Abstract
The house dust mite is one of the most common allergens worldwide. There is good evidence that house dust mite subcutaneous immunotherapy is efficacious and has long-term benefit in children. However, the evidence of the benefit of house dust mite sublingual immunotherapy (SLIT) is less convincing. The purpose of this meta-analysis was to evaluate that efficacy and safety of dust mite SLIT in children with asthma.
Medical Literature Analysis and Retrieval System Online, ISI Web of Knowledge, and Cochrane Central Register of Controlled Trials databases until February 2014 were searched. The primary outcome was mean change in asthma symptom score. Secondary outcomes included mean change in serum immunoglobulin G4 (sIgG4), specific Dermatophagoides pteronyssinus, immunoglobulin E (IgE) levels, and medication score. Safety was also assessed.
We found that SLIT significantly decreased asthma symptom score (P = 0.007) and increased sIgG4 levels (P = 0.011) greater than control in children (<18 years of age) with asthma. There was no difference between SLIT and control groups in specific D pteronyssinus IgE levels (P = 0.076) and medication score (P = 0.408). The safety profile was similar between groups.
Our study indicates that dust mite SLIT therapy was effective in reducing asthma symptoms and in increasing sIgG4 but did not significantly reduce medication scores or specific D pteronyssinus IgE levels. Our findings are not enough to support the use of dust mite SLIT in children with asthma.
INTRODUCTION
Allergen-specific immunotherapy along with allergy avoidance and patient education are mainstay approaches for treating allergic disease.1–4 Specific allergen immunotherapy is the sole treatment for changing the natural course of allergic disease and minimizing the risk of an exacerbation.4 Subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are often used in clinical practice, but the frequency of their use differs worldwide with SCIT being commonly used in the United States, whereas in Europe SLIT and SCIT are about equally prescribed.5–7
There has been an increase in the prevalence of asthma and allergies over the past few decades, and it is thought that it may, in part, be due to an increase in indoor environmental exposure.8 Allergens and irritants such as house dust mite, domestic pets, mold, cockroaches, mice, tobacco smoke, endotoxin, and air pollution are important indoor allergens.9 The house dust mite is one of the most common allergens worldwide, and the major strains are Dermatophagoides pteronyssinus and Dermatophagoides farinae.
SCIT and SLIT show efficacy in treating allergies in children.10 SCIT has been validated for the treatment of asthma and rhinitis, using standardized house dust mite extracts.11–14 However, in the pediatric age group, SCIT has some limitations due to the discomfort of repeated injections and side effects.15,16 Prior meta-analyses in children indicate that SLIT is an effective and safe alternative to SCIT in treating allergic respiratory symptoms.10,17 There is growing evidence that SLIT therapy is associated with a lower incidence of systemic reactions compared with control and that it reduces the durations and dose of inhaled corticosteroids used and improves lung function in children with asthma.5,16,18,19
A number of studies focused on house dust mite SCIT and house dust mite SLIT in treating asthma or rhinitis in general but not for a particular antigen. However, many of these studies were small and used variable doses of antigen. There is good evidence that house dust mite SCIT is efficacious and has long-term benefit in children.3 However, the evidence of the benefit of house dust mite SLIT is less convincing.3 The studies that have evaluated the efficacy and safety of house dust mite SLIT in treating asthma show high clinical and methodological heterogeneity, which make it difficult to determine the benefit of house dust mite SLIT as allergen immunotherapy.3 Many studies were underpowered to be able to make firm conclusions, and the efficacy findings across studies have been variable.3 The objective of this current meta-analysis was to further evaluate that efficacy and safety of dust mite SLIT in children with asthma.
METHODS
Search Strategy
Medical Literature Analysis and Retrieval System Online, ISI Web of Knowledge, and Cochrane Central Register of Controlled Trials databases until February 2014 were searched for randomized controlled trials that investigated the efficacy of SLIT in children with asthma. Search terms included asthma, sublingual, immunotherapy, mite allergen, and house dust mite. Included studies were randomized, controlled, and prospective in design and published in English. The studies had to have evaluated children (<18 years of age) with asthma who were treated with SLIT or control and must have reported clinical efficacy outcome, D pteronyssinus immunoglobulin E (IgE) levels, serum IgG4 levels, and safety. Studies that included only children with rhinitis or in which subjects received SCIT were excluded. Letters, comments, editorials, and case reports were also excluded. The approval by an institutional review board is not required for this study because human subjects were not studied.
Data Extraction
The following data was extracted from the different studies: name of the first author, type of patients studied, treatments, number of patients, duration of treatment, cumulative dose, sex, and mean age. Other information extracted included treatment outcomes (ie, specific D pteronyssinus IgE, serum immunoglobulin G4 [sIgG4], asthma symptom score, medication score) and adverse events. Two independent reviewers extracted the data from the eligible studies, and a third reviewer was consulted to resolve any disagreement(s).
Quality Assessment
The included studies were assessed for risk bias using the “Risk of Bias” assessment tool, Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011, and recommendations for judging risk of bias provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions.20
Statistical Analysis
The primary outcome was mean change in asthma symptom score. Secondary outcomes included mean change in medication score, specific D pteronyssinus IgE levels, and sIgG4 levels. Safety was also assessed. The mean ± standard deviation [SD] for the measurements of asthma symptom score and D pteronyssinus IgE levels were used for the meta-analysis because across the studies the data were reported in multiple ways (ie, mean ± SD or median [range: minimum, maximum]).21 Because the scale or unit across the studies for the efficacy outcomes (asthma symptom score, medication score, specific D pteronyssinus IgE level, and sIgG4) differed, the standardized differences in mean changes with 95% confidence intervals (CIs) were calculated.22 The values for the different outcomes when changed to the same unit (international units per milliliter) were diverse, making it not meaningful to change the standardized difference in means to the clinical values. The odds ratio (OR) with 95% CI between SLIT and control groups was calculated for the occurrence of adverse event among children treated with SLIT compared with the control group. Heterogeneity among the studies was assessed by calculating Cochran Q and the I2 statistic. For the Q statistic, P < 0.10 indicated statistically significant heterogeneity. I2 statistics indicate the percentage of the observed between-study variability caused by heterogeneity. Heterogeneity was determined using I2 statistics and was defined as follows: 0% to 24% = no heterogeneity, 25% to 49% = moderate heterogeneity, 50% to 74% = large heterogeneity, and 75% to 100% = extreme heterogeneity. The random effects model (DerSimonian–Laird method)23 was adopted for the current study because it assumes that different studies may have different underlying effects, and it also takes into consideration both within and between-study variation. Combined standardized differences in mean change or ORs were calculated, and a 2-sided P value < 0.05 was considered to indicate statistical significance.
Sensitivity analysis was performed for efficacy outcomes based on the leave-one-out approach. When at least 5 studies had sufficient data for the outcome, funnel plot analysis with 1-sided Egger tests were performed to evaluate the publication bias for the meta-analyses.24 All statistical analyses were performed using the Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
RESULTS
The database search identified 91 potential studies of which 57 were eliminated as not being relevant (Figure 1). The text of the remaining 34 studies were assessed in detail, and 23 were excluded due to the study being a duplication of an included study (n = 3), the full text or study design was not available (n = 5), the outcomes of interest were not reported (n = 2), the subjects evaluated were adults (n = 3), the patients were children without asthma (n = 4), SLIT was not compared with control (n = 3), and SLIT was not specified as the treatment (n = 3). The 11 remaining studies were included in the review.25–35
FIGURE 1.
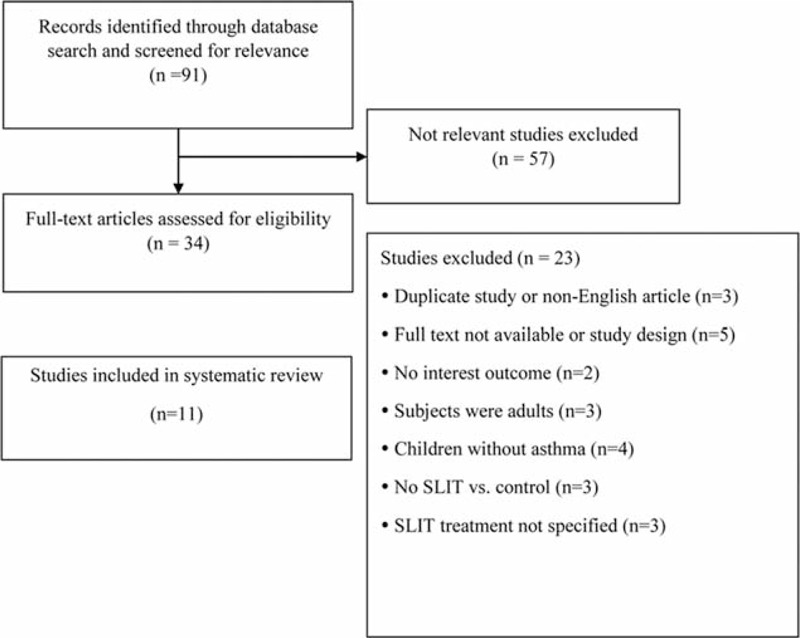
Flow diagram of study selection.
Study Characteristics
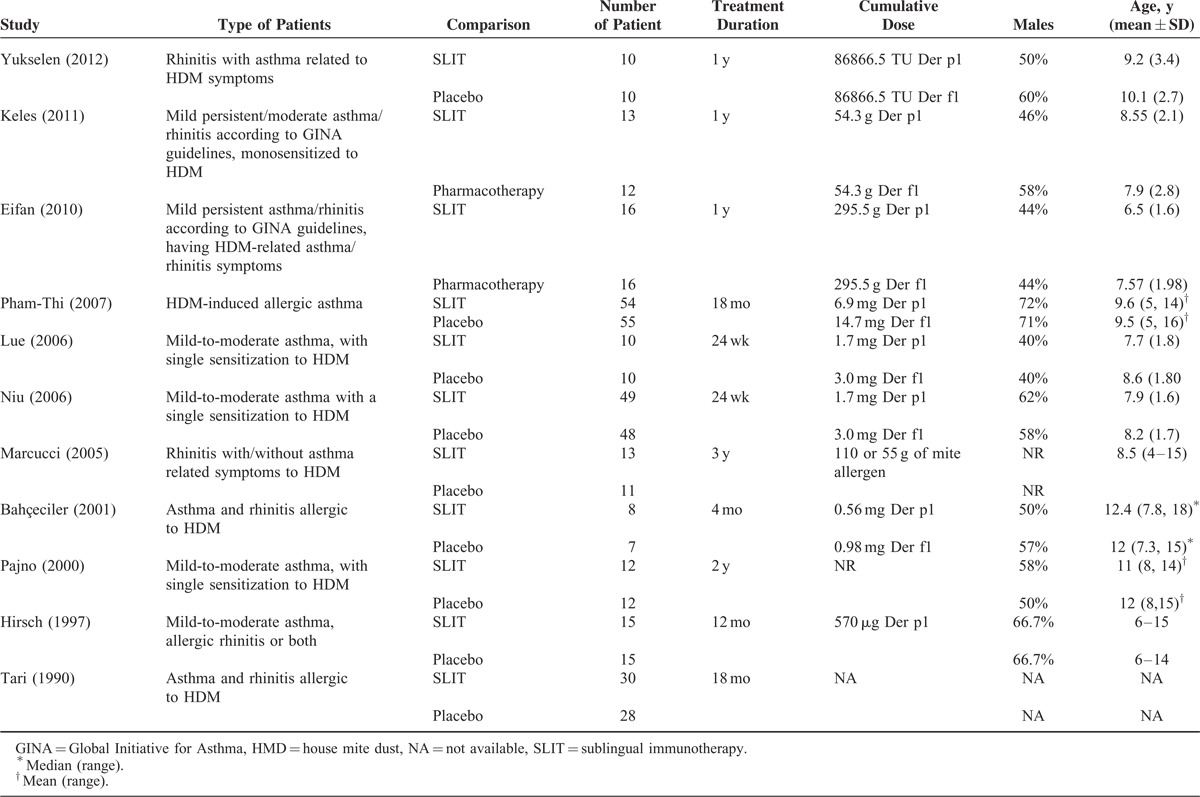
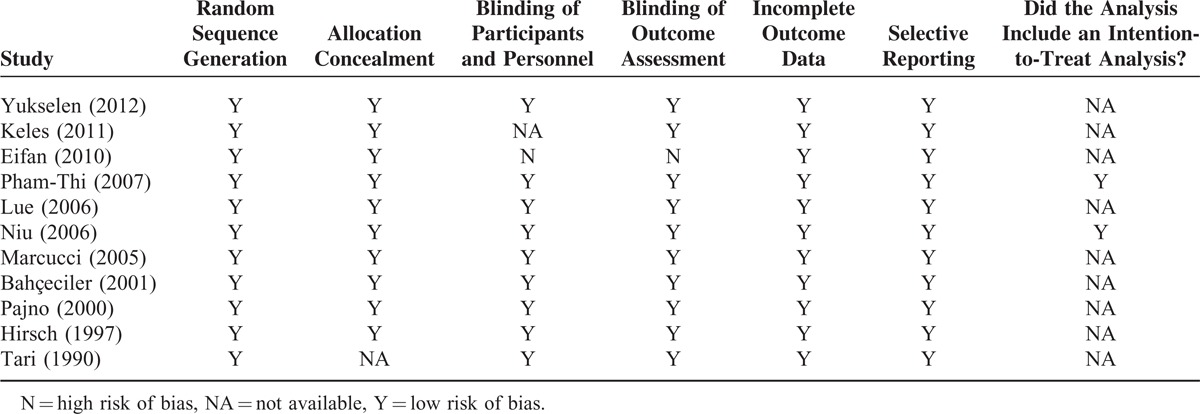
The 11 studies include a total of 454 children with asthma/rhinitis who were sensitized to house dust mites and were treated with SLIT or control for between 4 months to 3 years (Table 1). The total number of patients in each of the studies ranged from 15 to 109 patients. The proportion of patients who were boys ranged from 40% to 71%, and the mean age ranged from 6.5 to 15 years. In most of the studies, some additional therapies were allowed including a minimum dose of inhaled corticosteroids,26 rescue medication,25,28,29,32,35 and oral antihistamines.34 In the studies by Marcucci et al30 and Bahceciler et al,31 SLIT patients were also on pharmacotherapy. Across the entire population, 230 patients underwent SLIT treatment, and 224 were treated with placebo/pharmacotherapy.
TABLE 1.
Summary of Basic Characteristics of Included Studies
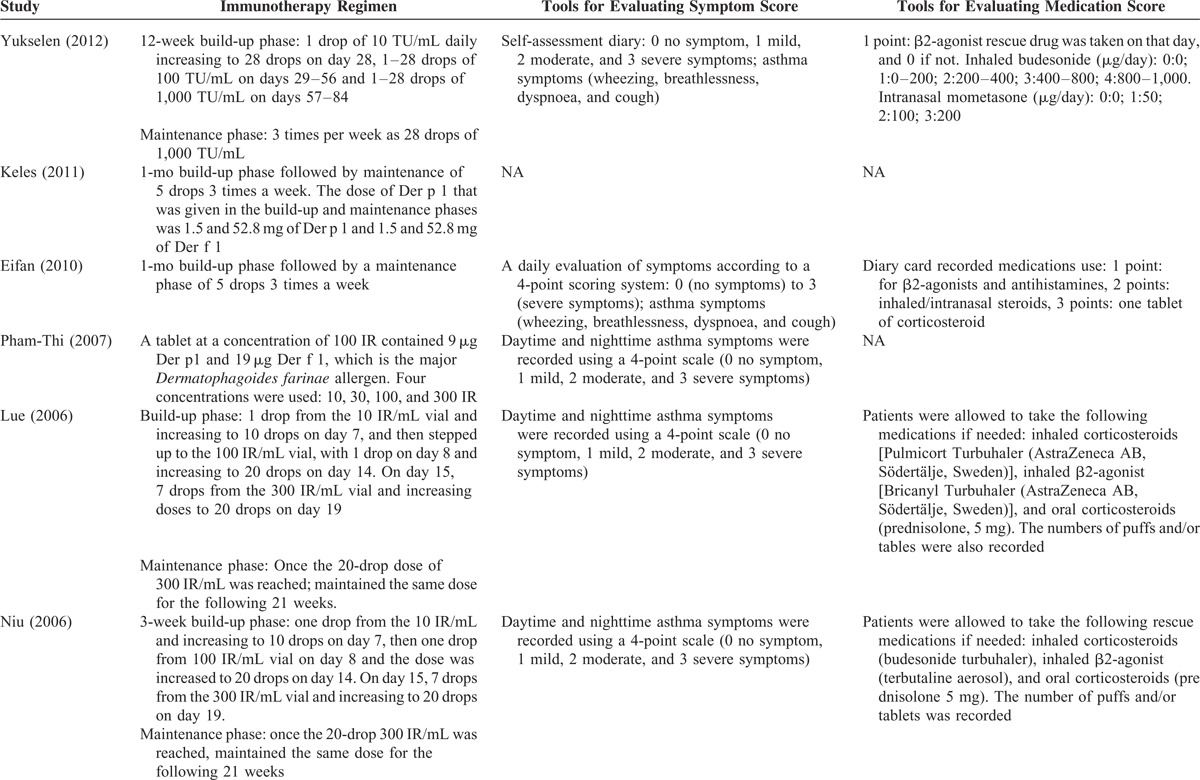
The types of asthma regimens and symptom and medications scores varied across studies (Table 2 ). The regimens differed in length of the build-up phase and the dose of Index of Reactivity (IR) during the build-up and maintenance phase. Most of the studies assessed asthma symptoms using a 4-point scale that in general was 0 for no symptoms to 3 for severe asthma symptoms. The symptoms were either reported as daily scores or yearly scores. Medication scores were reported using diaries, number of rescue medication puffs or tablets used, or use of a specific rescue medication.
TABLE 2.
Summary of Treatment and Evaluation Tools of Included Studies
Asthma symptom score and medication score decreased from baseline in the SLIT treatment group in the studies that reported these outcomes. The changes of specific D pteronyssinus IgE varied, and sIgG4 levels increased from baseline to post-SLIT treatment across all of the 11 studies (Table 3 ). Overall, the frequency of adverse events was low (<15%) in most of the studies. Of the studies, 3 reported higher frequencies of adverse event. The study by Pham-Thi et al27 reported a frequency of approximately 72% and 67% for SLIT and control groups, respectively. The studies by Pajno et al32 and Tari et al34 reported a frequency of approximately 50% in the SLIT group.
TABLE 2(Continued).
Summary of Treatment and Evaluation Tools of Included Studies
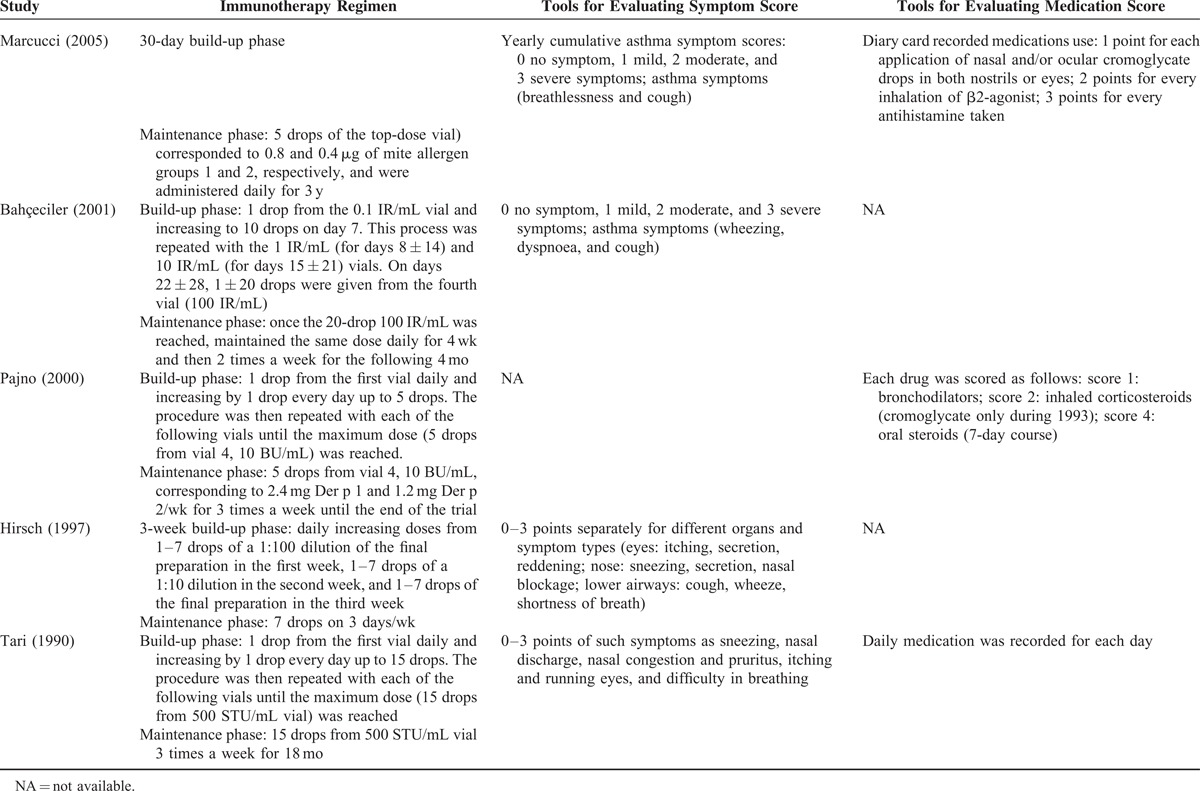
TABLE 3 (Continued).
Summary of Primary and Secondary Outcomes and Adverse Event
Four of the included studies also evaluated visual analogue score (VAS) for asthma symptoms.25,26,32,35 Three of the studies26,32,35 saw an improvement in VAS treatment group compared with control, whereas one study found no difference between the groups.25 The markers used across studies to assess inflammation were diverse making this information difficult to compare.
Asthma Symptom Score
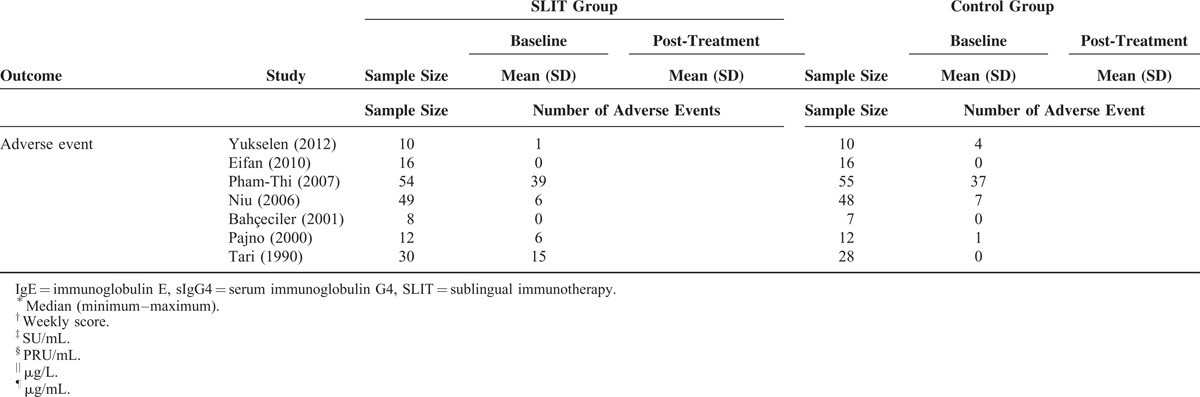
Eight of the included studies provided sufficient information regarding asthma symptom score before and after the treatment for analysis.27–31,33–35 Evaluation of the pooled data indicated there was extreme heterogeneity among the studies (Q = 91.19, df = 7, P < 0.001; I2 = 92.32%). The findings of the meta-analysis indicated that the asthma symptom score significantly decreased more among children treated with SLIT compared with those treated with control (standardized differences in mean change = −1.202, 95% CI −2.071 to −0.333, P = 0.007, Figure 2).
FIGURE 2.
Meta-analyses for the comparisons of asthma symptom score between 2 treatment groups. CI = confidence interval.
Medication Score
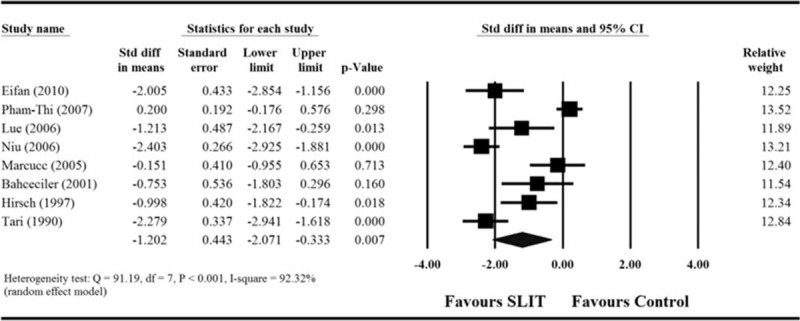
Among the 11 included studies, only 3 provided sufficient information regarding medication score before and after treatment.28,30,35 For these 3 studies, extreme heterogeneity among the studies was found after pooling of data (Q = 13.16, df = 2, P = 0.001; I2 = 84.8%). The results indicated that there was no difference in children treated with SLIT compared with those treated with control treatment (standardized differences in mean change = −0.52, 95% CI −1.753 to 0.713, P = 0.408, Figure 3A).
FIGURE 3.
Meta-analyses for the comparisons of (A) medication score, (B) specific Dermatophagoides pteronyssinus IgE levels, and (C) sIgG4 levels between 2 treatment groups. CI = confidence interval, IgE = immunoglobulin E, sIgG4 = serum immunoglobulin G4.
Specific D pteronyssinus IgE Levels
Of the 11 included studies, 7 provided sufficient information regarding specific D pteronyssinus IgE levels before and after treatment.26–29,32,34,35 Analysis of the pooled data from the 7 studies indicated there was an extreme degree of heterogeneity (Q = 26.32, df = 6, P < = 0.001; I2 = 77.21%). The results found that there was no difference in the mean change in specific D pteronyssinus IgE levels between children treated with SLIT and those treated with control treatment (standardized differences in mean change = 0.430, 95% CI −0.045 to 0.905, P = 0.076, Figure 3B).
sIgG4 Levels
Five of the 11 studies provided sufficient information regarding sIgG4 before and after treatment.25–28,34 Analysis of the pooled data showed there was extreme heterogeneity among the studies (Q = 45.53, df = 4, P < 0.001; I2 = 91.22%). The results indicate that the mean change in sIgG4 level was significantly greater among children treated with SLIT than those treated with control treatment (standardized differences in mean change = 1.456, 95% CI 0.334–2.578, P = 0.011, Figure 3C).
Safety
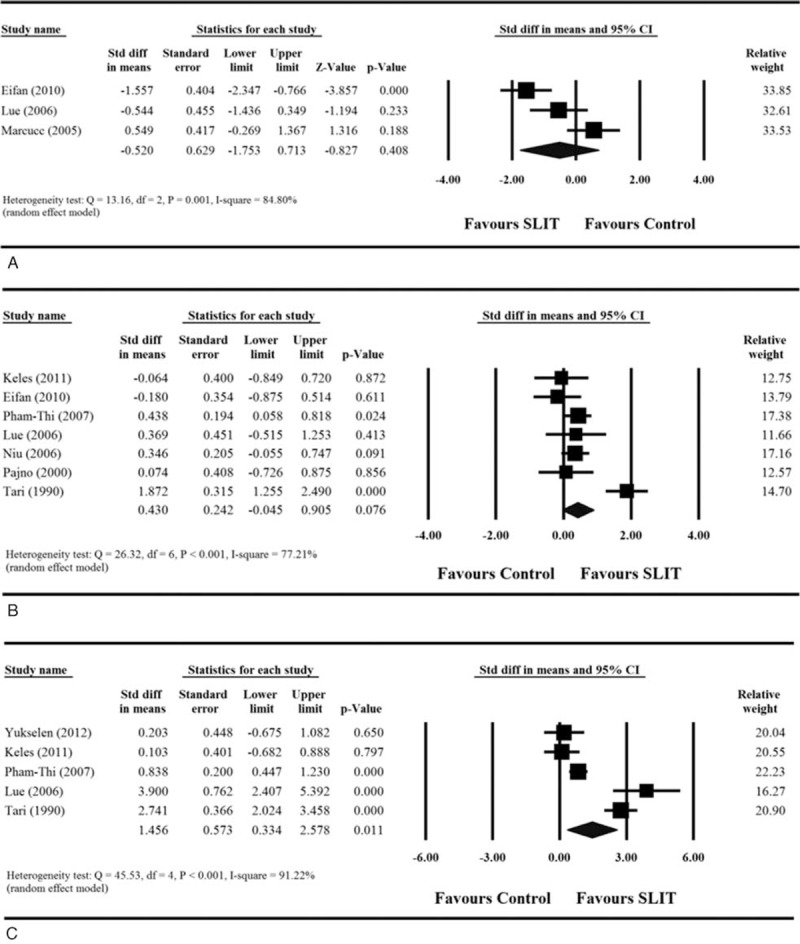
Seven of the 11 studies reported overall adverse event for both treatment and control groups.25,27,29,32,34 However, 2 studies of them were excluded in the meta-analysis because they reported that no adverse events occurred. Analysis of the pooled data from these studies found the presence of moderate heterogeneity (Q = 13.16, df = 4, P = 0.011; I2 = 69.60%). The results indicated that the occurrence of adverse events was not significantly different between children treated with SLIT and those treated with control treatment (combined OR 1.916, 95% CI 0.500–7.347, P = 0.343, Figure 4).
FIGURE 4.
Meta-analyses for comparison of the safety outcome (adverse event) between 2 treatment groups. CI = confidence interval.
Sensitivity Analysis and Quality Assessment
We performed sensitivity analysis where the results were analyzed when 1 study was removed in turn for asthma symptom score, medication score, D pteronyssinus IgE, sIgG4, and adverse events. The direction and magnitude of pooled estimates did not vary considerably for asthma symptom score (Figure 5 A) and adverse event (Figure 5 E), indicating that the meta-analysis had good reliability for these outcomes. However, the removal of some studies caused the pooled difference in means to become significant. Removal of Marcucci et al altered the medication score analysis (pooled standardized difference in means = −1.072, 95% CI −2.064 to −0.080, P = 0.034; Figure 5 B); removal of either Eifan et al35 or Tari et al34 affected the results with regard to specific D pteronyssinus IgE levels; (pooled standardized difference in means = −1.072, 95% CI −2.064 to −0.080, P = 0.034 and 0.269, 95% CI 0.044–0.494, P = 0.019, respectively; Figure 5 C); and removal of Pham-Thi et al27 or Lue et al28 changed the findings for sIgG4 levels (pooled standardized difference in means = 1.673, 95% CI −0.011 to 3.357, P = 0.051, and 0.983, 95% CI −0.090 to 2.056, P = 0.072, respectively; Figure 5 D). These results suggest that the meta-analysis had poor reliability in the findings for medication score, specific D pteronyssinus IgE level, and sIgG4 level.
FIGURE 5.
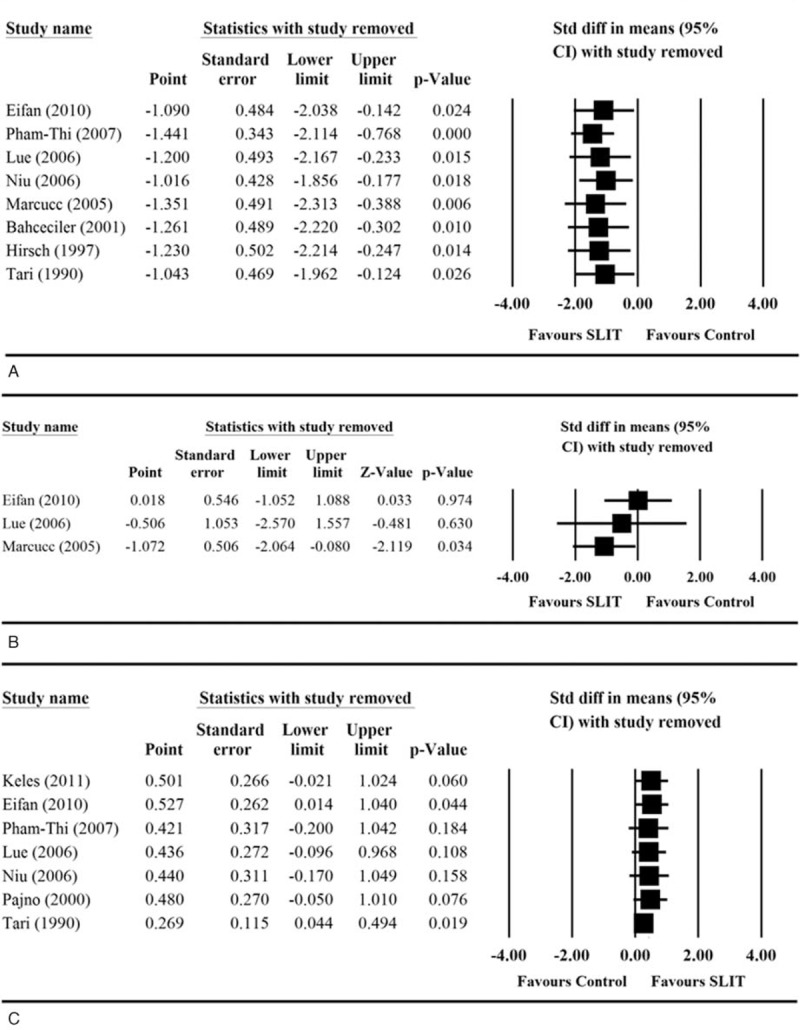
Sensitivity analyses of the comparisons of (A) asthma symptom score, (B) medication score, (C) specific Dermatophagoides pteronyssinus IgE levels, (D) sIgG4 levels, and (E) adverse event between two treatment groups. CI = confidence interval, IgE = immunoglobulin E, sIgG4 = serum immunoglobulin G4.
We evaluated the risk of bias of the included studies using the “Risk of Bias” assessment tool of Review Manager 5.1. Overall, there was low risk of bias across the studies (Table 4); all the studies were positive for all criteria except for that of Eifan et al35 in which participants or personnel were not blinded, and the assessment of outcomes was also not blinded.
TABLE 3.
Summary of Primary and Secondary Outcomes and Adverse Event
TABLE 4.
Quality Assessment of Included Studies
Publication Bias
For asthma symptom score, the Egger test for the intercept in the funnel plot showed no significant publication bias among the 8 studies (intercept = −3.66 with 1-tailed P value = 0.184, Figure 6A). For specific D pteronyssinus IgE levels, the Egger test for the intercept in the funnel plot also showed no significant publication bias among the 7 studies (intercept = −0.550 with 1-tailed P value = 0.423, Figure 6B).
FIGURE 5 (Continued).
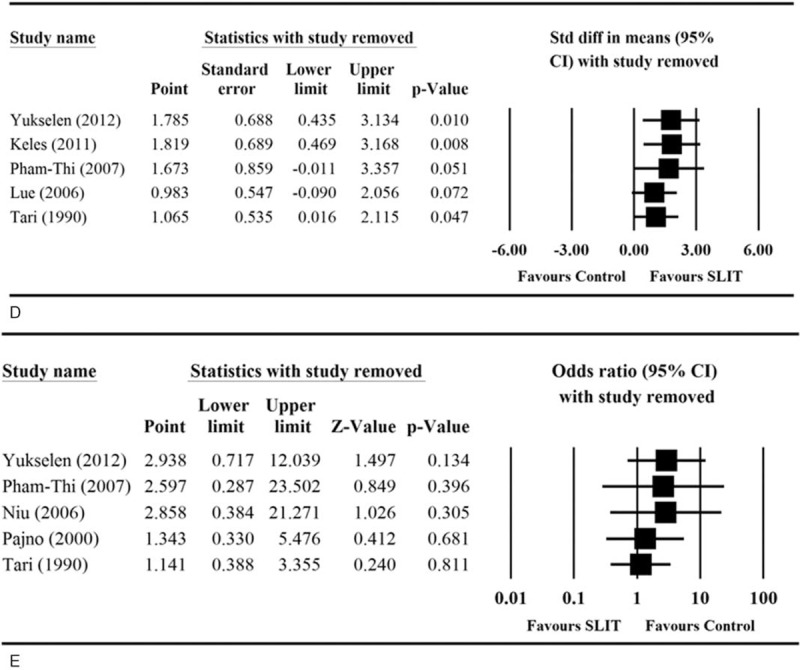
Sensitivity analyses of the comparisons of (A) asthma symptom score, (B) medication score, (C) specific Dermatophagoides pteronyssinus IgE levels, (D) sIgG4 levels, and (E) adverse event between two treatment groups. CI = confidence interval, IgE = immunoglobulin E, sIgG4 = serum immunoglobulin G4.
FIGURE 6.
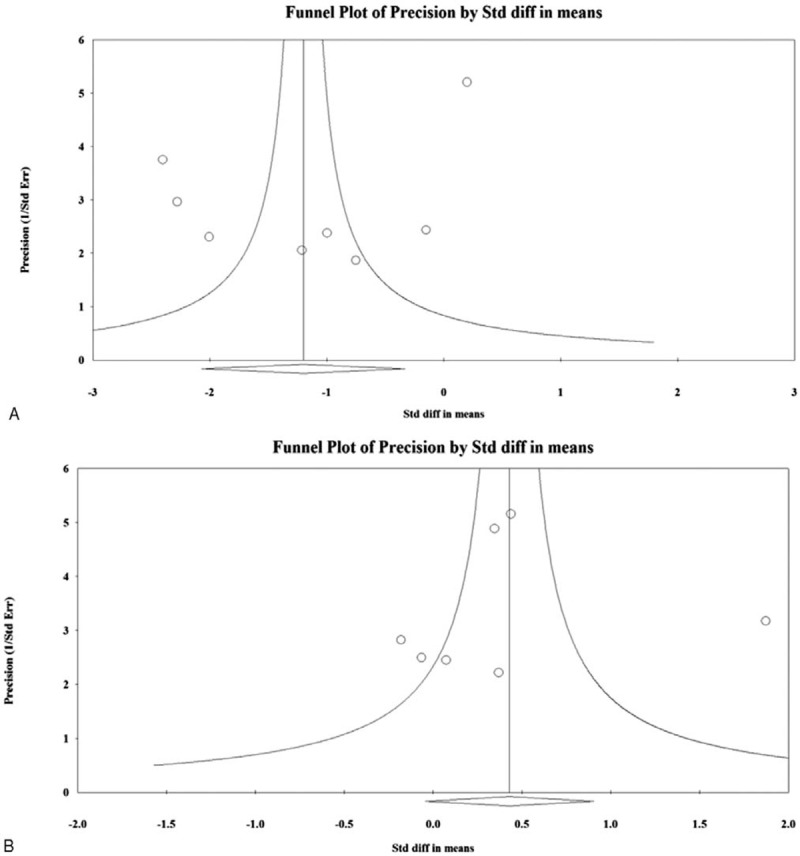
Funnel plots of evaluation of publication bias. (A) asthma symptom score and (B) specific Dermatophagoides pteronyssinus IgE levels. IgE = immunoglobulin E.
DISCUSSION
We evaluated the efficacy and safety of dust mite SLIT in asthmatic children. We found that the reduction in asthma symptom score and the increase in sIgG4 levels were significantly greater in children treated with dust mite SLIT than in children treated with control. Dust mite SLIT did not significantly decrease medication score or specific D pteronyssinus IgE compared with control. Dust mite SLIT was well tolerated by children, and in most studies the frequency of adverse events also did not differ between dust mite SLIT and control. Sensitivity analysis indicated that generally the finding for the primary analysis of asthma symptom score was not dependent on any 1 study, and there was no publication bias.
The prevalence of asthma and allergic diseases has increased in different parts of the world, including China. Allergen sensitization is closely related to the development of asthma, and house dust mites are the most common allergens worldwide and are the most prevalent allergen in Chinese children with asthma and/or rhinitis.3,36,37 It is thought that sensitization to house dust mites plays an important role in the development of asthma or allergic rhinitis.37 In support of this idea, sensitization to house dust mites is one of the key risk factors associated with increase in wheeze in secondary school children in Guangzhou, China.36
Several prior meta-analyses have assessed the use of SLIT in treating children with asthma or allergic rhinitis.10,38–41 Penagos et al evaluated the efficacy of SLIT in children with asthma (3–18 years of age) using randomized double-blind, placebo-controlled studies.38 Their meta-analysis included 9 studies that comprised 441 children; 232 of whom received SLIT and 209 of whom received placebo. They evaluated symptom score and use of rescue medicine. Although, the included studies used a wide range of scoring systems, they found, similar to our results, SLIT with standardized extracts was associated with an overall reduction in symptom score (P = 0.02) and use of rescue medication (P = 0.007).
Olaguibel and Alvarez Puebla10 performed a meta-analysis that assessed the efficacy of SLIT in children ≤14 years of age with either allergic rhinitis or asthma in randomized, double blind, and placebo-controlled trials. Seven studies were evaluated that included 256 children (129 treated with SLIT and 127 treated with placebo). They saw a decrease in symptoms scores for allergic rhinitis and asthma, but this did not reach statistical significance. There was a significant decrease in asthma allergy symptoms (P = 0.01) and medication scores (P = 0.026). No severe nor systemic reactions or oral and gastrointestinal complaints were seen.
The above-mentioned meta-analyses did not separately evaluate the effect of pollen or dust mite allergen. Only 1 prior meta-analysis assessed the efficacy of SLIT using dust mite extract compared with placebo.40 Compalati et al identified 12 randomized, placebo-controlled studies that assessed dust mite SLIT in patients with allergic rhinitis or asthma (382 patients with allergic rhinitis and 476 with allergic asthma).39 They found significant benefit in using dust mite SLIT compared with placebo for nasal symptom scores, bronchial symptom scores of allergic asthma, and decrease in rescue drug use for allergic rhinitis and asthma. Subgroup analysis also found a significant reduction in symptom scores for asthma and medication use in children with asthma.
In contrast to the previous meta-analyses, we did not find a significant decrease in medication score. Only the study by Olaguibel and Alvarez Puebla10 evaluated medication score, and they did find a significant benefit to SLIT for this outcome. The lack of significance in our analysis may reflect the fact that the methods for evaluating medication score differed across the included studies possibly confounding the findings. In contrast to our analysis, Olaguibel and Alvarez Puebla used allergic rhinitis as part of the search term and likely had a higher proportion of patients with allergic rhinitis compared with our study, which could have influenced the findings. In addition, both the study by Olaguibel et al10 and our study included only a small number of studies, which may have influenced the results.
Our study did not evaluate the efficacy of SCIT for asthma. Two prior systematic reviews evaluated the efficacy of SCIT in patients with rhinitis or asthma. Erekosima et al40 identified 61 studies that evaluated SCIT in children or adults, which included 3577 subjects. The included studies compared SCIT with placebo, pharmacotherapy, or SLIT. The majority of the studies (66%) assessed a single-allergen immunotherapy regimen. They found high-grade evidence that SCIT reduces asthma symptoms, asthma medication use, allergic rhinitis symptoms, and rhinitis disease-specific quality of life compared with placebo or usual care. They found that respiratory reactions were the most common systemic adverse event.
The systematic review of Chelladurai et al (2013)41 used only studies that performed head-to-head comparisons between SCIT and SLIT. They included 8 studies with 555 subjects. They found low-grade evidence that SCIT is more efficacious than SLIT for asthma symptom reduction, and in reducing symptoms and medication use of rhinitis symptoms. Moderate-grade evidence supported SCIT as being more effective than SLIT in reducing nasal and/or eye symptoms.
The studies included in our meta-analysis were heterogeneous. Some included children with allergic rhinitis, and the studies differed with regard to dose, dose frequency, and duration of treatment. To compensate for this, we used standardized mean difference and random effect model for combining the different type of data. We did not evaluate VAS or inflammatory markers due to insufficient data. We also did not assess change in symptoms of allergic rhinitis. Also, most of the included studies had small study populations with some having <20 people in the active treatment group. There was publication bias for the specific D pteronyssinus IgE levels.
In conclusion, our study indicates that dust mite SLIT therapy was effective in reducing asthma symptom score and increasing sIgG4, but did not significantly reduce medicine scores and specific D pteronyssinus IgE. Our findings are not enough to support the use of dust mite SLIT in children with asthma. However, the data in our meta-analysis, as well as others, suffers from the small number of clinical studies included and the small sample size of these studies. Larger well-designed studies that use similar scoring systems and monitor dust mite SLIT are necessary to further explore this question.
Footnotes
Abbreviations: SCIT = subcutaneous immunotherapy, SLIT = sublingual immunotherapy.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Compalati E, Penagos M, Tarantini F, et al. Specific immunotherapy for respiratory allergy: state of the art according to current meta-analyses. Ann Allergy Asthma Immunol 2009; 102:22–28. [DOI] [PubMed] [Google Scholar]
- 2.Passalacqua G. Specific immunotherapy in asthma: a comprehensive review. J Asthma 2014; 51:29–33. [DOI] [PubMed] [Google Scholar]
- 3.Eifan AO, Calderon MA, Durham SR. Allergen immunotherapy for house dust mite: clinical efficacy and immunological mechanisms in allergic rhinitis and asthma. Expert Opin Biol Ther 2013; 13:1543–1556. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998; 102 (4 pt 1):558–562. [DOI] [PubMed] [Google Scholar]
- 5.Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol 2012; 130:1097–1107. [DOI] [PubMed] [Google Scholar]
- 6.Vitaliti G, Pavone P, Guglielmo F, et al. Sublingual immunotherapy in preschool children: an update. Expert Rev Clin Immunol 2013; 9:385–390. [DOI] [PubMed] [Google Scholar]
- 7.Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J 2014; 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao D, Phipatanakul W. Impact of environmental controls on childhood asthma. Curr Allergy Asthma Rep 2011; 11:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright LS, Phipatanakul W. Environmental remediation in the treatment of allergy and asthma: latest updates. Curr Allergy Asthma Rep 2014; 14:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olaguibel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol 2005; 15:9–16. [PubMed] [Google Scholar]
- 11.Ameal A, Vega-Chicote JM, Fernandez S, et al. Double-blind and placebo-controlled study to assess efficacy and safety of a modified allergen extract of Dermatophagoides pteronyssinus in allergic asthma. Allergy 2005; 60:1178–1183. [DOI] [PubMed] [Google Scholar]
- 12.Varney VA, Tabbah K, Mavroleon G, et al. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy 2003; 33:1076–1082. [DOI] [PubMed] [Google Scholar]
- 13.Pichler CE, Marquardsen A, Sparholt S, et al. Specific immunotherapy with Dermatophagoides pteronyssinus and D. farinae results in decreased bronchial hyperreactivity. Allergy 1997; 52:274–283. [DOI] [PubMed] [Google Scholar]
- 14.Pichler CE, Helbling A, Pichler WJ. Three years of specific immunotherapy with house-dust-mite extracts in patients with rhinitis and asthma: significant improvement of allergen-specific parameters and of nonspecific bronchial hyperreactivity. Allergy 2001; 56:301–306. [DOI] [PubMed] [Google Scholar]
- 15.Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol 2006; 117:169–175. [DOI] [PubMed] [Google Scholar]
- 16.Ragusa VF, Massolo A. Non-fatal systemic reactions to subcutaneous immunotherapy: a 20-year experience comparison of two 10-year periods. Eur Ann Allergy Clin Immunol 2004; 36:52–55. [PubMed] [Google Scholar]
- 17.Penagos M, Compalati E, Tarantini F, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol 2006; 97:141–148. [DOI] [PubMed] [Google Scholar]
- 18.Di Rienzo V, Pagani A, Parmiani S, et al. Post-marketing surveillance study on the safety of sublingual immunotherapy in pediatric patients. Allergy 1999; 54:1110–1113. [DOI] [PubMed] [Google Scholar]
- 19.Larenas-Linnemann D, Blaiss M, Van Bever HP, et al. Pediatric sublingual immunotherapy efficacy: evidence analysis, 2009–2012. Ann Allergy Asthma Immunol 2013; 110:402–415. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. In: Higgens JPT, Green LA, eds: The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John Wiley & Sons, Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. 2011; 26. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yukselen A, Kendirli SG, Yilmaz M, et al. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol 2012; 157:288–298. [DOI] [PubMed] [Google Scholar]
- 26.Keles S, Karakoc-Aydiner E, Ozen A, et al. A novel approach in allergen-specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol 2011; 128:808–815. [DOI] [PubMed] [Google Scholar]
- 27.Pham-Thi N, Scheinmann P, Fadel R, et al. Assessment of sublingual immunotherapy efficacy in children with house dust mite-induced allergic asthma optimally controlled by pharmacologic treatment and mite-avoidance measures. Pediatr Allergy Immunol 2007; 18:47–57. [DOI] [PubMed] [Google Scholar]
- 28.Lue KH, Lin YH, Sun HL, et al. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, randomized, placebo-controlled study. Pediatr Allergy Immunol 2006; 17:408–415. [DOI] [PubMed] [Google Scholar]
- 29.Niu CK, Chen WY, Huang JL, et al. Efficacy of sublingual immunotherapy with high-dose mite extracts in asthma: a multi-center, double-blind, randomized, and placebo-controlled study in Taiwan. Respir Med 2006; 100:1374–1383. [DOI] [PubMed] [Google Scholar]
- 30.Marcucci F, Sensi L, Di Cara G, et al. Three-year follow-up of clinical and inflammation parameters in children monosensitized to mites undergoing sub-lingual immunotherapy. Pediatr Allergy Immunol 2005; 16:519–526. [DOI] [PubMed] [Google Scholar]
- 31.Bahceciler NN, Isik U, Barlan IB, et al. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double-blind, placebo-controlled study. Pediatr Pulmonol 2001; 32:49–55. [DOI] [PubMed] [Google Scholar]
- 32.Pajno GB, Morabito L, Barberio G, et al. Clinical and immunologic effects of long-term sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, placebo-controlled study. Allergy 2000; 55:842–849. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch T, Sahn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol 1997; 8:21–27. [DOI] [PubMed] [Google Scholar]
- 34.Tari MG, Mancino M, Monti G. Efficacy of sublingual immunotherapy in patients with rhinitis and asthma due to house dust mite. A double-blind study. Allergol Immunopathol (Madr) 1990; 18:277–284. [PubMed] [Google Scholar]
- 35.Eifan AO, Akkoc T, Yildiz A, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy 2010; 40:922–932. [DOI] [PubMed] [Google Scholar]
- 36.Li J1, Wang H, Chen Y, et al. House dust mite sensitization is the main risk factor for the increase in prevalence of wheeze in 13- to 14-year-old schoolchildren in Guangzhou city, China. Clin Exp Allergy 2013; 43:1171–1179. [DOI] [PubMed] [Google Scholar]
- 37.Arshad SH, Tariq SM, Matthews S, et al. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics 2001; 108:e33. [DOI] [PubMed] [Google Scholar]
- 38.Penagos M, Passalacqua G, Compalati E, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest 2008; 133:599–609. [DOI] [PubMed] [Google Scholar]
- 39.Compalati E, Passalacqua G, Bonini M, et al. The efficacy of sublingual immunotherapy for house dust mites respiratory allergy: results of a GA2LEN meta-analysis. Allergy 2009; 64:1570–1579. [DOI] [PubMed] [Google Scholar]
- 40.Erekosima N, Suarez-Cuervo C, Ramanathan M, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope 2014; 124:616–627. [DOI] [PubMed] [Google Scholar]
- 41.Chelladurai Y, Suarez-Cuervo C, Erekosima N, et al. Effectiveness of subcutaneous versus sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. J Allergy Clin Immunol Pract 2013; 1:361–369. [DOI] [PubMed] [Google Scholar]


