Abstract
Previous studies suggested that the incidental use of β-blockers might influence clinical outcome in solid tumors. We assessed the correlation between the incidental use of β-blockers and clinical outcome in colorectal cancer patients treated with first-line chemotherapy alone or in combination with bevacizumab in metastatic colorectal cancer patients.
We collected data from 235 metastatic colorectal cancer patients treated with first-line chemotherapy alone (128 patients) or with bevacizumab (107 patients). Patients were stratified for clinical factors such as β-blockers use, age, sex, and site of metastases, previous adjuvant chemotherapy and ECOG performance status.
In the chemotherapy alone group patients receiving β-blockers showed an improved overall survival (median OS 41.3 vs 25.7 months, P = 0.03, HR: 2.26, 95% CI: 1.05–3.24). A significant relationship with improved response rate was also evident for B-blocker users (P = 0.044).
On the contrary in the β-blockers users group treated with chemotherapy in combination with bevacizumab we observed a trend toward a worse overall survival although nonstatistically significant (median OS 18.5 vs 23.6 months, HR: 0. 89, 95% CI: 0.38–2.03, P = 0.77).
Our analysis confirmed a potential prognostic role for the use of β-blockers in colorectal cancer patients treated with chemotherapy. Our findings also suggest a potential worse outcome for patients on β-blockers receiving bevacizumab. Future prospective studies should include the incidental use of β-blockers as stratification factor for clinical outcome.
INTRODUCTION
Different growth factors have been shown to possess a crucial role in tumor development and progression. Recent data suggested that adrenergic activity might also influence tumor-related biological mechanisms. Both α- and β-adrenergic stimulation besides a well-known positive inotrope and chronotrope effects on cardiac muscle also have a postulated activity as growth factor. Several analyses in different tumor types, including colon cancer, demonstrated that tumor cells might express in fact either β- or α-adrenergic receptors1: On this basis researchers involved hypothesized that adrenergic activity might affect tumor cells survival and replication. In accordance with these findings cancer patients receiving incidental β-blockers treatment have been shown to experience an improved outcome.2–10 These data are lacking for metastatic colorectal cancer patients.2–10
In this latter patients’ population an increasing proportion of patients will experience arterial hypertension during the course of their disease, both as preexisting condition (as in primary hypertension) and as consequence of anticancer treatment (particularly for antiangiogenic treatments). In the present analysis we evaluated the role of the incidental use of an antihypertensive therapy (particularly β-blockers) in affecting clinical outcome for metastatic colorectal cancer patients receiving first-line treatment.
METHODS
Patients’ Characteristics
The present analysis was designed as retrospective cohort study that assumed as exposed patients those who were on incidental β-blockers use as antihypertensive therapy. All consecutive metastatic colorectal cancer patients treated with a first-line regimen including a chemotherapy doublet (capecitabine/5FU + either oxaliplatin or irinotecan) ± bevacizumab at our Institution between 2010 and 2013 were eligible. All patients received chemotherapy until first radiological evidence of disease progression, patients’ refusal, or unacceptable toxicity on an outpatient basis. All relevant patients’ characteristics and follow-up data were collected by patients’ clinical files.
The study was not deemed to be submitted to the local ethical committee due to the fact that all patients, at the time of clinical file creation, gave an informed consent for anonymized clinical data storage and analysis.
Patients were stratified on the basis of antihypertensive treatment (no treatment vs treatment with β-blockers vs treatment with antihypertensive drugs other than β-blockers). Other stratification factors were sex, age, performance status at the beginning of treatment, previous adjuvant chemotherapy, time of metastatic involvement (synchronous vs metachronous), sites of metastatic involvement, k-ras status.
Study Design
Primary aim of the study was to assess the impact of incidental β-blockers use on overall survival. Assuming that risk of death in the first 2 years of therapy was 50% in the cohort of metastatic colorectal cancer patients treated with a first-line regimen and that a clinically relevant relative risk of death for the use of β-blockers was 1.7 times or more, with an α-probability error at 0.05 and with a power of 0.80, at least 50 patients (25 in either group) were needed.
Overall survival was calculated through Kaplan–Meier method and was defined as the time between start of treatment and patients’ death or lost at follow-up, whichever came first.
Progression-free survival was also calculated through Kaplan–Meier method and was defined as the time between the start of treatment and the first radiological sign of progressive disease, patient's death, or lost at follow-up, whichever came first.
Response rates were evaluated on the basis of RECIST criteria (ver 1.1) and their association with categorical variables was assessed through χ2 test.
All statistical calculations were performed using MedCalc Statistical Software version 14.10.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014).
RESULTS
Patients’ Characteristics
Complete clinical data from 235 patients were available. One hundred twenty-eight patients received a first-line chemotherapy doublet whereas the remaining 107 received a chemotherapy doublet + bevacizumab.
In the global population, 142 patients (60%) were not receiving any antihypertensive treatment, 29 patients (12%) were on incidental therapy with β-blockers, and 65 patients (27%) were treated with antihypertensive therapy other than β-blockers.
In the group of patients treated with a chemotherapy doublet, 71 patients (55%) were not receiving any antihypertensive treatment, 20 patients (15%) were on treatment with β-blockers, and 37 patients (28%) were on treatment with other antihypertensive drugs.
In the bevacizumab treated group, 71 patients (65%) were not receiving any antihypertensive treatment, 9 (8%) patients were on treatment with β-blockers, and 28 (25%) were on treatment with other antihypertensive drugs. Patients groups were comparable for all clinical factors analyzed (Table 1).
Table 1.
Characteristics of the 2 Groups of Patients by Main Factors of Stratification
Survival Analysis in the Overall Population
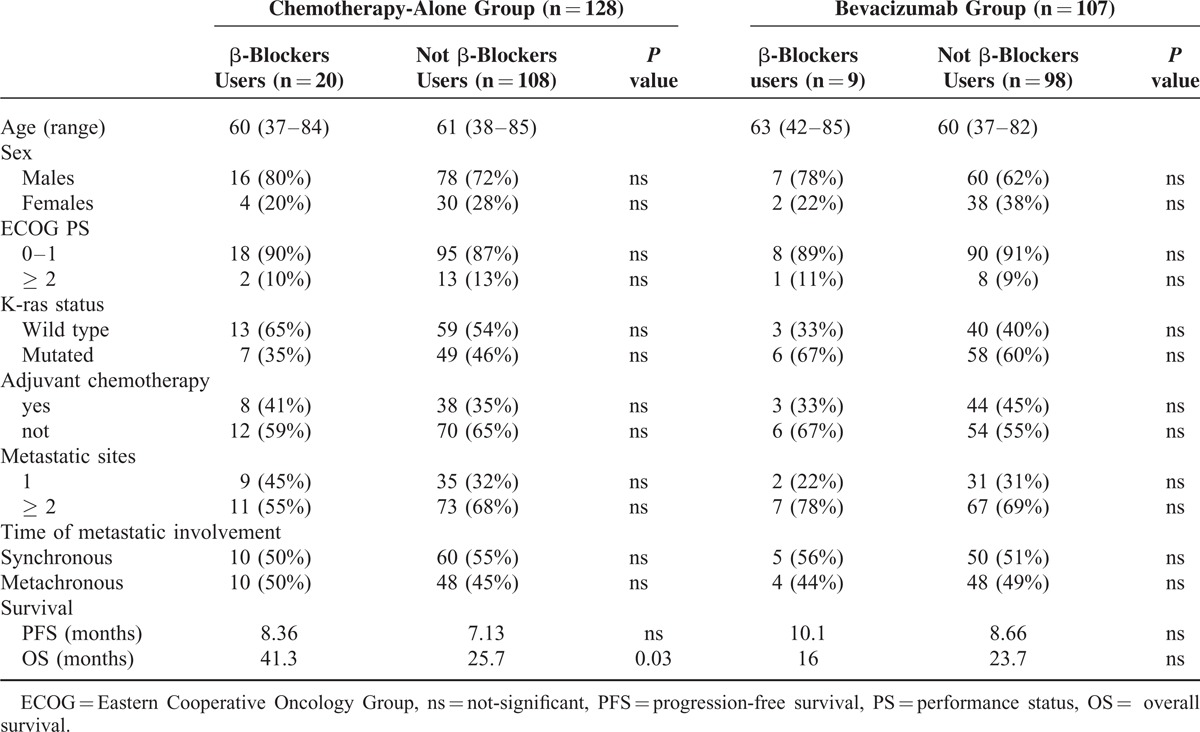
In the overall patients’ population we observed a trend toward an improved overall survival in patients receiving β-blockers, although this was not statistically significant (median OS: 34.4 vs 24.2 months, HR: 1.51, 95% CI: 0.88–2.31, P = 0.14) (Figure 1).
FIGURE 1.
Overall survival in the global population in β-blockers users (----) compared with non-β-blockers users (___) (34.4 vs 24.2 months, HR: 1.51, 95% CI: 0.88–2.31, P = 0.14).
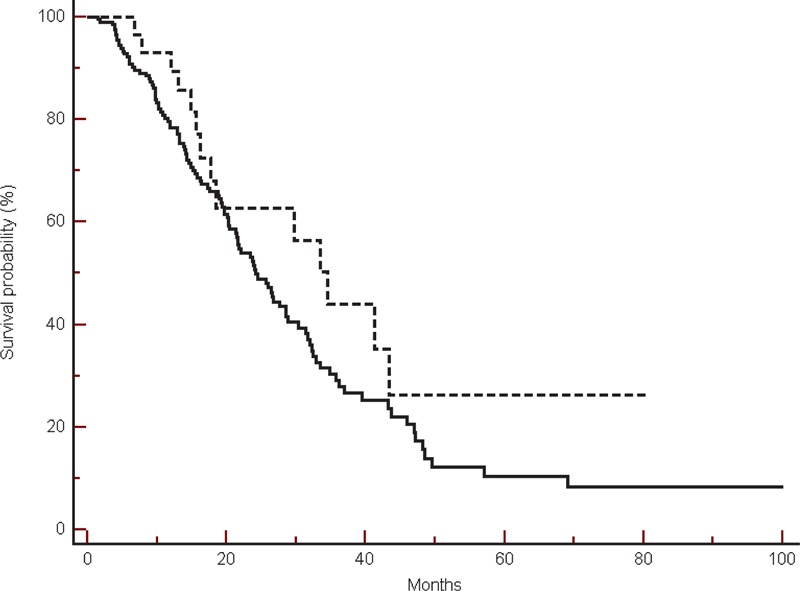
Progression-free survival was also slightly improved in β-blockers users, although once again this was not statistically significant (median PFS: 8.86 vs 7.93 months, HR: 1.19, 95% CI: 0.81–1.72, P = 0.38) (Figure 2).
FIGURE 2.
Progression-free survival in the global population in β-blockers users (----) compared with non-β-blockers users (___) (8.86 vs 7.93 months, HR: 1.19, 95% CI: 0.81–1.72, P = 0.38).
Ninety-seven (41%) patients obtained a complete/partial response. Sixteen responses were observed in the β-blockers users group, whereas 81 responses were seen in the remaining patients’ population. This difference was not statistically significant (P = 0.09).
Survival Analysis According to Treatment
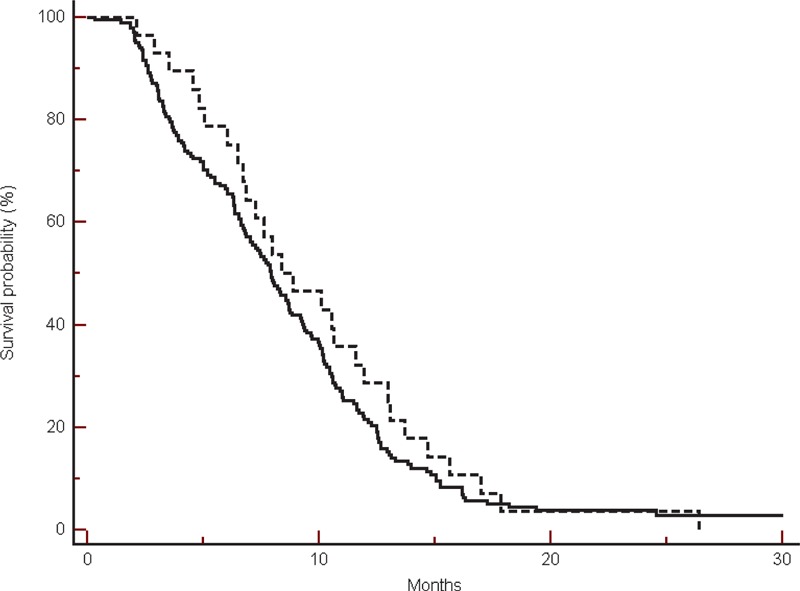
In the group of patients treated with a chemotherapy doublet, among the 20 β-blockers users we observed a significantly improved overall survival (median OS: 41.3 vs 25.7 months, HR: 2.25, 95% CI: 1.04–3.42, P = 0.03) (Figure 3).
FIGURE 3.
Overall survival in the group of patients treated without bevacizumab in β-blockers users (----) compared with non-β-blockers users (___) (41.3 vs 25.7 months, HR: 2.25, 95% CI: 1.04–3.42, P = 0.03).
In the same group of patients, the use of β-blockers was correlated to a nonstatistically significant trend toward an improved progression-free survival (median PFS: 8.36 vs 7.13 months, HR: 1.36, 95% CI: 0.85–2.11, P = 0.20) (Figure 4).
FIGURE 4.
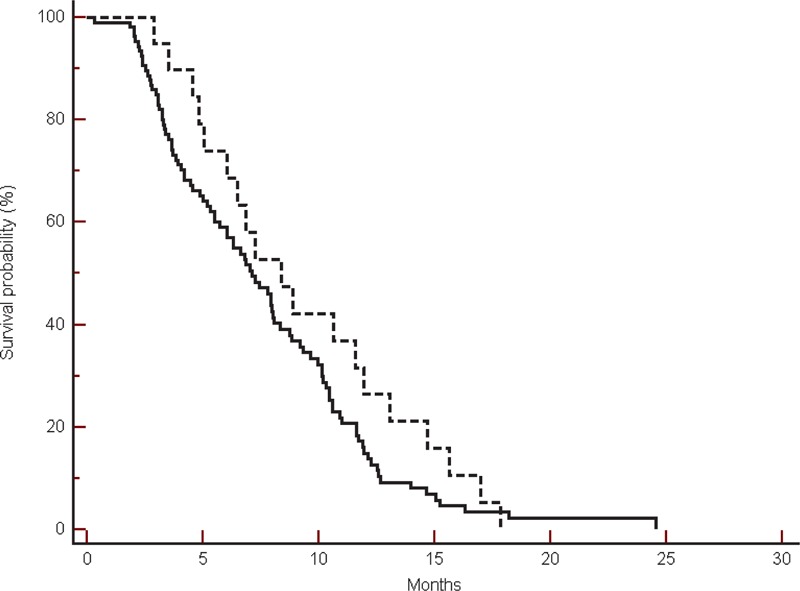
Progression-free survival in the group of patients treated without bevacizumab in β-blockers users (----) compared with non-β-blockers users (___) (8.36 vs 7.13 months, HR: 1.36, 95% CI: 0.85–2.11, P = 0.20).
Among 20 patients treated with β-blockers, we observed 12 (60%) complete/partial responses, whereas in the remaining 108 patients, we observed 36 (33%) responses (P = 0.04).
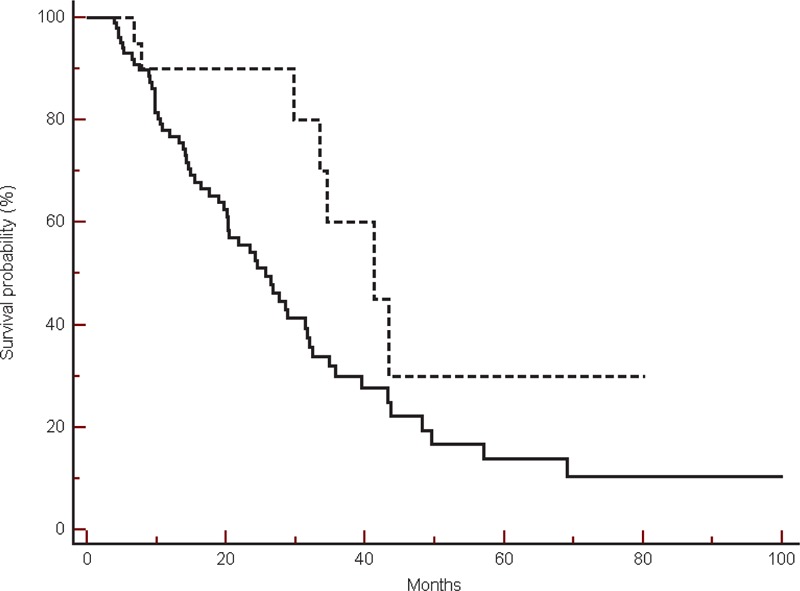
On the other hand in the bevacizumab treated group, among 9 patients on incidental treatment with β-blockers we observed a nonsignificant trend toward a worse overall survival (median OS: 18.5 vs 23.6 months, HR: 0. 89, 95% CI: 0.38–2.03, P = 0.77) (Figure 5).
FIGURE 5.
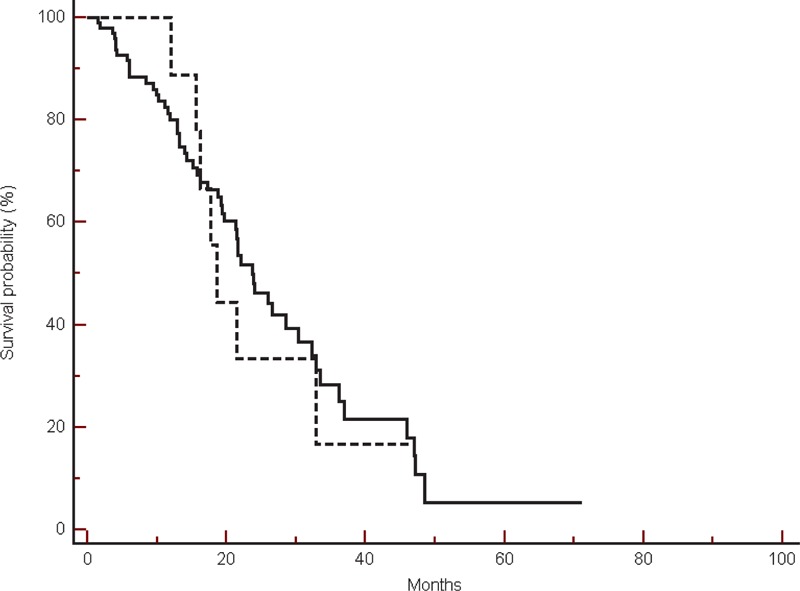
Overall survival in the group of patients treated with bevacizumab, in β-blockers users (----) compared with non-β-blockers users (___) (18.5 vs 23.6 months, HR: 0.89, 95% CI: 0.38–2.03, P = 0.77).
In the same group of patients, the use of β-blockers demonstrated a nonstatistically significant trend toward an improved progression-free survival (median PFS: 10.1 vs 8.66 months, HR: 1.35, 95% CI: 0.68–2.53, P = 0.40) (Figure 6).
FIGURE 6.
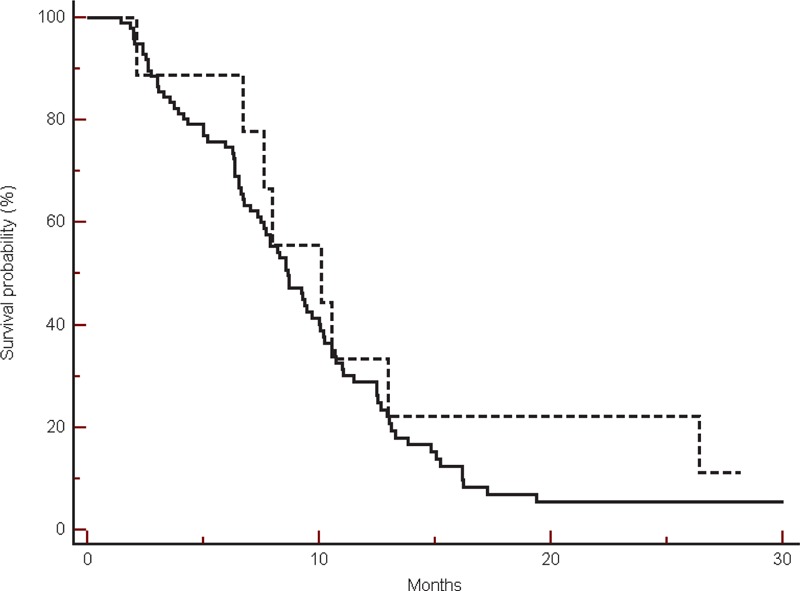
Progression-free survival in the group of patients treated with bevacizumab, in β-blockers users (----) compared with non-β-blockers users (___) (10.1 vs 8.66 months, HR: 1.35, 95% CI: 0.68–2.53, P = 0.40).
Among the 9 patients treated with β-blockers, we observed 6 (66%) complete/partial responses, whereas in the remaining 98 patients, 43 patients (43%) showed an objective response to treatment. This difference was not statistically significant (P = 0.06).
Among 70 patients who were not in treatment for high blood pressure at the start of bevacizumab-based therapy, 38 (54%) experienced hypertension requiring antihypertensive treatment: 22 patients received diuretics (mainly loop diuretics), 12 patients received calcium channel blockers (mainly lercanidipine), the other 4 patients were treated with AT1-receptor antagonists.
DISCUSSION
Besides a well-known effect on cardiac outflow, heartbeat frequency and a general impact on increased blood pressure adrenaline might affect metabolism and trophism of several tissues. Adrenaline also showed a role as a growth factor in many tumor types. Accordingly published data also demonstrated that cancer cells might express adrenergic receptors.
Abel et al11 demonstrated the presence of β2-adrenergic receptors on DiFi colon cancer cell line, thus hypothesizing that adrenergic stimulation may lead to increased proliferation and enhanced tumor aggressiveness.
Wong et al12 confirmed that observation. In particular, in colon cancer cell line HT-29, adrenaline determined an increase in cell proliferation and production of proinflammatory and proangiogenic factors such as PGE2 and VEGF. This activity was mediated through interaction of adrenaline with both β1 and β2-receptors, thus further suggesting a potential role of β-blockers in attenuating tumor aggressiveness.
Other authors suggest a possible role of adrenergic stimulation as chemotherapy resistance factor: In a study of Yao et al,13 in the HT-29 colon cancer cell line, the levels of ABCB1 gene expression (usually implied to have a role in the phenomenon of multidrug resistance) were linked to adrenergic stimulation. Pharmacological α2-adrenergic agonists (such as yohimbine) determined a net reduction in the levels of ABCB1. On the other hand, other β-adrenergic inhibitors such as atenolol or α1-inhibitors did not have significant effects on the expression of the gene.
A further analysis showed a high expression of β-type-receptors in SW-480 colon cancer cell line.14 In this study, exposure to norepinephrine determined a clear increase in tumor cell mobility. The introduction of the nonselective β-blocker propranolol in the growing medium was able to reverse this biological phenomenon. On the contrary, the β1-blocker atenolol only marginally influenced the norepinephrine-induced cancer cell migration.
Other analyses suggested that β-blockers use might negatively influence overall survival. In a study of Shah et al,15 authors observed a slightly decreased survival for β-blockers users in a series of different tumor types. However, no significant impact on survival was observed among the 619 colorectal cancer patients with the use of either β-blockers alone (103 patients) or β-blockers in combination with other antihypertensive drugs (162 patients) or who were on incidental use of other antihypertensive drugs (354 patients) (HR: 1.00, 95% CI: 0.77–1.30).
In another recently published paper, the authors assessed the impact of β-blockers in a large population-based case-control study (DACHS study).16 At the multivariate analysis β-blockers use was not correlated with overall lower risk of developing colorectal cancer (OR: 1.05, 95% CI: 0.86–1.29). On the other hand, a higher risk for stage IV colorectal cancer was observed for patients who were on β-blockers use for more than 6 years (OR: 2.02, 95% CI: 1.25–3.27).
Our study suggests that incidental β-blockers use could represent a favorable prognostic factor in the overall population, whereas in the group of patients who received bevacizumab-based chemotherapy some caution should be advised.
This apparent discrepancy can be related to the complex management of hypertension in bevacizumab-treated patients. In fact there is no conclusive consensus of opinion regarding the optimal class of antihypertensive drug to be used in this group of patients, who are on the contrary known to require a careful evaluation for the possible implication on the clinical outcome.17
The inhibition of β-adrenergic receptors could also make a higher concentration of adrenaline available for the interaction with α-adrenergic receptors.
This group of receptors is usually linked to smooth muscle contraction, particularly in blood vessels. On the other hand, bevacizumab is supposed to exert part of its role as inhibitor of the vascular endothelial growth factor (VEGF) by increasing nitric oxide concentrations that mediates vasodilatation.18 As a consequence, the net effect of VEGF inhibition is in the end similar to the interaction between adrenergic agonists and their α1-type receptor. An unusual stimulation of α-adrenergic receptors could lead to a reduced delivery of active chemotherapeutic drugs in a setting where, in the absence of VEGF-inhibition, chemotherapy would achieve optimal concentrations in tumor tissue.
Although further definitive data are necessary for definitive conclusions, the present analysis seems to suggest that extra attention should be given to the management of patients on incidental treatment with β-blockers and who are receiving a bevacizumab-based chemotherapy regimen for metastatic colorectal cancer, thus raising the issue of the need of established guidelines regarding optimal hypertension management in this group of patients.
Footnotes
Abbreviations: 5FU = 5fluorouracil, 95% CI = 95% confidence interval, ABCB1 = ATP-binding cassette, sub-family B1 (gene or protein encoded), ECOG = Eastern Cooperative Oncology Group, HR = hazard ratio, OR = odds ratio, OS = overall survival, PFS = progression-free survival, PGE2 = prostaglandin E2, VEGF = vascular endothelial growth factor.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Schuller HM. Beta-adrenergic signaling, a novel target for cancer therapy. Oncotarget 2010; 1:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powe DG, Entschladen F. Targeted therapies: using β-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol 2011; 8:511–512. [DOI] [PubMed] [Google Scholar]
- 3.Barron TI, Connolly RM, Sharp L, et al. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 2011; 29:2635–2644. [DOI] [PubMed] [Google Scholar]
- 4.Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011; 29:2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010; 1:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemeshow S, Sorensen HT, Phillips G, et al. β-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011; 20:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 2013; 24:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Wadei HAN, Ullah MF, Al-Wadei MH. Intercepting neoplastic progression in lung malignancies via the beta adrenergic ((-AR) pathway: implications for anticancer drug targets. Pharmacol Res 2012; 66:33–40. [DOI] [PubMed] [Google Scholar]
- 9.Schuller HM, Porter B, Riechert A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol 2000; 126:624–630. [DOI] [PubMed] [Google Scholar]
- 10.Lutgendorf SK, Sood AK. Antoni MH Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol 2010; 28:4094–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel PW, Zeng W, Wildrick DM, et al. Characterization of beta-adrenergic receptors in DiFi and HT-29 cells. Anticancer Res 1992; 12:1655–1658. [PubMed] [Google Scholar]
- 12.Wong HP, Ho JW, Koo MW, et al. Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci 2011; 88:1108–1112.doi: 10.1016/j.lfs.2011.04.007. Epub 2011 Apr 30. [DOI] [PubMed] [Google Scholar]
- 13.Yao H, Duan Z, Wang M, et al. Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet Cytogenet 2009; 190:81–87.doi: 10.1016/j.cancergencyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Masur K, Niggemann B, Zanker KS, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 2001; 61:2866–2869. [PubMed] [Google Scholar]
- 15.Shah SM, Carey IM, Owen CG, et al. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol 2011; 72:157–161.doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen L, Below J, Chang-Claude J, et al. Beta blocker use and colorectal cancer risk: population-based case-control study. Cancer 2012; 118:3911–3919.doi: 10.1002/cncr.26727. Epub 2012 May 14. [DOI] [PubMed] [Google Scholar]
- 17.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 2009; 20:227–230. [DOI] [PubMed] [Google Scholar]
- 18.Giampieri R, Scartozzi M, Del Prete M, et al. The “angiogenetic ladder”, step-wise angiogenesis inhibition in metastatic colorectal cancer. Cancer Treat Rev 2014; 40:934–941. [DOI] [PubMed] [Google Scholar]


