Abstract
The prognosis of advanced gastrointestinal stromal tumors (GISTs) was dramatically improved in the era of imatinib. Cytoreduction surgery was advocated as an additional treatment for advanced GISTs, especially when patients having poor response to imatinib or developing resistance to it. However, the efficacy and benefit of cytoreduction were still controversial. Likewise, the sequence between cytoreduction surgery and imatinib still need evaluation. In this study, we tried to assess the feasibility and efficiency of cytoreduction in advanced GISTs. Furthermore, we analyzed the impact of timing of the cytoreduction surgery on the prognosis of advanced GISTs.
We conducted a prospective collecting retrospective review of patients with advanced GISTs (metastatic, unresectable, and recurrent GISTs) treated in Chang Gung memorial hospital (CGMH) since 2001 to 2013. We analyzed the impact of cytoreduction surgery to response to imatinib, progression-free survival (PFS), and overall survival (OS) in patients with advanced GISTs. Moreover, by the timing of cytoreduction to imatinib, we divided the surgical patients who had surgery before imatinib use into early group and those who had surgery after imatinib into late. We compared the clinical response to imatinib, PFS and OS between early and late cytoreduction surgical groups.
Totally, 182 patients were enrolled into this study. Seventy-six patients underwent cytoreduction surgery. The demographic characteristics and tumor presentation were similar between surgical and non-surgical groups. The surgical group showed better complete response rate (P < 0.001) and partial response rate (P = 0.008) than non-surgical group. The 1-year, 3-year, and 5-year PFS were significantly superior in surgical group (P = 0.003). The 1-year, 3-year, and 5-year OS were superior in surgical group, but without statistical significance (P = 0.088). Dividing by cytoreduction surgical timing, the demographic characteristics and tumor presentation were comparable in early and late groups. The late cytoreduction group presented higher R0 resection rate (59.1% vs 31.5%, P = 0.025). However, the PFS and OS were comparable in both groups.
Combining imatinib with cytoreduction increased the response rate to imatinib and prolonged PFS in patients with advanced GISTs. Moreover, early and late cytoreduction surgery was comparable in prognosis, although late cytoreduction revealed higher complete resection rate.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, and frequently result from activating mutations in KIT (CD117) or platelet-derived growth factor receptor alpha.1,2 Complete resection remains the standard treatment of primary GISTs. Before the introduction of the tyrosine-kinase inhibitor (TKI) imatinib, the prognosis of advanced (metastatic or recurrent) GISTs was poor.3–6 Following the establishment of imatinib as a treatment choice, the median survival of patients with advanced GISTs has increased to beyond 24 months, based on the potential of the medication to downsize the tumor.3,5 However, not all GISTs respond to targeted therapy, and approximately 20% of patients do not tolerate specific TKIs.1,6–8 In addition, the majority of patients initially benefiting from imatinib eventually develop resistance.9–11 Therefore, cytoreduction surgery for advanced GISTs is thought to be an additional therapy for patients responding to imatinib. Some authors advocate surgery for patients with metastatic GISTs on imatinib to delay or prevent progression due to drug resistance. However, others feel that cytoreduction surgery might not benefit patients owing to its limited prolongation of survival accompanied by a high possibility of surgical complications.
The aim of this study was to investigate whether patients with advanced GISTs benefit from cytoreduction surgery. In addition, we examined the timing of surgery, that is, if it is more beneficial to apply it before or after treatment with imatinib in patients with advanced GISTs.
PATIENTS AND METHODS
Study Design
This was a prospective data collection and retrospective review study. We prospectively collected medical data from all patients with GISTs treated at CGMH, Linkou, Taiwan between January 2001 and June 2013. The Chang Gung Medical Foundation Institutional Review Board has approved this study. Demographic information and medical, operative, postoperative, and pathological records were prospectively collected in a computerized GIST database. In addition, we retrospectively reviewed patients with advanced GISTs (including metastatic GISTs, local recurrence after initial resection, and combination of metastasis and recurrence) who received imatinib (Gleevec®; Novartis, Basel, Switzerland) therapy. Patients with advanced GISTs started receiving imatinib at a dose of 400 mg once daily, and all patients continued imatinib until they experienced disease progression or unacceptable toxicity.
Demographic information was collected, along with the results of clinical analyses and the location of the primary tumor. All images such as chest radiographs and abdominal computed tomography (CT) scans were reviewed. Metastasis was defined as a tumor presenting in a location in addition to the gastrointestinal (GI) tract. Tumor recurrence was defined as evidence of tumor existence by means of imaging studies during regular follow-up after previous surgery. The response to imatinib was evaluated according to the Response Evaluation Criteria in Solid Tumors with CT Scans guidelines (RECIST),12 including complete response (CR), partial response (PR), stable disease (SD), or progression of disease (PD). Tumor size was measured in at least 5 target lesions with the sum of the largest dimension that was used as a response evaluation indicator as described in RECIST as the total size of the tumor burden.13 The response to imatinib was assessed every 3 months after imatinib use for first 3 years and then follow-up every 6 months thereafter.
We performed cytoreduction surgery in advanced GISTs to remove the tumor whether it was a primary tumor, a recurrence, or a metastatic site, extending the surgery as radically as possible. On the basis of whether cytoreduction surgery was performed, we divided the patients into surgical and nonsurgical groups. In the surgical group, on the basis of the timing of the surgery, we classified patients into a group with metastatic and/or recurrent GISTs who received cytoreduction surgery before imatinib use (early group), and a group who received surgery after imatinib use (late group). In early cytoreduction group, patients underwent cytoreduction surgery first. After patients recovered from surgery, we performed the CT and started imatinib. In late group, we begin imatinib and assessed the response then performed cytoreduction surgery. The timing and follow-up protocol were the same between 2 groups. In the late group, we performed the operation when patients had experienced clinical benefit following imatinib use, including CR, PR, or SD. Those who showed PD after imatinib use were excluded from this portion of the study to compare and clarify the timing of surgery between the early and late groups. The primary goal of surgery was to achieve complete microscopic resection (R0). In those patients with multiple metastases, debulking surgery was performed to as great an extent as possible, but sparing the function was the ultimate goal. The resection status was classified as R0, complete macroscopic resection (R1), and residual macroscopic resection (R2), according to the histopathological examination.
Progression-free survival (PFS) was defined as the time from the date when intervention (surgery or Imatinib) was initiated to the date of documented progression of residual disease or recurrent disease. Overall survival (OS) was defined as the length of time from the date when intervention was initiated to the date of patient death from any cause or the end of this review. Surgical morbidity was defined as the complications related to surgery including leakage, intra-abdominal abscess, or surgical site infection. Surgical mortality was defined as death within 90 days after the surgery.
Statistics
All numerical continuous data were compared using an independent Student t test, and categorical variables were analyzed using Pearson χ2 test or Fisher exact test and multiple forward stepwise logistic regression analysis when needed. The survival rates were calculated with the Kaplan–Meier method, and survival analysis was conducted using the log-rank test. The Cox proportional hazards model was used for multivariate regression analysis. SPSS v. 20.0 for Macintosh (SPSS Inc, Chicago, IL) was employed for statistical analysis. The definition of statistical significance was P < 0.05.
RESULTS
Study Population
Table 1 summarizes the clinical features of a total of 182 patients with metastatic or recurrent GISTs. There were 76 of 182 patients in the surgery group and 106 in the non-surgery group. In the surgery group, 54 patients underwent surgery before imatinib treatment (early group); in contrast, 22 patients underwent surgery followed by imatinib treatment (late group). There was no patient with advanced GIST undergoing cytoreduction surgery without imatinib use.
TABLE 1.
Demographic Characteristics and Prognosis of Surgical vs Nonsurgical Groups
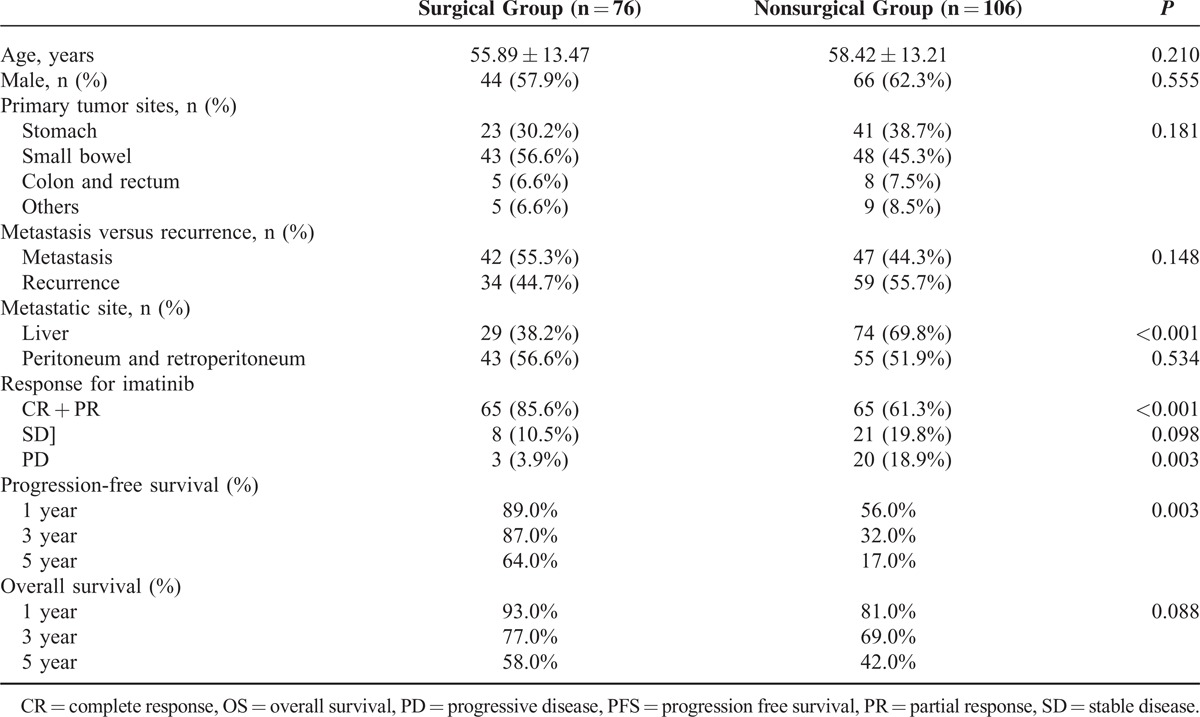
There were 110 male and 72 female patients, with a mean age of 55.89 years in the surgery group and 58.42 years in the non-surgery group. The primary tumor site for the GISTs was the stomach in 64 patients, whereas the tumors were small intestinal in 91 patients, colorectal in 13, and located in other sites in 14. A total of 89 (48.9%) patients presented with metastatic GISTs; 93 patients experienced recurrence of GISTs during follow-up. Between the surgery and non-surgery groups, there were no significant differences regarding age (P = .21), sex (P = .56), primary tumor sites (P = .18), or tumor distribution (P = .15). Comparing the response to imatinib, the CR and PR rates were higher in the surgical group than in the non-surgical group (P < 0.001 and P = 0.008, respectively). The SD was higher in non-surgical group, however, not reaching statistical significance (19.8% vs 10.5%, P = 0.098). Furthermore, the PD rate was lower in the surgical group than in the nonsurgical group (3.9% vs 18.9%, P = 0.003). The 1-year, 3-year, and 5-year PFS rates were better in the surgical group than in the nonsurgical group (89% vs 56%, 87% vs 32%, 64% vs 17%, respectively; P = 0.003) (Figure 1). The 1-year, 3-year, and 5-year OS rates were also better in the surgical group than in the nonsurgical group (93% vs 81%, 77% vs 69%, 58% vs 42%, respectively), although there was no significant difference (P = 0.088, Figure 2).
FIGURE 1.
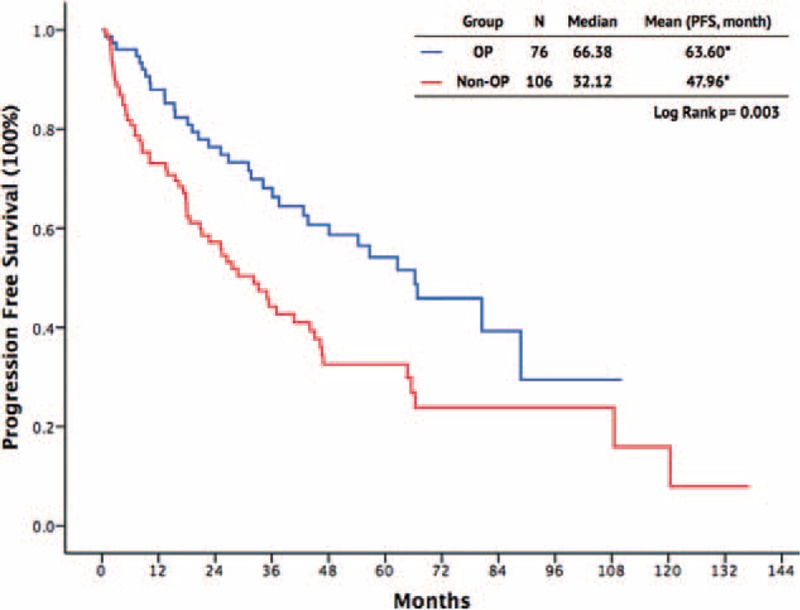
Progression-free survival outcomes of patients with advanced gastrointestinal stromal tumor.
FIGURE 2.
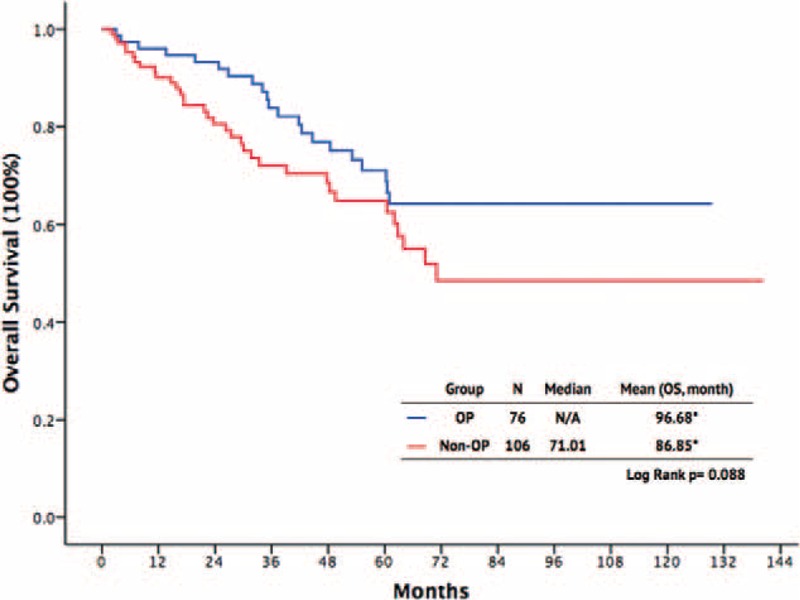
Overall survival outcomes of patients with advanced gastrointestinal stromal tumor.
Timing of Cytoreductive Surgery
To clarify the timing of cytoreduction surgery combined with imatinib for patients with advanced GISTs, we divided the patients into early and late cytoreduction groups. Table 2 shows a summary of these patients. The early group comprised 54 patients who underwent cytoreduction surgery before treatment with imatinib, whereas the other 22 patients, who received surgery after imatinib therapy, constituted the late group. The age, sex, primary tumor location, distribution of metastasis or recurrence, tumor size, maximal size, and metastatic/recurrent site were similar between the 2 groups (Table 2). Comparing these 2 groups, the R0 resection rate was higher in the late group than in the early group (59.1% vs 31.6%, P = 0.027). The surgical morbidity was similar between the 2 groups (9.3% vs 9.1%, P = 0.86). There was no surgical mortality in either of the 2 groups. The length of total hospital stay and postoperative stay were comparable between the 2 surgical groups. The response to imatinib, including CR and PR, was higher in the late group than in the early group (100% vs 79.6%, P = 0.022). The SD was higher in early group, however, not reaching statistical significance (Table 2). The 1-year, 3-year, and 5-year PFS rates in the early group and late group were 83.0% versus 100.0%, 67.8% versus 69.3%, and 55.7% versus 49.6%, respectively (P = .63, Figure 3). The 1-year, 3-year, and 5-year OS rates in the early group and late group were 92.6% versus 100%, 76.6% versus 84.2%, and 64.7% versus 66.9%, respectively (P = 0.56, Figure 4).
TABLE 2.
Summaries of Clinical Characteristics and Prognosis of Early and Late Cytoreduction Groups
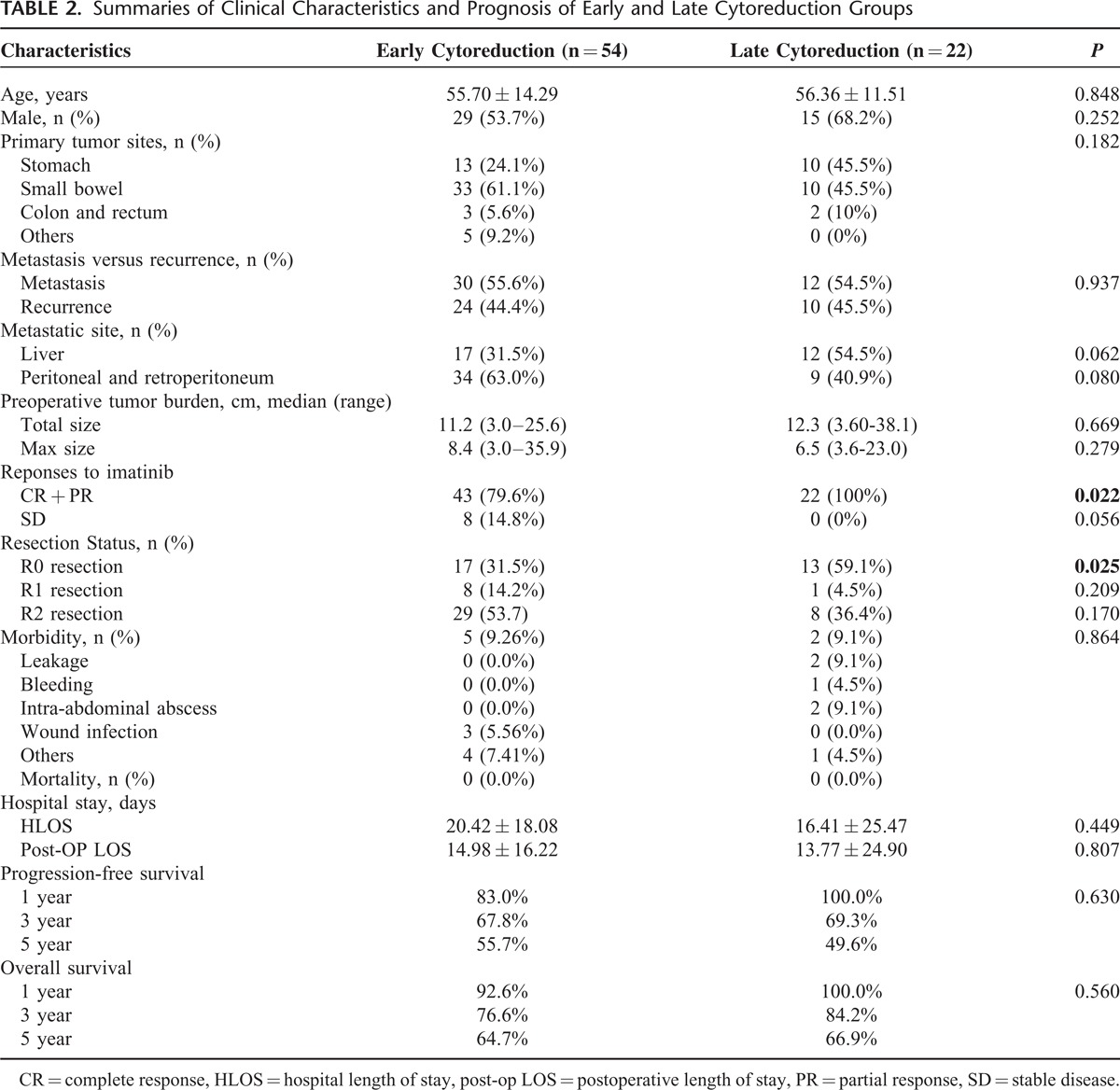
FIGURE 3.
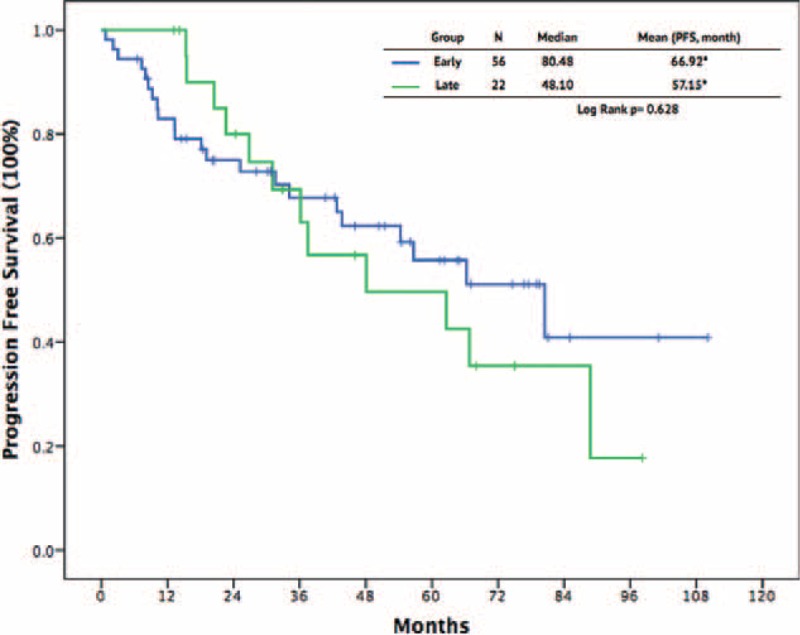
Progression-free survival outcomes between patients with early and late cytoreduction surgery.
FIGURE 4.
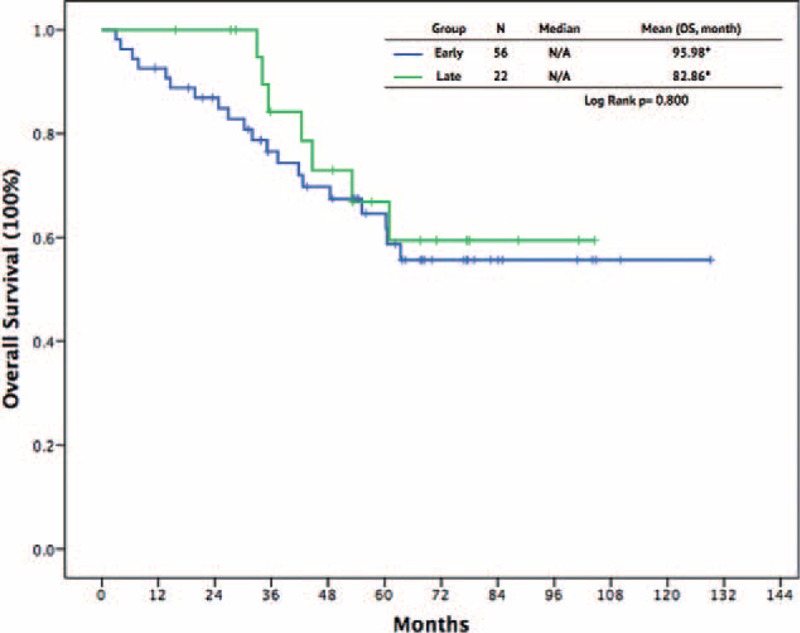
Overall survival outcomes between patients with early and late cytoreduction surgery.
DISCUSSION
After the development of imatinib, surgery still remained one of the main treatment modalities for GISTs, either in primary, recurrent, or metastatic tumors. Furthermore, published evidence has shown that <5% of the lesions responding to imatinib exhibit a complete pathologic response, suggesting that medical management is only part of the optimal therapeutic strategy.14 Progression eventually develops because of drug resistance. An increasing amount of literatures suggests that cytoreduction surgery or metastasectomy in combination with TKIs may increase PFS and OS in selected patients.6,10,12,14 However, conflicting data regarding the role of surgical cytoreduction in advanced GISTs have also been reported.15
The remarkable fact in this study is the trend of cytoreduction surgery achieving higher rates of CR, PR, or SD and further lowering the rate of PD. Furthermore, the results of the present study support the conclusion that cytoreduction surgery combined with imatinib does prolong survival in patients with metastatic or recurrent GISTs when compared with imatinib use alone. This is reflected by the fact that PFS in the surgery group was significantly prolonged compared with those in the non-surgery group (P = 0.003). Surgery reduces the tumor burden and delays the time to the development of secondary resistance to TKI therapy, contributing to prolong PFS.10,12,14,16 Consequently, it prolongs the time to tumor progression, and increases the possibility of patients’ response, and even cure. Similar to previous evidence, the OS of patients with advanced GISTs in our study was strongly related to the preoperative response to TKI therapy.6,17,18 However, in our study, the OS in the surgery group tended to be superior to that in the non-surgery group, although statistical significance was not reached. Therefore, we considered that cytoreduction surgery could benefit the advanced GIST patients.
As the cytoreduction surgery might prolong the survival, the timing of the cytoreduction surgery became another issue. In the present study, we performed analysis to clarify the timing of cytoreduction surgery. The patient characteristics were comparable in the early and late cytoreduction groups, especially considering the tumor burden, including the maximal tumor size and total tumor size, which minimized the selection bias in this study. The early or late surgery achieved comparable surgical morbidity, mortality, and length of stay. Higher R0 resection rates as well as CR and PR rates were found in the late group. However, the PFS and OS were similar between the 2 groups. We demonstrated that early cytoreduction surgery is difficult to achieve R0 resection when compared with late cytoreduction. It might be partly explained by the huge tumor size or disseminated tumor distribution before imatinib use. Previous studies have demonstrated that R0 resection of advanced GISTs serves as an important prognostic factor and is correlated with the outcome.6,19 Hard to achieve R0 resection in early cytoreduction, theoretically, result in poor prognosis. In this series, we identified the lower R0 resection rate in the early group, but the survival is similar between both groups, which might be controversial to other results.6,19 There are several explanations for these findings. First, early surgery may lessen the tumor burden earlier to reduce the secondary resistance and increase survival. Second, for advanced GISTs, the nutrition absorption, motivation, and integrity of the GI tract might be compromised by the tumor, and early cytoreduction may help to recover the function of the GI tract and improve survival. Therefore, with caution, early cytoreduction surgery may be comparable with late cytoreduction in PFS and OS irrespective of whether complete resection is achieved.
Although our results may support the impact of cytoreduction surgery on advanced GISTs, there were several limitations inherent to this study. First, this was a retrospective study, although all data were collected prospectively and the characteristics of the groups were similar and homogeneous, selective, and recall bias could not be completely prevented. Second, lacking evidence from prospective randomized study, the role of cytoreduction surgery in advanced GISTs with response after imatinib use is still unknown. There were some patients hesitating to take cytoreduction surgery after imatinib use, contributing to the bias of patient's selection. Third, for patient with good response to imatinib, we usually performed surgery for these patients to reduce the tumor burden and prevent from the development of resistance to imatinib. Theoretically, this group of patients might have better prognosis, which lead another bias in our study. Fourth, the sequence of imatinib and cytoreduction surgery could not be controlled in this retrospective study, which induced crucial selection bias. Although we identified the factors influencing prognosis in early and late groups were comparable, which might minimize the bias induced by patient's selection. Another limitation of this study is that it was not double-blinded. Therefore, biases owing to patient and surgeon attitudes are likely to arise. The unblinded patients may have had more motivation to receive therapy because they thought they could undergo surgery. Unblinded surgeons may have been more aggressive in facilitating therapy after the operation. Hence, the placebo effect could not be completely avoided in this study. To overcome these limitations, our results should be confirmed by further prospective randomized controlled trials that examine the benefits of cytoreduction surgery in advanced GISTs.
In conclusion, compared with imatinib alone, combining imatinib with cytoreduction surgery significantly prolongs the PFS and lowers the rate of PD in advanced GISTs. With caution, early cytoreduction may be comparable with late cytoreduction in PFS and OS irrespective of whether complete resection is achieved.
Footnotes
Abbreviations: CR = complete response, CT = computed tomography, GIST = gastrointestinal stromal tumor, OS = overall survival, PD = progression of disease, PFS = progression-free survival, PR = partial response, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease, TKI = tyrosine-kinase inhibitor.
S-CC and C-HL contributed equally to this manuscript.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Demetri GD, Mehren von M, Antonescu CR, et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010; 8 Suppl 2:S1–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol 2000; 15:1293–1301. [DOI] [PubMed] [Google Scholar]
- 3.Barnes G, Bulusu VR, Hardwick RH, et al. A review of the surgical management of metastatic gastrointestinal stromal tumours (GISTs) on imatinib mesylate (Glivec). Int J Surg 2005; 3:206–212. [DOI] [PubMed] [Google Scholar]
- 4.Nunobe S, Sano T, Shimada K, et al. Surgery including liver resection for metastatic gastrointestinal stromal tumors or gastrointestinal leiomyosarcomas. Jpn J Clin Oncol 2005; 35:338–341. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaydfudim V, Okuno SH, Que FG, et al. Role of operative therapy in treatment of metastatic gastrointestinal stromal tumors. J Surg Res 2012; 177:248–254. [DOI] [PubMed] [Google Scholar]
- 7.Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist 2004; 9:271–281. [DOI] [PubMed] [Google Scholar]
- 8.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007; 370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh C-N, Chen Y-Y, Tseng J-H, et al. Imatinib Mesylate for Patients with Recurrent or Metastatic Gastrointestinal Stromal Tumors Expressing KIT: A Decade Experience from Taiwan. Transl Oncol 2011; 4:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh C-N, Chen T-W, Tseng J-H, et al. Surgical management in metastatic gastrointestinal stromal tumor (GIST) patients after imatinib mesylate treatment. J Surg Oncol 2010; 102:599–603. [DOI] [PubMed] [Google Scholar]
- 11.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004; 364:1127–1134. [DOI] [PubMed] [Google Scholar]
- 12.Blanke CD, Demetri GD, Mehren von M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008; 26:620–625. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Canc 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 14.Bamboat ZM, DeMatteo RP. Metastasectomy for gastrointestinal stromal tumors. J Surg Oncol 2014; 109:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An HJ, Ryu M-H, Ryoo B-Y, et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol 2013; 20:4212–4218. [DOI] [PubMed] [Google Scholar]
- 16.Vadakara J, Mehren von M. Gastrointestinal stromal tumors: management of metastatic disease and emerging therapies. Hematol Oncol Clin North Am 2013; 27:905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006; 24:2325–2331. [DOI] [PubMed] [Google Scholar]
- 18.DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007; 245:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib - analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014; 40:412–419. [DOI] [PubMed] [Google Scholar]


