progression-free survival, PR = partial response, RR = relative risk.
DL, CC, and YZ contributed equally to this study.
Author contributions: Conception and design—YL, DL, and CC; Administrative support—DL, CC, RC, ZB, YL, and ZL; Provision of study materials or patients—YZ and XF; Collection and assembly of data: CC, YZ, and XF; Data analysis and interpretation—DL, CC, RC, ZB, YZ, XF, and YL; Manuscript writing—all authors; Final approval of manuscript—all authors.
This study was not supported by any pharmaceutical company or grants; the cost was borne by the authors’ institutions. All authors approved the report. The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ 2012; 344:e2476. [DOI] [PubMed] [Google Scholar]
- 3.Feliu J, Borrega P, Leon A, et al. Phase II study of a fixed dose-rate infusion of gemcitabine associated with erlotinib in advanced pancreatic cancer. Cancer Chemother Pharmacol 2011; 67:215–221. [DOI] [PubMed] [Google Scholar]
- 4.Cascinu S, Graziano F, Catalano G. Chemotherapy for advanced pancreatic cancer: it may no longer be ignored. Ann Oncol 1999; 10:105–109. [DOI] [PubMed] [Google Scholar]
- 5.Burris HR, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 7.Saif MW, Syrigos KN, Katirtzoglou NA. S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs 2009; 18:335–348. [DOI] [PubMed] [Google Scholar]
- 8.Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 2009; 39:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno H, Okusaka T, Ikeda M, et al. An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology 2005; 68:171–178. [DOI] [PubMed] [Google Scholar]
- 10.Okusaka T, Funakoshi A, Furuse J, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 2008; 61:615–621. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Yamaguchi T, Ishihara T, et al. Phase I trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 2005; 92:2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Yamaguchi T, Ishihara T, et al. Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 2006; 94:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno H, Okusaka T, Ikeda M, et al. A phase I study of combination chemotherapy with gemcitabine and oral S-1 for advanced pancreatic cancer. Oncology 2005; 69:421–427. [DOI] [PubMed] [Google Scholar]
- 14.Kim MK, Lee KH, Jang BI, et al. S-1 and gemcitabine as an outpatient-based regimen in patients with advanced or metastatic pancreatic cancer. Jpn J Clin Oncol 2009; 39:49–53. [DOI] [PubMed] [Google Scholar]
- 15.Lee GW, Kim HJ, Ju JH, et al. Phase II trial of S-1 in combination with gemcitabine for chemo-naive patients with locally advanced or metastatic pancreatic cancer. Cancer Chemother Pharmacol 2009; 64:707–713. [DOI] [PubMed] [Google Scholar]
- 16.Oh DY, Cha Y, Choi IS, et al. A multicenter phase II study of gemcitabine and S-1 combination chemotherapy in patients with unresectable pancreatic cancer. Cancer Chemother Pharmacol 2010; 65:527–536. [DOI] [PubMed] [Google Scholar]
- 17.Lee ES, Han EM, Kim YS, et al. Occurrence of c-kit+ tumor cells in hepatitis B virus-associated hepatocellular carcinoma. Am J Clin Pathol 2005; 124:31–36. [DOI] [PubMed] [Google Scholar]
- 18.Luzzi KJ, MacDonald IC, Schmidt EE, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998; 153:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai Y, Isayama H, Sasaki T, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 2012; 106:1934–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudo K, Ishihara T, Hirata N, et al. Randomized controlled study of gemcitabine plus S-1 combination chemotherapy versus gemcitabine for unresectable pancreatic cancer. Cancer Chemother Pharmacol 2014; 73:389–396. [DOI] [PubMed] [Google Scholar]
- 22.Ozaka M, Matsumura Y, Ishii H, et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol 2012; 69:1197–1204. [DOI] [PubMed] [Google Scholar]
- 23.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31:1640–1648. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Ozaki Y, Saida S, et al. Retrospective study of gemcitabine plus S-1 versus gemcitabine alone in cases with unresectable advanced pancreatic cancer. Hepatogastroenterology 2013; 60:916–920. [DOI] [PubMed] [Google Scholar]
- 25.Ueda A, Hosokawa A, Ogawa K, et al. Treatment outcome of advanced pancreatic cancer patients who are ineligible for a clinical trial. Onco Targets Ther 2013; 6:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savovic J, Weeks L, Sterne JA, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev 2014; 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morizane C, Okusaka T, Ueno H, et al. Phase I/II study of gemcitabine as a fixed dose rate infusion and S-1 combination therapy (FGS) in gemcitabine-refractory pancreatic cancer patients. Cancer Chemother Pharmacol 2012; 69:957–964. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima H, Itoh A, Ohno E, et al. Prospective multicenter study to investigate the introduction rate of second-line S-1 in gemcitabine-refractory unresectable pancreatic cancer. Cancer Chemother Pharmacol 2011; 68:677–683. [DOI] [PubMed] [Google Scholar]
- 29.Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 2005; 16:1639–1645. [DOI] [PubMed] [Google Scholar]
- 30.Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 2006; 95:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009; 27:3778–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 33.Saif MW, Chabot J. Chemotherapy: metastatic pancreatic cancer—is FOLFIRINOX the new standard? Nat Rev Clin Oncol 2011; 8:452–453. [DOI] [PubMed] [Google Scholar]
- 34.Tatsumi K, Fukushima M, Shirasaka T, et al. Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rat liver extracts. Jpn J Cancer Res 1987; 78:748–755. [PubMed] [Google Scholar]
- 35.Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 1993; 53:4004–4009. [PubMed] [Google Scholar]
- 36.Yamaue H, Satoi S, Kanbe T, et al. Phase II clinical study of alternate-day oral therapy with S-1 as first-line chemotherapy for locally advanced and metastatic pancreatic cancer. Cancer Chemother Pharmacol 2014; 73:97–102. [DOI] [PubMed] [Google Scholar]
- 37.Yanagimoto H, Ishii H, Nakai Y, et al. Improved survival with combined gemcitabine and S-1 for locally advanced pancreatic cancer: pooled analysis of three randomized studies. J Hepatobiliary Pancreat Sci 2014; 21:761–766. [DOI] [PubMed] [Google Scholar]
- 38.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014; 12:1083–1093. [DOI] [PubMed] [Google Scholar]
- 39.Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol 2008; 26:2118–2123. [DOI] [PubMed] [Google Scholar]
- 40.Chuah B, Goh BC, Lee SC, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 2011; 102:478–483. [DOI] [PubMed] [Google Scholar]


