Abstract
Dercum's disease is a rare condition of painful subcutaneous growth of adipose tissue. Etiology is unknown and pain is difficult to control. We report the case of a 57-year-old man with generalized diffuse Dercum's disease, who improved after the treatment with transcutaneous frequency rhythmic electrical modulation system (FREMS).
Treatment consisted in 4 cycles of 30 minutes FREMS sessions over a 6-month period. Measures of efficacy included pain assessment (visual analogue scale, VAS), adipose tissue thickness by magnetic resonance imaging, total body composition and regional fat mass by dual-energy X-ray absorptiometry, physical disability (Barthel index), and health status (Short Form-36 questionnaire).
After FREMS treatment the patient's clinical conditions significantly improved, with reduction of pain on the VAS scale from 64 to 17 points, improvement of daily life abilities (the Barthel index increased from 12 to 18) and amelioration of health status (higher scores than baseline in all Short Form-36 domains). Furthermore, we documented a 12 mm reduction in subcutaneous adipose tissue thickness at the abdominal wall and a 7040 g decrease in total body fat mass.
FREMS therapy proved to be effective and safe in the treatment of this rare and disabling condition.
INTRODUCTION
Dercum's disease, also known as adiposis dolorosa or lipomatosis dolorosa, is a rare disorder characterized by a generalized and painful growth of the subcutaneous adipose tissue.1 The etiology is unknown. The disease is listed in Orphanet1 and the National Organization for Rare Disorders.2 No epidemiological data are available,1,2 however, based on individual case reports, Dercum's disease occurs more frequently in women than men, and during middle age (35–50 years).1 Based on the distribution of the affected adipose tissue and the association with lipomas, Dercum's disease is classified into 4 types: generalized diffuse (widespread painful adipose tissue with no lipomas), generalized nodular (generalized painful adipose tissue, more intense within and around lipomas), localized nodular (painful adipose tissue exclusively within and around lipomas), and juxtaarticular (painful solitary fat deposition in the proximity of large joints).1
In the absence of specific markers of disease, the diagnosis is clinical and based on a minimal set of criteria, including overweight or obesity and chronic (>3 months) pain of the subcutaneous tissue. In general pain is symmetrical, often disabling, and resistant to analgesics.1 Diagnosis is confirmed by excluding other conditions, such as fibromyalgia, lipoedema, panniculitis, Cushing's syndrome, primary psychiatric disorders, adipose tissue tumors, and familial multiple lipomatosis.1 No specific treatment exists for this disease,2 but several approaches directed to pain symptoms have been reported. Surgical removal of adipose tissue with liposuction3,4 was proposed as a successful treatment to alleviate pain, but even if the positive effect persisted during a 5-year follow-up period, it seemed to diminish overtime.3 There are reports of treatments based on manual lymphatic drainage in addition to pregabalin5 or on cycling hypobaric pressure:6 these physical methods seemed to be effective in relieving pain, however randomized, controlled trials are needed to confirm these data. Many drugs (local and systemic analgesics, steroid and nonsteroidal anti-inflammatory drugs, calcium-channel modulators as pregabalin, methotrexate, infliximab, interferon, and metformin) have been used as therapeutic strategies.1 Lidocaine has been administered by intralesional injections1 as topical analgesic7 and intravenous systemic therapy;8 this last route of administration resulted in pain relief lasting from 9 to 12 months.9 An apparent permanent pain relief has been observed with the administration of infliximab and methotrexate10 and with interferon alfa,11 but these drugs have many side effects and have benefitted only few cases to date.
We here report the case of a patient with Dercum's disease treated with frequency rhythmic electrical modulation system (also known as frequency-modulated electromagnetic neural stimulation, FREMS), a novel transcutaneous electrotherapy consisting of a sequence of modulated electrical stimuli automatically varying as for pulse frequency, duration, and voltage amplitude. In our patient FREMS was attempted empirically, based on its established efficacy in diabetic neuropathy and other painful conditions,12–17 easiness to perform and overall safety.
PATIENT HISTORY AND CLINICAL FINDINGS
A 57-year-old Caucasic obese (body mass index [BMI] 38) male with a recent diagnosis of Dercum's disease was referred to the San Raffaele Hospital. Patient's past medical history was characterized by partial gastrectomy for gastric neoplasia (pT1N0G1) with negative follow-up, cholecystectomy for gallbladder chronic inflammation, and multiple lumbar discal herniations. The patient was hospitalized for the first time elsewhere in May 2013 with a 2-month history of fever (up to 38°C) and a painful, hard, subcutaneous swelling, that from the right shoulder extended to the trunk and proximal portion of both arms and legs, and associated with local signs of inflammation. The patient also noted a 19 kg increase in body weight in the 9 months prior to admission at our hospital. A skin and soft tissue biopsy of the right shoulder documented normal epidermis, dermal, and adipose tissue, with no evidence of panniculitis or other inflammatory conditions. Blood tests were normal with the exception of increased C reactive protein (6.3 mg/dL, normal < 0.5) and presence of nuclear antibodies (low/medium titer [1:160, nucleolar pattern]), HCV infection was excluded. A whole-body CT scan and a gastroscopy were negative. A diagnosis of Dercum's disease was presumed and then confirmed by a dermatology consultant of the Centre for Rare Dermatological Diseases (University of Milano, School of Medicine), after other possible differential diagnosis were excluded (ie, paraneoplastic syndrome and panniculitis). The patient was treated with prednisone (75 mg/day, progressively tapered to 12.5 mg/day), with fever resolution and transient reduction of pain. Aiming at pain relief the patient was prescribed different combinations of acetaminophen, codeine, fentanyl, and methotrexate, without noticeable effects. The patient experienced a progressive worsening of his functional status, forcing him to take a leave from work.
Upon admission the clinical picture was dominated by a diffuse and painful subcutaneous swelling, with hard thickened skin, involving the entire body surface, sparing only the head, neck, and the distal portion of extremities (Figure 1). Pain was graded as intolerable, exacerbated by digital pressure, and prevented the patient to lie on his back, wear a T-shirt and bend forward, making difficult to sit. Medical treatment included fentanyl 50 mcg/hours transdermal patches (1 skin patch applied every 72 hours), acetaminophen 3 g/day, and prednisone 12.5 mg/day.
FIGURE 1.
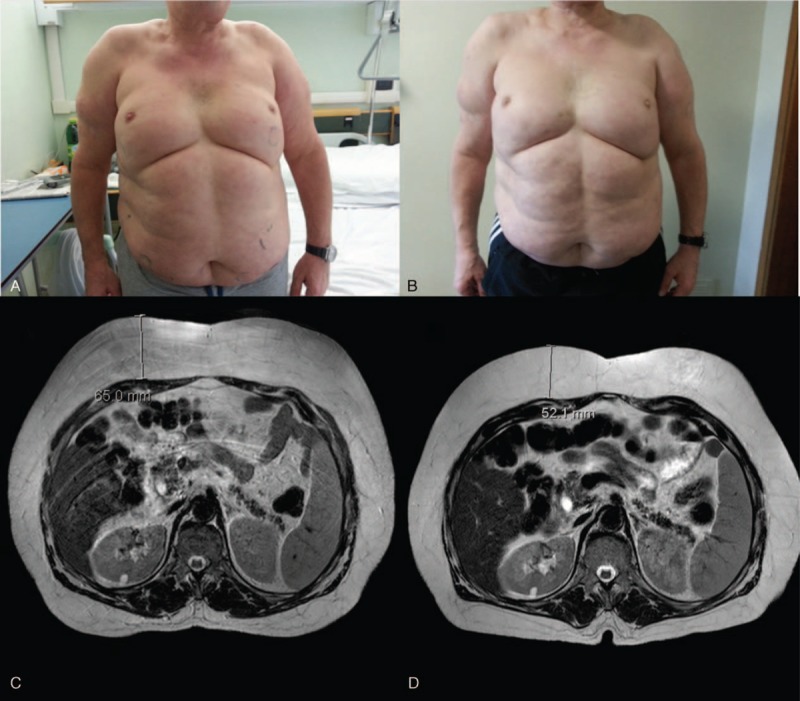
Patient appearance upon admission (October 2013) (panel A) and after FREMS treatment (April 2014) (panel B): a homogenous decrease in subcutaneous swelling was observed in the trunk and proximal portion of the upper limbs. MRI scan of the abdomen with T2-weighted Spin Echo respiratory-triggered sequences at baseline (panel C) and after FREMS treatment (panel D): a significant reduction in fat was documented in the subcutaneous tissue of the abdomen, while no changes were observed in visceral fat. FREMS = frequency rhythmic electrical modulation system, MRI = magnetic resonance imaging.
ELECTROTHERAPY
Based on our experience in using FREMS to treat diabetic neuropathy and other painful and/or inflammatory conditions, we offered the patient a cycle of FREMS treatment, performed as compassionate therapy (ie, off label), after obtaining the patient's written informed consent.
FREMS is a transcutaneous electrical stimulation consisting of a sequence of software-driven modulated electrical stimuli that vary automatically as of pulse frequency, duration, and voltage amplitude.4 FREMS treatment was administered using the Aptiva device (Lorenz Lifetech, Ozzano dell’Emilia [formerly Lorenz Biotech, Medolla], Italy). In detail, the electrical stimulation delivered during each 30 minutes FREMS session is characterized by sequences of biphasic (negative and positive), asymmetric and electrically balanced pulses, each consisting of an active phase of high negative voltage spike (variable, max −300 V) of extra short duration (variable, ∼40 μseconds), and a recharging phase of low voltage activity of longer duration (0.9–999 milliseconds). Pulse frequency is variable, ranging 1 to 1000 Hz, mainly in the low range 1 to 50 Hz. During treatment sessions the patient was invited to set the threshold of maximal electrical stimulation by progressively increasing the voltage from 0 to 300 V through a hand-held remote control device that increases the nominal voltage by 1 V per step to the maximum allowed corresponding to a burning sensation. The patient mostly used a threshold of 180 V.
To treat the entire disease extension, FREMS was delivered through 3 channels of 4 pair of electrodes, for a total of 24 electrodes applied to the surface of the trunk, back, shoulders, upper, and lower limbs (Figure 2). Over a 6 months period the patient underwent 4 cycles of FREMS, starting during hospitalization and continuing on an outpatient basis, each consisting of 10 consecutive sessions, administered 24 hours apart. The first 2 cycles were administered consecutively, the remaining ones 2 to 3 months apart. We arbitrarily choose to use the same time intervals between cycles used in the FREMS treatment of diabetic neuropathy, a conditions also characterized by chronic pain.
FIGURE 2.
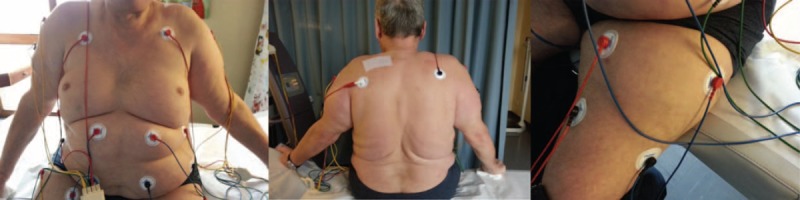
Positioning of electrodes for FREMS treatment. Electrodes were applied to the surface of the trunk, back, shoulders, upper, and lower limbs for a total of 24 electrodes. FREMS = frequency rhythmic electrical modulation system.
CLINICAL ASSESSMENT
The following measurements were obtained to document baseline conditions and monitor efficacy of FREMS treatment: anthropometrics (body weight, body mass index); intensity of pain using a 0 to 100 mm visual analogue scale, ranging from “no pain” (score 0) to “worst pain imaginable” (score 100); physical disability using the Barthel Index of Activities of Daily Living questionnaire; health status using the Short Form 36 questionnaire; subcutaneous adipose tissue thickness by total body MR, with measurements expressed in millimeters in a defined area of interest of the abdominal wall; and total body composition and regional fat mass by dual-energy X-ray absorptiometry, with results expressed in grams and percentage of fat or lean mass. Serial pictures of the patient were taken with permission.
RESULTS
Efficacy measures at baseline and months 3 and 6 are shown in Table 1. After starting FREMS treatment we observed the improvement of the patient's clinical conditions with amelioration of all measured variables. By month 3 the patient reported a dramatic and ubiquitous reduction of pain. We observed a decrease in subcutaneous fat consistency, from hard to soft, making possible for him to lie on his back, bend forward, and raise his arms over the head. By month 4 his conditions had improved to the extent that he was able to return to work. By month 6 pain was further reduced. Prescription of anti-inflammatory and analgesics was progressively decreased: prednisone was rapidly tapered and stopped within 2 weeks from the start of FREMS treatment, while transdermal fentanyl was reduced to 25 mcg/hours patch applied every 72 hours, associated to indomethacin 50 mg on demand. Analgesic drugs were needed mainly because of multiple lumbar herniated disks causing pain on his back irradiated to lower limbs. By month 6 body weight had progressively decreased to 90 kg (BMI 33) with no changes in dietary habits. Disability and health status scores improved accordingly.
TABLE 1.
Anthropometrics, Efficacy Measures, and Medications at Baseline, Month 3 and Month 6 After FREMS Treatment
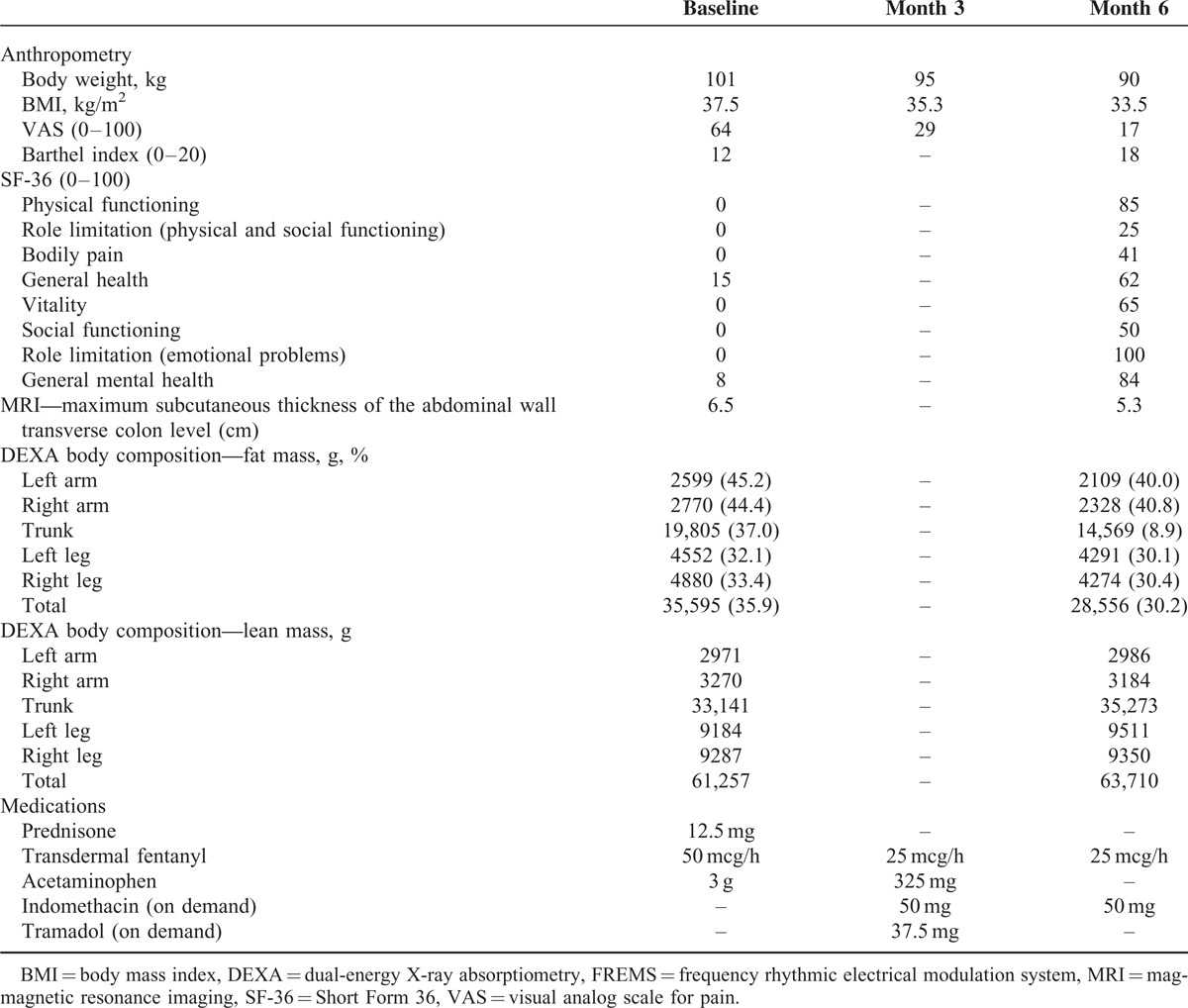
The marked improvement of clinical signs and symptoms was paralleled by the quantitative reduction of adipose tissue. At baseline, magnetic resonance imaging showed a diffuse and homogeneous increase of the subcutaneous adipose tissue, particularly pronounced in the abdominal wall, up to a maximum thickness of 65 mm in the upper abdomen (normal values 20–30 mm for a male of similar age and BMI). The increase in adipose tissue was detected only within the subcutaneous district, with no quantitative abnormalities of visceral fat. At month 6, magnetic resonance imaging showed a uniform reduction of the subcutaneous adipose tissue thickness to 53 mm in the upper abdomen, that is, a 12 mm decrease compared to baseline (Figure 1). No changes in visceral fat were documented. Body composition by dual-energy X-ray absorptiometry at month 6 showed a reduction of total fat from 35.596 at baseline to 28.556 g (−19.8%), with the percentage of fat mass decreasing from 35.9% to 30.2%. The total fat reduction was detected in all affected body segments and was more pronounced in the arms and trunk.
No major adverse events were recorded during FREMS treatment. The patient occasionally reported a slight burning sensation and skin redness at the site of electrode placement, both resolving spontaneously within few hours after ending FREMS treatment.
DISCUSSION
This report documents a case of Dercum's disease, a rare disabling condition, in which FREMS electrotherapy significantly reduced pain, decreased subcutaneous adipose tissue thickness and fat compactness, and remarkably improved functional and health status.
FREMS has proved effective and safe in the treatment of painful diabetic neuropathy,12,13 chronic painful leg ulcers,14,15 myofascial pain syndrome,16 and more recently, in a single case of scleredema diabeticorum.17 The mechanism of action of FREMS has still to be elucidated, as for many of the new and largely unexplored medical treatments based on electrical impulses.18 Previous reports showed that FREMS treatment enhances microvascular blood flow,19 increases vasomotor activity mediated by smooth cells,20 releases vascular endothelial growth factor,21 and changes the amplitude of Hoffmann (H)-reflex.22
The observation of a beneficial effect of FREMS in a case of Dercum's disease is intriguing. In fact, it has been reported that electrical impulses delivered by artificial devices or procedures, such as acupuncture, may act on disease-specific or disease-altered neural circuits, ultimately modulating immune response and controlling inflammation.23–27 In our case of Dercum's disease the target of electrotherapy was the subcutaneous adipose tissue, altered by an overgrowth of unknown origin, associated with pain, and local signs of inflammation. Although there is no evidence of any involvement of subcutaneous neural circuits in Dercum's disease, we can speculate that the modulation of such circuits by electrotherapy may provide local benefit. Interestingly, in an early case report sympathetic dysregulation causing abnormal subcutaneous blood flow was postulated as the pathogenic mechanism of Dercum's disease.28 Furthermore, FREMS is a unique form of electrotherapy, in which the automatic, software-driven modulation of frequency, duration, and amplitude of electrical stimulation might be relevant for its therapeutic effects. Indeed, modulation is important when delivering electrical impulses, and FREMS overarches a large spectrum of frequencies (ranging 1–1000 Hz, mainly in the low range 1–50 Hz), possibly including also those responsible for disease interference and for restoring healthy neural circuits. Another possible mechanism to explain decrease in pain perception may involve increased blood flow; in fact, pain in lipedema has been associated with hypoxia, inflammation, and necrosis of adipocytes;29 therefore, the release of vascular endothelial growth factor by FREMS therapy21 may improve blood flow and reduce hypoxia in painful adipose tissue. A similar mechanism has been postulated to explain the positive effect of cyclic variations in altitude conditioning (CVAC) process6 in a pilot study on 10 participants with Dercum's disease.
We acknowledge that the clinical benefits of FREMS treatment observed in our patient may be transient: whether additional FREMS sessions will be efficacious in case of recurrence is unknown. We cannot rule out that the improvement in pain is also due to a placebo effect or the natural history of the disease, that is why additional studies, possibly randomized and on a larger number of patients are needed.
In view of the observed efficacy in the absence of relevant side effects, FREMS treatment may be considered a therapeutic option for Dercum's disease, to improve patients’ pain, functional status, and quality of life.
Footnotes
Abbreviations: BMI = body mass index, FREMS = frequency rhytmic electrical modulation system.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Hansson E, Svensson H, Brorson H. Review of Dercum's disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis 2012; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Organization for Rare Disorders (NORD), Report on Dercum's Disease. http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/490/viewAbstract (Accessed November 11, 2014) [Google Scholar]
- 3.Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum's disease (adiposis dolorosa). Pain Med 2011; 12:942–952. [DOI] [PubMed] [Google Scholar]
- 4.Hansson E, Manjer J, Svensson H, et al. Quality-of-life in patients with Dercum's disease – before and after liposuction. J Plast Surg Hand Surg 2012; 46:252–256. [DOI] [PubMed] [Google Scholar]
- 5.Lange U, Oelzner P, Uhlemann C. Dercum's disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int 2008; 29:17–22.in press. [DOI] [PubMed] [Google Scholar]
- 6.Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res 2010; 3:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reggiani M, Errani A, Staffa M, et al. Is EMLA effective in Dercum's disease? Acta Derm Venereol 1996; 76:170–171. [DOI] [PubMed] [Google Scholar]
- 8.Petersen P, Kastrup J. Dercum's disease (adiposis dolorosa). Treatment of the severe pain with intravenous lidocaine. Pain 1987; 28:77–80. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson RL. Intravenous lidocaine for the treatment of intractable pain of adiposis dolorosa. Int J Obes 1982; 6:351–357. [PubMed] [Google Scholar]
- 10.Singal A, Janiga JJ, Bossenbroek NM, et al. Dercum's disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venereol 2007; 21:717. [DOI] [PubMed] [Google Scholar]
- 11.Gonciarz Z, Mazur W, Hartleb J, et al. Interferon alfa-2b induced long-term relief of pain in two patients with adiposis dolorosa and chronic hepatitis C. J Hepatol 1997; 27:1141. [DOI] [PubMed] [Google Scholar]
- 12.Bosi E, Conti M, Vermigli C, et al. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia 2005; 48:817–823. [DOI] [PubMed] [Google Scholar]
- 13.Bosi E, Bax G, Scionti L, et al. Frequency-modulated electromagnetic neural stimulation (FREMS) as a treatment for symptomatic diabetic neuropathy: results from a double-blind, randomised, multicentre, long-term, placebo-controlled clinical trial. Diabetologia 2013; 56:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janković A, Binić I. Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Arch Dermatol Res 2008; 300:377–383. [DOI] [PubMed] [Google Scholar]
- 15.Santamato A, Panza F, Fortunato F, et al. Effectiveness of the frequency rhythmic electrical modulation system for the treatment of chronic and painful venous leg ulcers in older adults. Rejuvenation Res 2012; 15:281–287. [DOI] [PubMed] [Google Scholar]
- 16.Farina S, Casarotto M, Benelle M, et al. A randomized controlled study on the effect of two different treatments (FREMS AND TENS) in myofascial pain syndrome. Eura Medicophys 2004; 40:293–301. [PubMed] [Google Scholar]
- 17.Gandolfi A, Pontara A, Di Terlizzi G, et al. Improvement in clinical symptoms of scleredema diabeticorum by frequency-modulated electromagnetic neural stimulation: a case report. Diabetes Care 2014; 37:e233–e234. [DOI] [PubMed] [Google Scholar]
- 18.Famm K, Litt B, Tracey KJ, et al. Drug discovery: a jump-start for electroceuticals. Nature 2013; 496:159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti M, Peretti E, Cazzetta G, et al. Frequency-modulated electromagnetic neural stimulation enhances cutaneous microvascular flow in patients with diabetic neuropathy. J Diabetes Complications 2009; 23:46–48. [DOI] [PubMed] [Google Scholar]
- 20.Bocchi L, Evangelisti A, Barrella M, et al. Recovery of 0.1 Hz microvascular skin blood flow in dysautonomic diabetic (type 2) neuropathy by using Frequency Rhythmic Electrical Modulation System (FREMS). Med Eng Phys 2010; 32:407–413. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua M, Dominguez LJ, Barrella M, et al. Induction of vascular endothelial growth factor release by transcutaneous frequency modulated neural stimulation in diabetic polyneuropathy. J Endocrinol Invest 2007; 30:944–947. [DOI] [PubMed] [Google Scholar]
- 22.Barrella M, Toscano R, Goldoni M, et al. Frequency rhythmic electrical modulation system (FREMS) on H-reflex amplitudes in healthy subjects. Eura Medicophys 2007; 43:37–47. [PubMed] [Google Scholar]
- 23.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med 2012; 209:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson U, Tracey KJ. A new approach to rheumatoid arthritis: treating inflammation with computerized nerve stimulation. Cerebrum 2012; 2012:3. [PMC free article] [PubMed] [Google Scholar]
- 25.Levine YA, Koopman FA, Faltys M, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One 2014; 9:e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavan SS, Tracey KJ. Regulating innate immunity with dopamine and electroacupuncture. Nat Med 2014; 20:239–241. [DOI] [PubMed] [Google Scholar]
- 27.Rosas-Ballina M, Olofsson PD, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skagen K, Petersen P, Kastrup J, et al. The regulation of subcutaneous blood flow in patient with Dercum's disease. Acta Derm Venereol 1986; 66:337–339. [PubMed] [Google Scholar]
- 29.Fife CE, Maus EA, Carter MJ. Lipedema: a frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv Skin Wound Care 2010; 23:81–920. [DOI] [PubMed] [Google Scholar]


