Abstract
Previous studies have shown that metformin or statins may decrease hepatocellular carcinoma (HCC) in diabetic patients. Accordingly, this article evaluates whether combination therapy may further reduce HCC.
Newly diagnosed type 2 diabetes mellitus (DM) patients, excluding those with history of malignancy prior to the date of DM diagnosis, were recruited to a DM cohort. DM patients developed HCC as the cancer cohort and the date for HCC diagnosis as index date. Non-cancer cohort was frequency matched with 4:1 according to age, sex, DM-year, and index date as case group from DM cohort.
Patients who were treated with statins showed a 63% decreased risk of HCC (odds ratio [OR] = 0.37; 95% confidence interval [CI] = 0.27–0.49). Patients who consumed simvastatin, atorvastatin, or rosuvastatin significantly decreased risk for HCC (OR = 0.32, 0.31, and 0.22; 95% CI = 0.18–0.58, 0.19–0.52, and 0.08–0.61, respectively). Metformin combinations with simvastatin, atorvastatin, or rosuvastatin may decrease HCC (OR = 0.30, 0.30, and 0.24; 95% CI = 0.15–0.59, 0.16–0.54, and 0.08–0.70, respectively). The comorbidities for HCC were decreased by consuming simvastatin and atorvastatin (OR = 0.31 and 0.29; 95% CI = 0.14–0.67 and 0.15–0.57, respectively). Only combination therapy of metformin and simvastatin may significantly decreased HCC comorbidities (OR = 0.26; 95% CI = 0.11–0.60) in our study.
In Asia, not all metformin combinations with statins may reduce the incidence of HCC and not all of this kind of combination therapy may decrease the HCC comorbidities.
INTRODUCTION
The 4 most critical diseases, as emphasized by the World Health Organization in 2011, are diabetes mellitus (DM), cancer, heart disease, and lung disease. However, DM can increase the comorbidities and mortality rates of all these diseases.
Since 1959, studies have reported an association between DM and certain cancers.1–3 The mortality rate among diabetic patients with cancer is more than 1.28 times that of diabetic patients who lack cancer.4 Previous studies have also shown that DM can increase the risk of hepatocellular carcinoma (HCC) independently of the hepatitis B virus (HBV), hepatitis C virus (HCV), cirrhosis, heavy alcohol consumption, and non-alcoholic fatty liver disease (NAFLD).5,6 More than 90% of patients with HCC in Taiwan test positive for the HBV surface antigen or HCV antibodies, and these carriers generate the severe public health problems as DM. Most guidelines recommend administration of metformin and statins as first-line medications for the treat of DM and dyslipidemia, ensuring primary or secondary prevention. Abundant literature has discussed the additional benefits provided by metformin or statins such as anti-cancer and anti-inflammatory activity. This article examines whether metformin combined with statins may further reduce HCC.
METHODS
For this nested-case–control study, we referenced the Longitudinal Health Insurance Database 2000 (LHID2000), which was established by the Taiwan Bureau of National Health Insurance. The Taiwan National Health Insurances is a single-payer program that covers over 99% of the population in Taiwan. The LHID2000 contains data of 1 million insurants. Beneficiaries of this program were randomly selected from the 2000 registry. This database contains all medical records of each insured people from 1996 to 2010. The insurant identities re-coded before the data samples were released to researchers. This research was approved by the institutional review board of the China Medical Hospital, Taiwan. Details of the LHID2000 database are presented on the National Health Research Institute's website (http://w3.nhri.org.tw/nhird/date_01.html).
Patients newly diagnosed with type 2 DM (International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM): 250.X0 and 250.X2) between 2000 and 2010, excluding those with a history of malignancy (ICD-9-CM: 140–208) before the DM diagnosis date, were recruited to forma DM cohort (Figure 1). DM patients who were diagnosed with HCC (ICD-9-CM: 155) were classified as the case group and the date of HCC diagnosis was defined as the index date. Controls were randomly assigned after being selected among DM patients who had not developed HCC, and matched at a 4:1 frequency according to age (stratum 5 years), sex, the year of DM diagnosis, and index date.
FIGURE 1.

Flowchart of the patient selection process.
The risk factors in this investigation comprised comorbidities and medication use. The comorbidities included HBV (ICD-9CM: 070.2, 0710.3, and V02.61), HCV (ICD-9-CM: 070.41, 070.44, 070.51, 070.54, and V02.62), NAFLD (ICD-9-CM: 571.8), and alcoholic liver damage (ALD; ICD-9-CM: 571.0, 571.1, and 571.3). Because there was a higher relationship between HCC and cirrhosis duration, the duration between the date for cirrhosis diagnosed (ICD-9-CM: 571.2, 571.5, and 571.6) and the index date was as a cofounder. All comorbidities were defined before the index date. Regarding medication use, we evaluated statins and metformin. Metformin use was defined before the index date, and statin use was defined between January 1, 2000 and the index date. We considered the 6 common statins simvastatin, atorvastatin, pravastatin, fluvastatin, lovastatin, and rosuvastatin. Medication users were further divided into 2 groups: “usually” and “occasionally” based on their duration rate of medication use during the study period. A duration rate of <50% was defined as occasionally, and a rate of ≥50% was defined as usually.
Logistic regression was used to assess the odds ratio (OR) and 95% confidence interval (CI) for patients with HCC in statin users compared with non-users. The association between HCC and the duration of each statin exposure was assessed. The relevant variables in Table 1 were included in the adjusted logistic regression analysis. The trend test results were also assessed using adjusted logistic regression. The statistical significance level was set as a 2-tailed P > .05. Furthermore, all analyses were performed using SAS software, Version 9.3 (SAS Institute, Carry, NC).
TABLE 1.
Distribution of Risk Factor and Odds Ratio for HCC
RESULTS
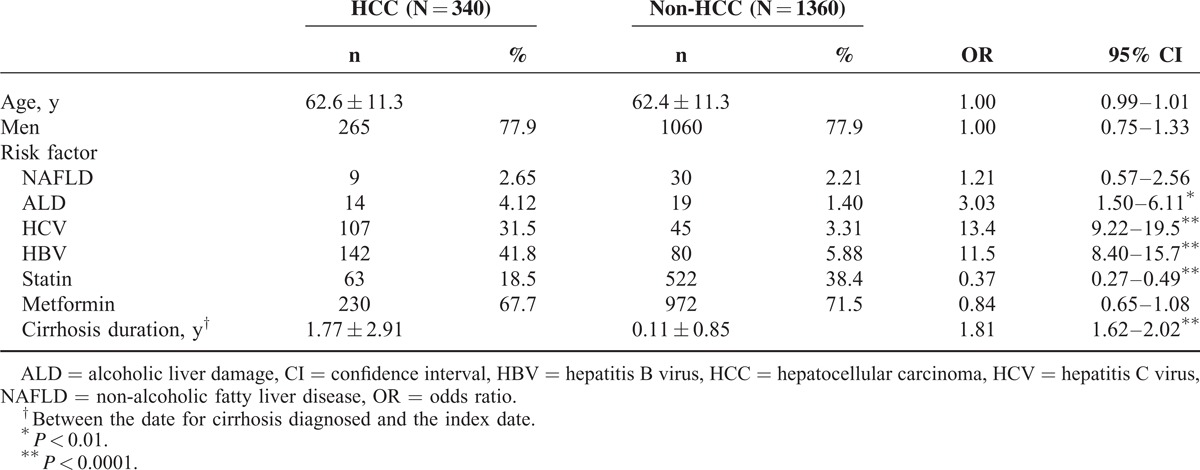
The DM cohort in this study comprised 340 patients with HCC and 1360 controls. The mean patient age was 62.6 years (standard deviation = 11.3). The proportion of men exceeded that of women (77.9% vs 22.1%) in the HCC group (Table 1). The logistic regression analysis results indicated that patients with cirrhosis duration (per year), ALD, HCV, or HBV demonstrated an increased risk of HCC (OR = 1.81, 3.03, 13.4, and 11.5, respectively). However, patients who received statins showed a 63% decreased risk of HCC (OR = 0.37; 95% CI = 0.27–0.49).
Compared with patients who did not receive metformin and statins, no significant difference was observed for various duration rates of statin or metformin use after controlling for cirrhosis duration, ALD, HCV, and HBV (Table 2). However, a significant decrease was observed as the duration rates of statin use increased among patients who received no or occasional metformin treatment (trend test P = 0.01 and 0.0008).
TABLE 2.
Adjusted Odds Ratio for HCC Among Metformin and Statin Use Duration Rate
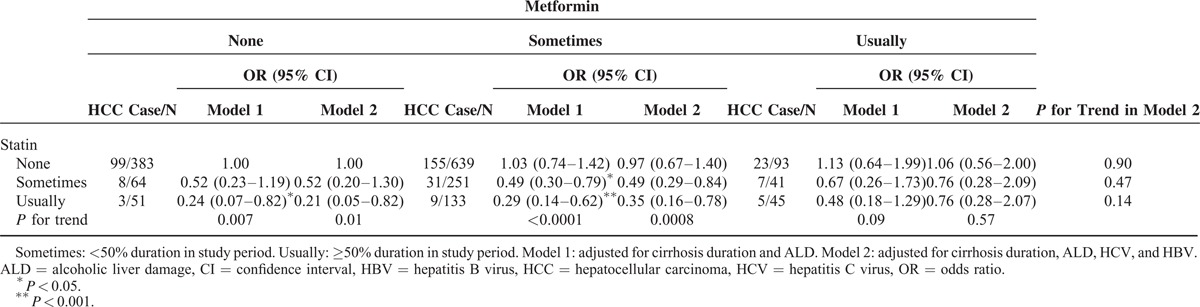
Table 3 shows the association between HCC and various statins. Compared with patients who did not receive statins, those who consumed simvastatin, atorvastatin, or rosuvastatin exhibited a significantly reduced risk of HCC (OR = 0.32, 0.31, and 0.22; 95% CI = 0.18–0.58, 0.19–0.52, and 0.08–0.61, respectively). The same trend was observed in patients who received metformin (OR = 0.30, 0.30, and 0.24; 95% CI = 0.15–0.59, 0.16–0.54, and 0.08–0.70). The risk of HCC decreased with the exposure duration (per month) of simvastatin, atorvastatin, and rosuvastatin which was increased compared without statin treatment (OR = 0.82, 0.95, and 0.79; 95% CI = 0.73–0.94, 0.92–0.99, and 0.63–0.99). In patients with metformin treatment, the risk reduced with the duration of simvastatin and atorvastatin (OR = 0.81 and 0.95; 95% CI = 0.70–0.93 and 0.92–0.99, respectively).
TABLE 3.
Association Between HCC and Different Statin Use and Exposure Duration
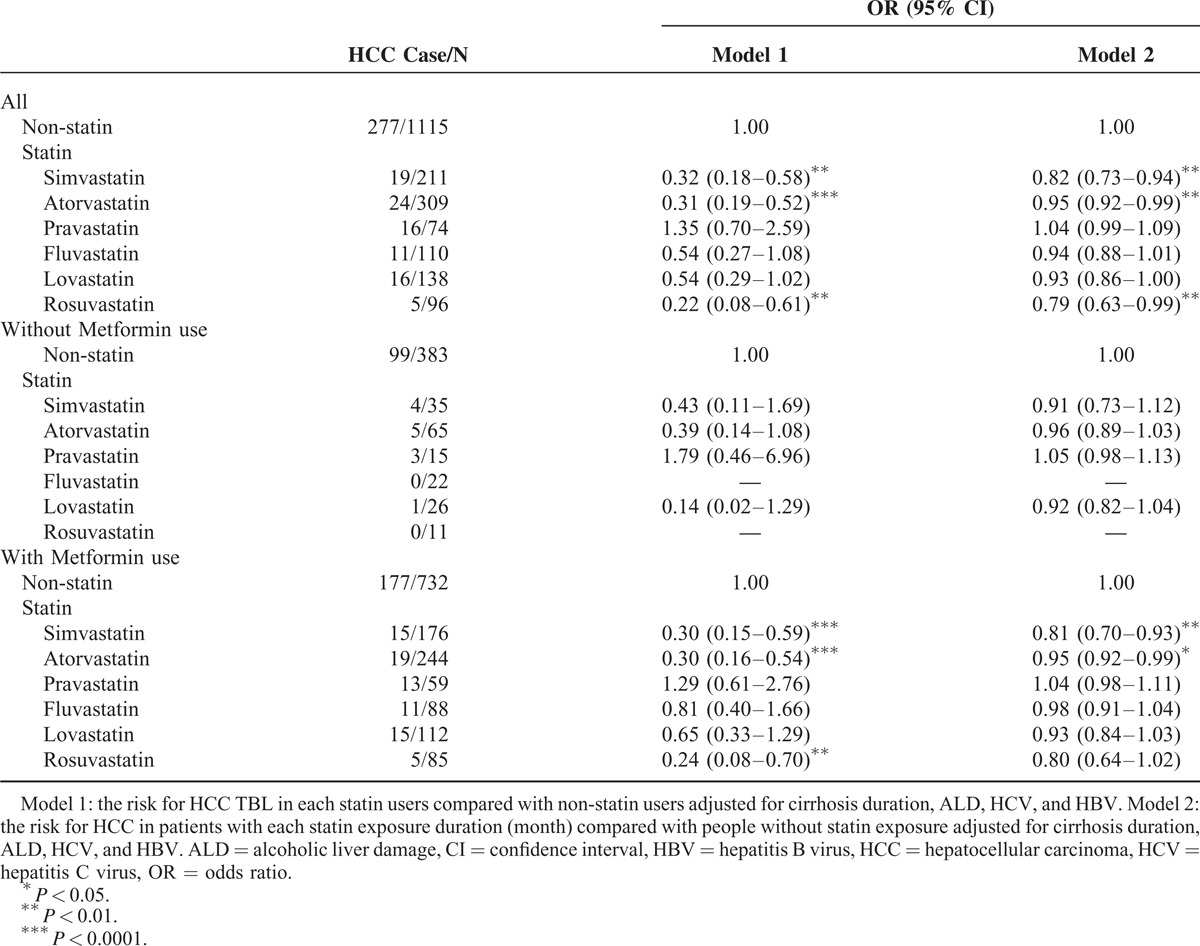
Table 4 shows the association between HCC and various statins in patients with any comorbidities included NAFLD, ALD, HCV, HBV, and cirrhosis. Overall, the risk of HCC was decreased among patients treated with simvastatin and atorvastatin compared with patients did not receive statin (OR = 0.31 and 0.29; 95% CI = 0.14–0.67 and 0.15–0.57, respectively). Compared to non-statin use, longer duration of simvastatin and atorvastatin use could reduce HCC risk (OR = 0.81 and 0.96; 95% CI = 0.68–0.97 and 0.92–0.99, respectively). The patients treated with metformin showed similar results.
TABLE 4.
Association Between HCC and Different Statin Use in Patients With Comorbidity

DISCUSSION
DM and HCC
The relationship between DM and HCC was identified in 1986.7 One systematic review8 showed that DM patients are 3.64 times more likely to develop HCC compared with non-DM patients. Other studies have also suggested that DM is a potential risk factor for HCC.9 The possible reasons for this inference are explained as follows: First, liver cirrhosis may cause glucose intolerance and up to 30% of cases may become diabetic.10 Terminal or end-stage hepatic disease can further increase glucose intolerance, which eventually progresses into overt DM.11 Second, non-alcoholic steatohepatitis (NASH)12 can lead to liver fibrosis, cirrhosis, and subsequently HCC. NASH is among the most severe manifestations of NAFLD, which is a risk factor for HCC. NAFLD can cause a wide range of liver disorders, including steatosis and steatohepatitis, demonstrating pathological features similar to those of alcohol-related liver injury, fibrosis, and occasionally cirrhosis. Unfortunately, DM is a risk factor for NAFLD. Third, both HCV and hemochromatosis can cause liver disease, and have been associated with an increased risk of DM.13 Fourth, insulin is produced and secreted by β-cells located in the pancreas. High concentrations of endogenous insulin pass directly into portal circulation in the liver, potentially explaining the association between DM and HCC.3 Fifth, other factors including obesity and inflammation may exert influence; these factors have been reported for DM and cancer. Furthermore, chronic inflammation can stimulate, promote, and advance cancer cell development. In this research, the risk factors for HCC were cirrhosis, HBV, HCV, and ALD, rather than NAFLD and metformin use (Table 1). However, additional studies may be required to clarify the effects on Asian DM patients with HCC.
Statins and HCC
Previous studies have reported that statins can not only reduce low-density lipoprotein (LDL) levels but also decrease HCC, infection, or inflammation.14–16 Statins reduce HCC cells by inducing cell apoptosis. Pravastatin can be prescribed in chemotherapy treatments for HCC to improve the survival rates of patients with advanced HCC.17,18 The enzyme 3-hydroxy-3-methylglutaryl coenzyme-A reductase (HMG-CoAR), which is a rate-limiting step in the mevalonate pathway for LDL reduction, can induce the production of isoprenoids, which contribute to the activation of Ras.19 HMG-CoAR is a rational molecular target for innovative anti-neoplastic treatments of HCC. Chemotherapy treatment that employs statin therapy can decrease resistance to cytotoxic drugs by activating the Ras/Raf/MEK/ERK signal transduction cascade20 and increasing cholesterol levels in cancer cells.21 Other statins such as fluvastatin and cerivastatin have been reported to inhibit hepatic tumor cell growth in rats22 and to induce tumor-specific apoptosis.23 One study showed that pitavastatin and atorvastatin can effectively and safely reduce elevated hepatic enzyme levels in patients with NAFLD.23 The results of our analyses supported the findings of previous studies. Specific statins, such as simvastatin, atorvastatin, and rosuvastatin, may reduce the risk of HCC (Tables 1 and 3).24,25 These findings indicate that various statins demonstrate distinct potential anti-cancer effects, and that the 2 statins investigated herein can yield benefits for Asian patients with HCC. The limitation of our patient numbers in different statin groups can also offer the same results as the previous studies for different statins.
Metformin and HCC
A systematic review and meta-analysis26 showed that metformin can reduce the mortality rates and incidence of all cancers compared with other treatments for DM. Metformin significantly reduced the risk of colorectal cancer, liver tumors, and lung cancer.27 One article stated that DM patients treated with metformin were associated with an estimated 70% reduction in HCC risk.28 Another systematic review showed that metformin yields more benefits for treating pancreatic and hepatic cancers compared with colon, breast, and prostate cancers.29 Several possible mechanisms for the anti-cancer effects of metformin have been discussed. Indirect pathways include the avoidance of weight gain,30 as compared with other anti-diabetic medications, and the amelioration of insulin resistance by reducing hyperinsulinemia. However, both pathways may promote carcinogenesis.31 The direct pathways of metformin can stimulate AMP-activated protein kinase (AMPK) through LKB-1 (serine–threonine liver kinase B1), which is a tumor-suppressing protein kinase. AMPK inhibits protein synthesis and hepatic glucose output during cellular stress and inhibits the mammalian target of rapamycin (mTOR), which is a downstream effector of growth factor signaling commonly present in malignant cells.32 Regarding breast cancer, metformin can inhibit mTOR activity, thereby reducing HER-2 protein expression. Metformin can also induce cell-cycle arrest and apoptosis, reducing growth factor signaling with hyperinsulinemia in diabetic or non-diabetic patients, thereby decreasing the risk of colorectal cancer.33 These articles have suggested other possible mechanisms by which metformin can decrease HCC risk, including reducing inflammation and endogenous reactive oxygen species.34 Studies have reported varying results regarding the anti-cancer effects of metformin, including no apparent benefit for reducing the risk of colorectal cancer.35 A recent nested case–control study conducted in Asia determined no significant relationship between metformin use and the incidence of cancer (such as liver cancer and female breast cancer).36 According to these articles, it remains ambiguous whether metformin exerts anti-neoplasm effects in DM patients. Our analysis showed that metformin cannot reduce HCC (Table 1). The possible reasons for this finding are explained as follows: First, diabetes is a progressive disease accompanied by continuous chronic inflammation that results from hyperglycemia or hyperinsulinemia, which play key roles in cancer cell activity, including its initiation, promotion, and progression.37 Metformin can decrease insulin resistance but cannot directly reduce abnormal insulin secretion. Second, high concentrations of insulin secreted by β-cells pass directly into liver tissues and increase the risk of HCC. Metformin has no effect on this pathway. Third, DM results from chronic inflammation and can cause additional oxidative stress. Compared with metformin, statins more effectively reduce inflammation. Forth, the sub-analysis results (Table 2) showed that the duration of metformin use may be another issue worth exploration in relation to its anti-cancer effects. Certain studies have highlighted that low concentrations of metformin (<0.5 mM) selectively inhibit CD133+ cell proliferation and reduce cancer stem cell activity. The cancer stem cell hypothesis suggests that cancer stem cells play a key role in tumor genesis, recurrence, and the resistance to adjuvant cancer therapies.38 Another study stated that low doses of metformin can induce p53-dependent senescence in hepatoma cells.39 One study conducted in Asia showed that low doses of metformin (<1000 mg/day) may be associated with a reduced risk of cancer in diabetic patients,40 and another article reported a trend of dose–response relations to cancers29 (treatment that employed less than 250 mg/day for 1 month were discussed). The results of the current study indicated a significant trend of decreased HCC risk in DM patients who received occasional metformin treatment (Table 2, trend test P = .03). This may imply that low doses or short durations of metformin use are sufficient to yield anti-cancer effects in Asian DM patients with HCC. Fifth, according to the results in Table 1, NAFLD and metformin do not contribute to the risk of HCC. Racial or genetic differences may be a possible explanation.
Metformin Combined With Statins and HCC
Our research did not involve observing all the positive effects of combining metformin and statins regarding reduced HCC risk. However, specific statins, such as simvastatin and atorvastatin alone or combined with metformin, may lower the risk of HCC (Table 3). We infer that this may be caused by the interaction of metformin with simvastatin or atorvastatin. DM patients treated with metformin combined with simvastatin exhibited a relatively low risk of comorbidities when they presented with HCC (Table 4). This finding indicates that administering this type of combination therapy to DM patients with HCC is fairly beneficial in Asia.
SUMMARY
Our study showed no benefit of metformin to HCC. Treatment with pravastatin alone or with metformin also showed no benefit to HCC. Further basic research is required for clarification.
STUDY LIMITATIONS
Two limitations to our studies should be considered. First, the LHID2000 cannot offer detailed information such as alcohol consumption, which may be risk factor for HCC. Second, all patient data in the LHID2000 are anonymous. Relevant clinical clues, such as child score for liver cirrhosis, imaging results, or pathologic findings, cannot be followed in our study.
CONCLUSION
According to the research results, not all metformin combined with statins may reduce the incidence of HCC in Asia and not all of this combination therapy may decrease the development of comorbidities in diabetic patients with HCC.
Footnotes
Abbreviations: CI = confidence interval, DM = diabetes mellitus, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NAFLD = non-alcoholic fatty liver disease, NHIRD = National Health Insurance Research Database, OR = odds ratio.
M-C L and H-H C contributed equally to this work.
Author contributions: Conception and design: H-H C, C-H K; Administrative support: F-C S, C-H K; collection and assembly of data: all authors; data analysis and interpretation: H-H C, M-C L, C-H M, C-H K; manuscript writing: all authors; final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
All authors state that they have no conflicts of interest.
REFERENCES
- 1.Joslin EP, Lombard HL, Burrows RE, et al. Diabetes and cancer. N Engl J Med 1959; 260:486–488. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010; 33:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigneri P, Frasca F, Sciacca L, et al. Diabetes and cancer. Endocr Relat Cancer 2009; 16:1103–1123. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care 2004; 27:1605–1609. [DOI] [PubMed] [Google Scholar]
- 5.Nomura K, Hamamoto Y, Takahara S, et al. Relationship between carotid intima-media thickness and silent cerebral infarction in Japanese subjects with type 2 diabetes. Diabetes Care 2010; 33:168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int 2009; 8:465–473. [PubMed] [Google Scholar]
- 7.Lawson DH, Gray JM, McKillop C, et al. Diabetes mellitus and primary hepatocellular carcinoma. Q J Med 1986; 61:945–955. [PubMed] [Google Scholar]
- 8.Noto H, Osame K, Sasazuki T, et al. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications 2010; 24:345–353. [DOI] [PubMed] [Google Scholar]
- 9.Davila JA, Morgan RO, Shaib Y, et al. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 2005; 54:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol 2009; 15:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrides AS, Vogt C, Schulze-Berge D, et al. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology 1994; 19:616–627. [DOI] [PubMed] [Google Scholar]
- 12.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002; 123:134–140. [DOI] [PubMed] [Google Scholar]
- 13.Davila JA, Morgan RO, Shaib Y, et al. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 2005; 54:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doshi SM, Kulkarni PA, Liao JM, et al. The impact of statin and macrolide use on early survival in patients with Pneumococcal pneumonia. Am J Med Sci 2013; 345:173–177. [DOI] [PubMed] [Google Scholar]
- 15.Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007; 28:664–672. [DOI] [PubMed] [Google Scholar]
- 16.Sutter AP, Maaser K, Hopfner M, et al. Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors: synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J Hepatol 2005; 43:808–816. [DOI] [PubMed] [Google Scholar]
- 17.Tatsuta M, Iishi H, Baba M, et al. Suppression by pravastatin, an inhibitor of p21ras isoprenylation, of hepatocarcinogenesis induced by N-nitrosomorpholine in Sprague–Dawley rats. Br J Cancer 1998; 77:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf H. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 2008; 78:34–38. [DOI] [PubMed] [Google Scholar]
- 19.Jakobisiak M, Golab J. Potential antitumor effects of statins. Int J Oncol 2003; 23:1055–1069. [PubMed] [Google Scholar]
- 20.Wong WW, Tan MM, Xia Z, et al. Cerivastatin triggers tumor-specific apoptosis with higher efficacy than lovastatin. Clin Cancer Res 2001; 7:2067–2075. [PubMed] [Google Scholar]
- 21.Weinstein-Oppenheimer CR, Henriquez-Roldan CF, Davis JM, et al. Role of the Raf signal transduction cascade in the in vitro resistance to the anticancer drug doxorubicin. Clin Cancer Res 2001; 7:2898–2907. [PubMed] [Google Scholar]
- 22.Paragh G, Kertai P, Kovacs P, et al. HMG CoA reductase inhibitor fluvastatin arrests the development of implanted hepatocarcinoma in rats. Anticancer Res 2003; 23:3949–3954. [PubMed] [Google Scholar]
- 23.Han KH, Rha SW, Kang HJ, et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J Clin Lipidol 2012; 6:340–351. [DOI] [PubMed] [Google Scholar]
- 24.Lai SW, Liao KF, Lai HC, et al. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol 2013; 28:485–492. [DOI] [PubMed] [Google Scholar]
- 25.Chiu HF, Ho SC, Chen CC, et al. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol 2011; 106:894–898. [DOI] [PubMed] [Google Scholar]
- 26.Franciosi M, Lucisano G, Lapice E, et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS ONE 2013; 8:e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS ONE 2012; 7:e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZJ, Zheng ZJ, Shi R, et al. Kip metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:2347–2353. [DOI] [PubMed] [Google Scholar]
- 29.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010; 3:1451–1461. [DOI] [PubMed] [Google Scholar]
- 30.Krakoff J, Clark JM, Crandall JP, et al. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity (Silver Spring) 2010; 18:1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White MF. The insulin signalling system and the IRS proteins. Diabetologia 1997; 40 (suppl 2):S2–S17. [DOI] [PubMed] [Google Scholar]
- 32.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012; 2:778–790. [DOI] [PubMed] [Google Scholar]
- 33.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog 2010; 49:662–671. [DOI] [PubMed] [Google Scholar]
- 34.Algire C, Moiseeva O, Deschenes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012; 5:536–543. [DOI] [PubMed] [Google Scholar]
- 35.Bodmer M, Becker C, Meier C, et al. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev 2012; 21:280–286. [DOI] [PubMed] [Google Scholar]
- 36.Wang SY, Chuang CS, Muo CH, et al. Metformin and the incidence of cancer in patients with diabetes: a nested case-control study. Diabetes Care 2013; 36:e155–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Pelzer AM, Kiang DT, et al. Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res 2007; 67:391–397. [DOI] [PubMed] [Google Scholar]
- 38.Grotenhuis BA, Wijnhoven BP, van Lanschot JJ. Cancer stem cells and their potential implications for the treatment of solid tumors. J Surg Oncol 2012; 106:209–215. [DOI] [PubMed] [Google Scholar]
- 39.Yi G, He Z, Zhou X, et al. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int J Oncol 2013; 43:1503–1510. [DOI] [PubMed] [Google Scholar]
- 40.Chung HH, Moon JS, Yoon JS, et al. The relationship between metformin and cancer in patients with type 2 diabetes. Diabetes Metab J 2013; 37:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]


