Abstract
The neutrophil/lymphocyte ratio (NLR), lymphocyte/monocyte ratio (LMR), and absolute lymphocyte count/absolute monocyte count prognostic score (ALC/AMC PS) have been described as the most useful prognostic tools for patients with diffuse large B-cell lymphoma (DLBCL). We retrospectively analyzed 148 Taiwanese patients with newly diagnosed diffuse large B-cell lymphoma under rituximab (R)-CHOP-like regimens from January 2001 to December 2010 at the Tri-Service General Hospital and investigated the utility of these inexpensive tools in our patients. In a univariate analysis, the NLR, LMR, and ALC/AMC PS had significant prognostic value in our DLBCL patients (NLR: 5-year progression-free survival [PFS], P = 0.001; 5-year overall survival [OS], P = 0.007. LMR: PFS, P = 0.003; OS, P = 0.05. ALC/AMC PS: PFS, P < 0.001; OS, P < 0.001). In a separate multivariate analysis, the ALC/AMC PS appeared to interact less with the other clinical factors but retained statistical significance in the survival analysis (PFS, P = 0.023; OS, P = 0.017). The akaike information criterion (AIC) analysis produced scores of 388.773 in the NLR, 387.625 in the LMR, and 372.574 in the ALC/AMC PS. The results suggested that the ALC/AMC PS appears to be more reliable than the NLR and LMR and may provide additional prognostic information when used in conjunction with the International Prognostic Index.
INTRODUCTION
The clinical outcome of diffuse large B-cell lymphoma (DLBCL) has been significantly improved by the introduction of rituximab (R).1–4 Current prognostic models, including the International Prognostic Index (IPI),5 incorporate patient and tumor characteristics. However, with improved outcomes, the identification of a high-risk subset of patients with an anticipated 5-year survival of less than 50% remains a challenge with the use of these models alone. Over the past 2 decades, many studies have been conducted to identify novel biomarkers characterizing patients with a poor prognosis. Gene expression profiling (GEP),6 mutational analyses,7 immunohistochemistry (IHC)-based detection,8–11 and early interim analysis with positron emission tomography (PET)12,13 have provided crucial information about several new prognostic parameters for the response to therapy in DLBCL. Although they are promising, many of these methods are costly, difficult to obtain, not easily interpreted, and require further validation. Therefore, the evaluation of a patient's prognosis using simple, inexpensive, and easily interpreted clinical parameters warrants investigation.
The complete blood cell (CBC) count and its components may be the most useful tools available. As a surrogate marker of inflammation, the baseline neutrophil count has been associated with survival in patients with malignancies.14,15 The absolute lymphocyte count (ALC) is a marker of host immunity and has also been reported to be a prognostic factor for survival in patients with DLBCL at diagnosis or after first relapse.16–18 Additionally, monocytes and lymphoma-associated macrophages (LAMs), which are considered immunologically relevant and are regarded to be a surrogate marker of the tumor microenvironment, have also recently been reported to be prognostic factors in DLBCL.19 Some applications derived from these blood cells have also been studied, including the neutrophil/lymphocyte ratio (NLR),20 lymphocyte/monocyte ratio (LMR),21,22 and the absolute lymphocyte count/absolute monocyte count prognostic score (ALC/AMC PS);19 all of these items were considered to be independent prognostic factors in DLBCL patients.
Therefore, we reviewed the clinicopathological characteristics of the patients with DLBCL in our institution and collected easily obtained data such as the NLR, LMR, and ALC/AMC PS. A retrospective analysis was performed in Taiwanese patients with DLBCL at the Tri-Service General Hospital.
MATERIALS AND METHODS
Patient Selection
The institutional review board (IRB) of Tri-Service General Hospital (TSGH), National Defense Medical Center approved the study (TSGHIRB No.: 2–103–05–149), and all procedures were carried out in accordance with the Declaration of Helsinki. The 2008 World Health Organization (WHO) classification23 was used to assess the clinical results in this retrospective analysis of treatment outcomes. A total of 268 patients with newly diagnosed DLBCL were identified between January 2001 and December 2010; the histological subtypes and clinical characteristics of these patients were assessed at the Tri-Service General Hospital. We reviewed medical records, demographic data, presentations, physical examinations, radiological findings, laboratory results, and pathology reports in addition to reanalyzing the clinical data of these patients, including age at diagnosis, sex, B symptoms (fever, night sweating, or weight loss), Ann Arbor stage, extranodal involvement, lactate dehydrogenase (LDH), hemoglobin (HgB), Eastern Cooperative Oncology Group performance status (ECOG PS), NLR, LMR, ALC/AMC PS, and IPI. The exclusion criteria included: patients with primary central nervous system (CNS) lymphoma who underwent aggressive intrathecal therapy; patients with primary testicular lymphoma, primary breast lymphoma, or who had paranasal involvement24 and received CNS prophylactic treatment; or patients who underwent not more than 4 cycles of standard R-chemotherapy. Of the 268 patients enrolled, 256 had complete clinical information; 148 patients were included in the final analysis.
Follow-Up
Regular radiographic examinations were performed after treatment, and disease status was determined and recorded based on the results of the imaging analysis. Progression was defined as an increase in size of the original lesion or the development of a new lesion. A relapse was defined as recurrence after an apparent recovery. The progression-free duration was defined as the time between the day of diagnosis and the day of disease progression, relapse, death, or the last day the patient was known to be alive. The final date for overall survival (OS) was defined as the day of death from any cause or the last day the patient was known to be alive.
Statistical Analysis
The receiver operating characteristic curve (ROC) method (with a determination of the sensitivity and specificity of all cut-off values) was applied in resetting an adequate value for the extranodal (EN) involvement number and generating the optimal cut-off point for the ALC, AMC, NLR, and LMR. The distribution of patient characteristics in relation to the ALC and AMC was also evaluated using a polychoric or tetrachoric correlation for categorical variables. Data were assessed using the Kaplan-Meier method, and the log-rank test was utilized to compare survival time between the groups. For the univariate analysis, P < 0.05 was considered statistically significant. Multivariate analysis using the Cox proportional hazards model (including all factors with P < 0.1 from the univariate analysis) was performed to determine the impact of any associated factors. Additionally, the akaike information criterion (AIC) was used to compare the 3 prognostic models of the NLR, LMR, and ALC/AMC PS. All study data were analyzed using the Statistical Package for the Social Sciences (SPSS, IBM PASW Statistics 18, ver 18.0.0, WinWrap Basic, copyright 1993–2007 Polar Engineering and Consulting).
RESULTS
Patient Cohorts and Characteristics
A total of 148 patients with newly diagnosed DLBCL under R-CHOP-like regimens were identified and analyzed in this study. The median observation period (from the day of diagnosis to the final date) for the entire study population was 53.28 months. Clinical data were available for all patients and are summarized in Table 1 . The median age at diagnosis was 61.0 years (range 16–88), and 46.6% of the patients were over 60 years of age at diagnosis. Of the 148 patients, 80 (54.1%) were males and 68 (45.9%) were females. B symptoms were observed in 49 (33.1%) patients. The distributions of the enrolled patients using the Ann Arbor staging system were: stage I, 26 (17.6%) patients; stage II, 34 (23.0%); stage III, 34 (23.0%), and stage IV, 54 (36.5%).
TABLE 1.
Baseline Characteristics of Diffuse Large B-Cell Lymphoma Patients (No. = 148)
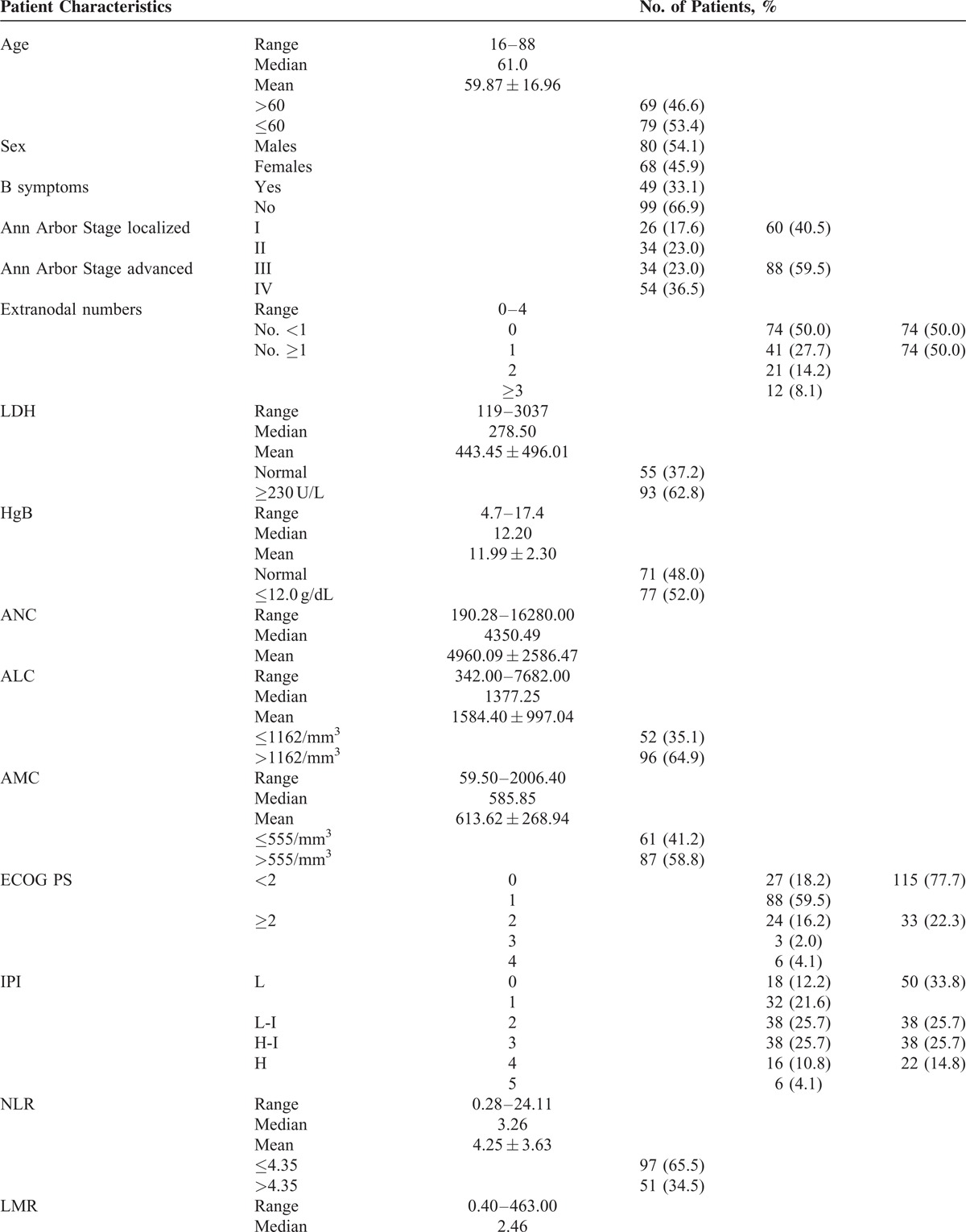
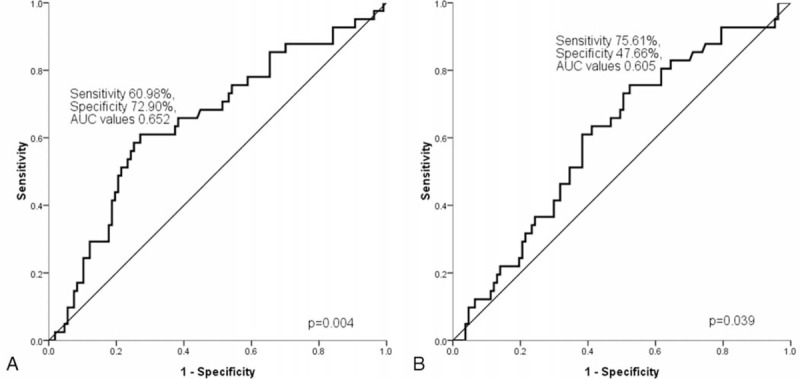
The revised cut-off value for EN involvement was set at ≥1 based on our previous analysis25 (cut-off value: 0.50, sensitivity 70.73% and specificity 57.94%; area under the curve [AUC] value: 0.642, 95% confidence interval [CI] 0.543–0.740, P = 0.008) and previous studies.26,27 There were 74 (50.0%) patients with EN lymphoma. LDH ≥ 230 U/L was detected in 93 (62.8%) patients, and HgB ≤ 12.0 g/dL was recorded in 77 (52.0%) patients. The median ALC was 1377.25/mm3 with a range from 342.00 to 7682.00/mm3. For the ALC, a cut-off of 1162/mm3 was generated according to the ROC analysis in the training set (sensitivity 60.98% and specificity 72.90%; AUC value 0.652, 95% CI 0.551–0.753, P = 0.004) (Figure 1A). Fifty two (35.1%) patients had a low ALC (≤1162/mm3) at diagnosis. The median AMC was 585.85/mm3 with a range from 59.50 to 2006.40/mm3. The cut-off value of the AMC selected using the ROC analysis in this study was 555/mm3 (sensitivity 75.61% and specificity 47.66%; AUC value 0.605, 95% CI 0.521–0.684, P = 0.039) (Figure 1B). There were 87 (58.8%) patients with a high AMC (>555/mm3). An ECOG PS ≥ 2 was identified in 33 (22.3%) patients, and the distribution of patients in relation to the IPI were: low risk, 50 (33.8%); low-intermediate risk, 38 (25.7%); high-intermediate risk, 38 (25.7%); and high risk, 22 (14.8%). The discriminative cut-off value in this study was 4.35 for the NLR (sensitivity 51.22% and specificity 72.90%; AUC values 0.621, 95% CI 0.538–0.699, P = 0.016) (Figure 2A) and 2.11 for the LMR (sensitivity 63.41% and specificity 66.36%; AUC values 0.680, 95% CI 0.598–0.754, P < 0.001) (Figure 2B). Fifty one (34.5%) patients had a high NLR (>4.35), and a low LMR (≤2.11) was noted in 60 (40.5%) patients. The ALC/AMC PS was identified as follows: low-risk, ALC > 1162/mm3 as well as AMC ≤ 555/mm3; intermediate risk, ALC ≤ 1162/mm3 as well as AMC ≤ 555/mm3 or ALC > 1162/mm3 plus AMC > 555/mm3; and high risk, ALC ≤ 1162/mm3 as well as AMC > 555/mm3. Through a simple calculation, the patients were divided into a low risk group, 39 (26.4%); intermediate risk group, 79 (53.4%); and high risk group, 30 (20.3%).
FIGURE 1.
(A) For the ALC, a cut-off point of 1162/mm3 was generated according to the receiver operating characteristic curve (ROC) analysis in the training set (sensitivity 60.98% and specificity 72.90%; AUC values 0.652, 95% CI 0.551–0.753, P = 0.004); (B) The cut-off value for the absolute monocyte count selected via the ROC analysis in this study was 555/mm3 (sensitivity 75.61% and specificity 47.66%; AUC values 0.605, 95% CI 0.521–0.684, P = 0.039). ALC = absolute lymphocyte count, AUC = area under the curve.
FIGURE 2.
(A) The discriminative cut-off value for the NLR was 4.35 (sensitivity 51.22% and specificity 72.90%; AUC values 0.621, 95% CI 0.538–0.699, P = 0.016); and (B) for the LMR was 2.11 (sensitivity 63.41% and specificity 66.36%; AUC values 0.680, 95% CI 0.598–0.754, P < 0.001). AUC = area under the curve, CI = confidence interval, LMR = lymphocyte/monocyte ratio, NLR = neutrophil/lymphocyte ratio.
Over the course of the study, 41 (27.7%) deaths occurred, of which 23 (56.1%) were due to lymphoma progression without other comorbidities, 16 (39.0%) were due to infectious diseases (pneumonia, number [N] = 8 patients; urinary tract infection [UTI], N = 3; intra-abdominal infection, N = 2; hepatitis B reactivation, N = 2; viremia due to cytomegalovirus, N = 1), 1 (2.4%) secondary to cardiac dysfunction; and 1 (2.4%) due to severe gastrointestinal (GI) tract bleeding.
The Distribution of Patients in Relation to ALC and AMC
The descriptive characteristics and distribution of patients in relation to ALC and AMC are shown in Tables 2 and 3.
TABLE 1 (Continued).
Baseline Characteristics of Diffuse Large B-Cell Lymphoma Patients (No. = 148)
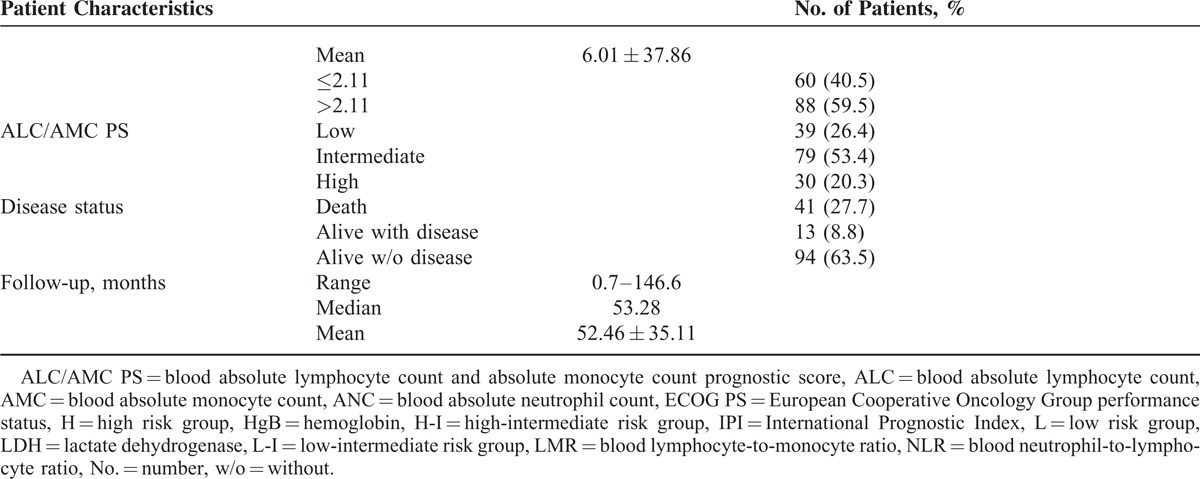
TABLE 2.
Descriptive Characteristics and Distribution of Patients in Relation to ALC
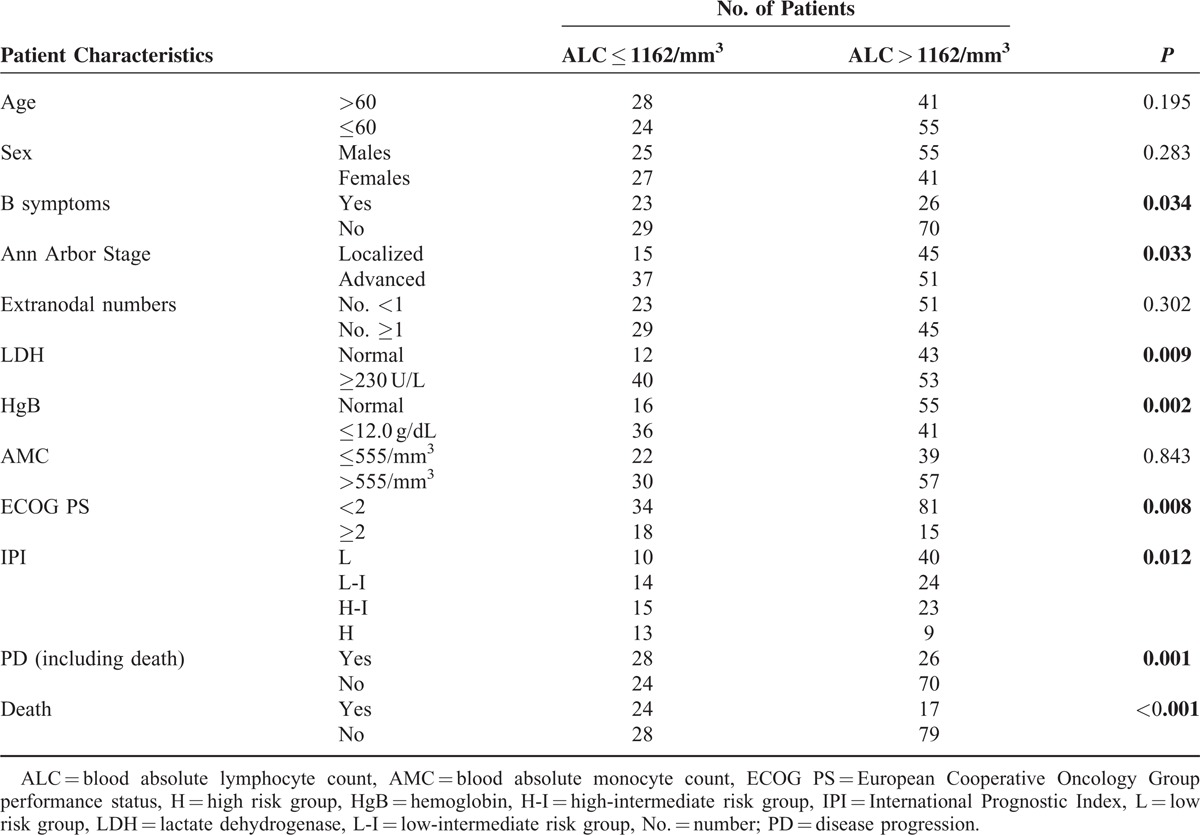
A significant difference was found between the patients with ALC values ≤1162/mm3 and those with ALC values >1162/mm3 in relation to B symptoms (P = 0.034), the Ann Arbor localized/advanced stage (P = 0.033), LDH (P = 0.009), HgB (P = 0.002), ECOG PS (P = 0.008), and IPI risk groups (P = 0.012). The correlation coefficients between the ALC and HgB (ρ = −0.253, P = 0.002) as well as the ALC and IPI risk groups (ρ = −0.260, P = 0.001) were of a fair degree. Additionally, the ALC was weakly correlated with B symptoms (ρ = −0.174, P = 0.034), localized/advanced stage (ρ = −0.175, P = 0.033), LDH (ρ = −0.215, P = 0.009), and the ECOG PS (ρ = −0.218, P = 0.008).
In contrast, there was a significant difference between the patients with AMC values ≤555/mm3 and those with AMC values >555/mm3 in relation to sex (P = 0.043), B symptoms (P = 0.004), localized/advanced stage (P < 0.001), ECOG PS (P = 0.025), and the IPI risk groups (P = 0.035). AMC was fairly correlated with localized/advanced stage (ρ = 0.315, P < 0.001), and weakly correlated with sex (ρ = 0.166, P = 0.044), B symptoms (ρ = 0.239, P = 0.003), ECOG PS (ρ = 0.185, P = 0.025), and the IPI risk groups (ρ = 0.234, P = 0.004).
Analysis of Outcomes
The estimated 5-year progression-free survival (PFS) of the 148 patients was 64.2%, and the 5-year OS was 71.4% (Figure 3). In the univariate analysis, all of the other clinical factors with the exception of sex (PFS, P = 0.993; OS, P = 0.798) were significantly associated with PFS and OS, including age (PFS, P = 0.046; OS, P = 0.003), B symptoms (PFS, P = 0.016; OS, P = 0.010), localized/advanced stage (PFS, P = 0.002; OS, P = 0.004), EN number (PFS, P = 0.003; OS, P = 0.004), LDH (PFS, P = 0.003; OS, P = 0.013), HgB (PFS, P = 0.050; OS, P = 0.018), ECOG PS (PFS, P < 0.001; OS, P < 0.001), ALC (PFS, P = 0.001; OS, P < 0.001), AMC (PFS, P = 0.007; OS, P = 0.012), and the IPI risk groups (PFS, P < 0.001; OS, P < 0.001). The prognostic models derived from the ALC and AMC (including the NLR [Figure 4A and B], LMR [Figure 5A and B], and ALC/AMC PS [Figure 6A and B]) were also significantly related to 5-year PFS and OS (NLR: PFS, P = 0.001; OS, P = 0.007. LMR: PFS, P = 0.003; OS, P = 0.05. ALC/AMC PS: PFS, P < 0.001; OS, P < 0.001) (Table 4). All of the significant parameters in the univariate analysis were included in the multivariate analysis. An advanced analysis was conducted separately with the ALC plus AMC, NLR, LMR, and ALC/AMC PS (Tables 5–8). The results showed that both the ALC (PFS, P = 0.038; OS, P = 0.017) and AMC (PFS, P = 0.028; OS, P = 0.026) are independent risk factors in patients with DLBCL under R-CHOP-like chemotherapy. The ALC/AMC PS was also the most reliable tool to predict outcome in DLBCLs (PFS, P = 0.023; OS, P = 0.017). However, in the separate multivariate analysis, the NLR and LMR lost their predictive capacities and did not reach statistical significance (NLR: PFS, P = 0.053; OS, P = 0.159. LMR: PFS, P = 0.281; OS, P = 0.242). The AIC was 388.773 in NLR, 387.625 in LMR, and 372.574 in ALC/AMC PS.
FIGURE 3.
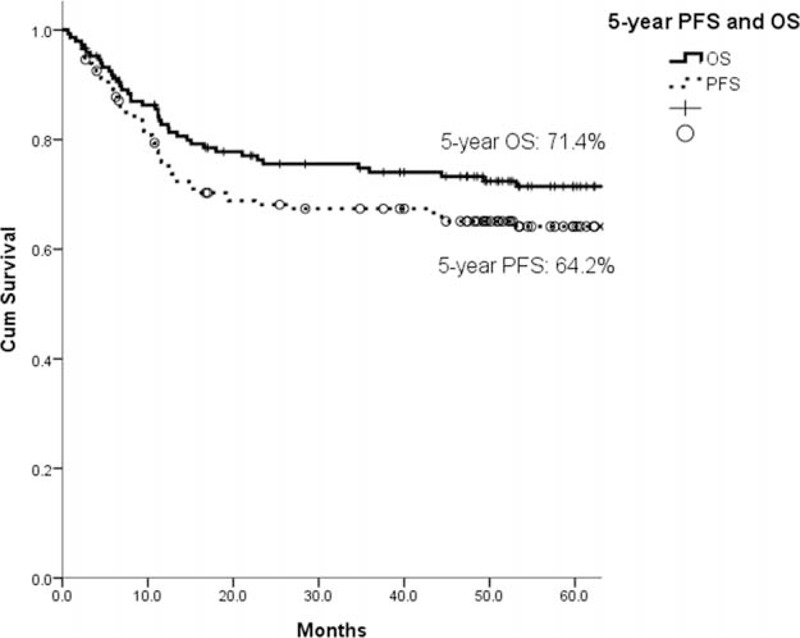
The estimated 5-year PFS of the 148 total patients was 64.2%, and the 5-year OS of these patients was 71.4%. OS = overall survival, PFS = progression-free survival.
FIGURE 4.
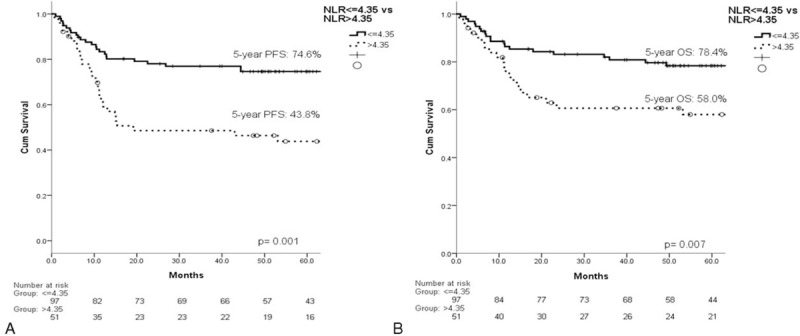
The NLR was significantly related to the (A) 5-year PFS (P = 0.001) and (B) OS (P = 0.007). NLR = neutrophil/lymphocyte ratio, PFS = progression-free survival.
FIGURE 5.
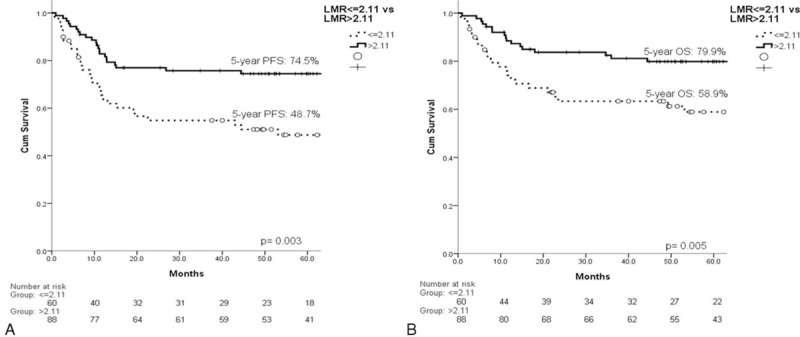
The LMR was significantly related to the (A) 5-year PFS (P = 0.003) and (B) OS (P = 0.005). LMR = lymphocyte/monocyte ratio, PFS = progression-free survival.
FIGURE 6.
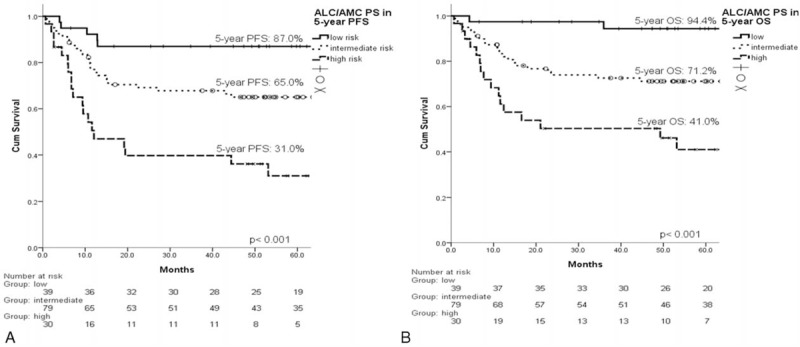
The ALC/AMC PS was significantly related to the (A) 5-year PFS (P < 0.001) and (B) OS (P < 0.001). ALC/AMC PS = absolute lymphocyte count/absolute monocyte count prognostic score, OS = overall survival, PFS = progression-free survival.
TABLE 3.
Descriptive Characteristics and Distribution of Patients in Relation to AMC
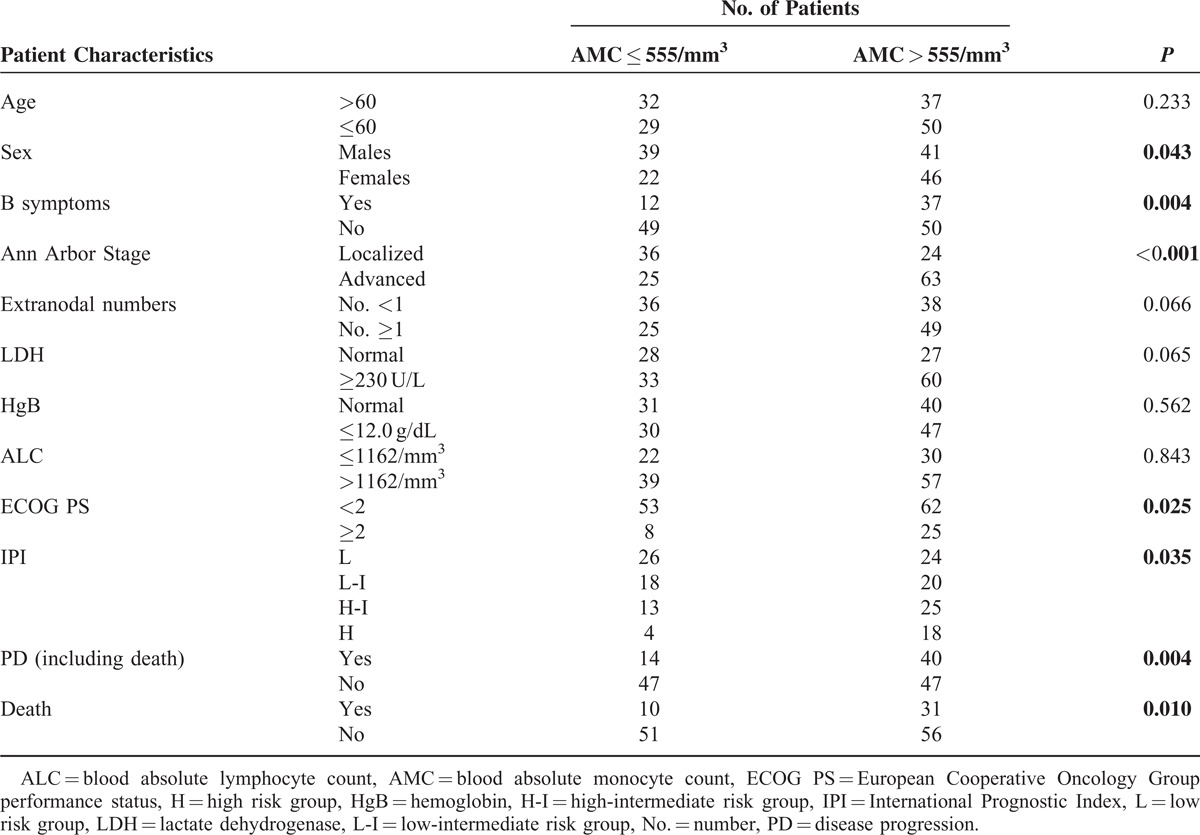
TABLE 4.
Univariate Cox Regression Analysis of 5-Year Progression-Free Survival and Overall Survival in Relation to Patient Characteristics
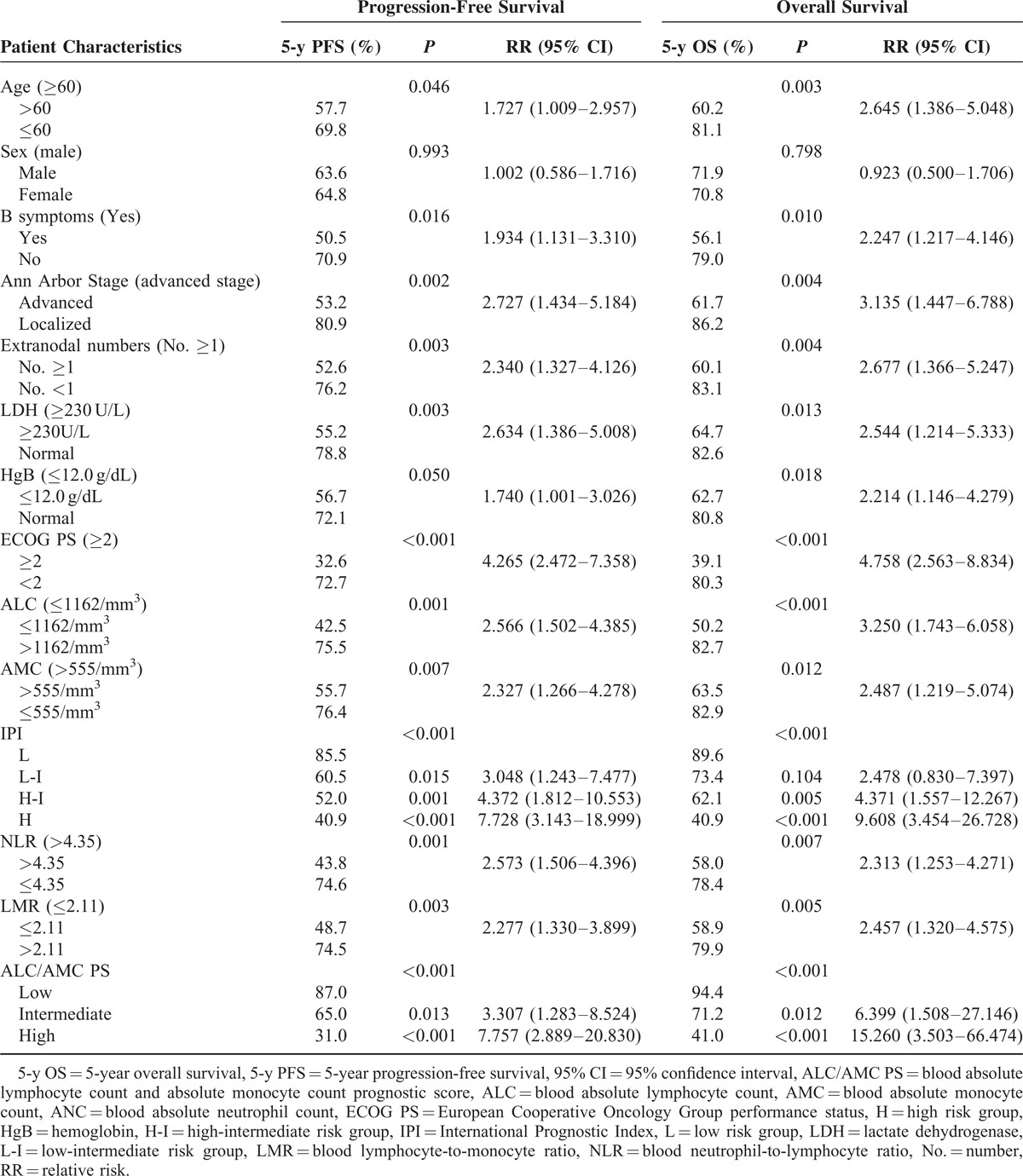
TABLE 7.
Multivariate Cox Regression Analysis of 5-Year Progression-Free Survival and Overall Survival in Relation to LMR
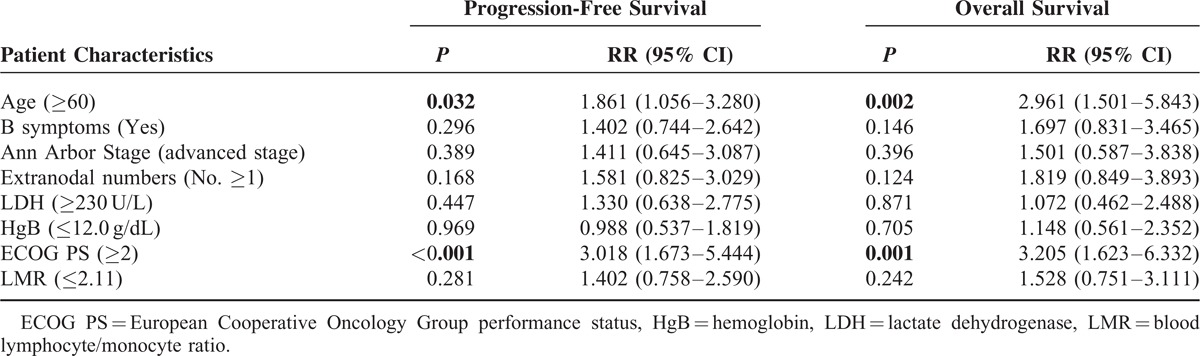
TABLE 5.
Multivariate Cox Regression Analysis of 5-Year Progression-Free Survival and Overall Survival in Relation to ALC and AMC
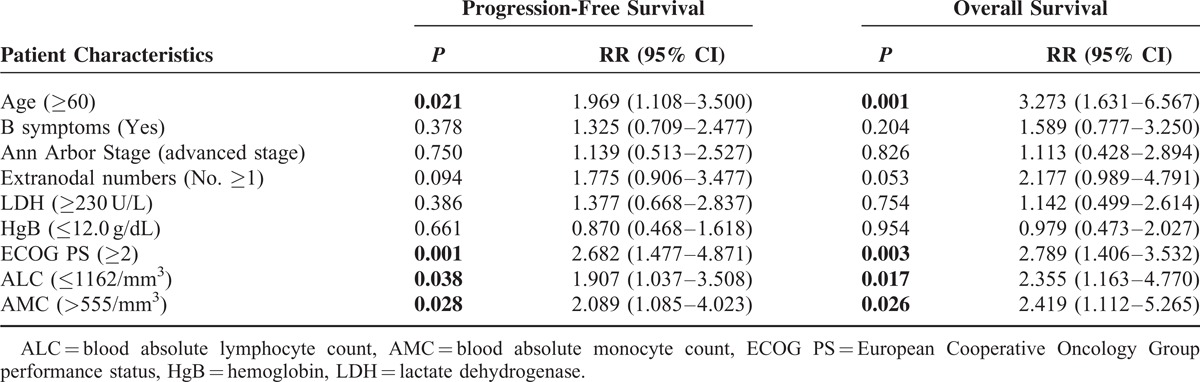
TABLE 6.
Multivariate Cox Regression Analysis of 5-Year Progression-Free Survival and Overall Survival in Relation to NLR
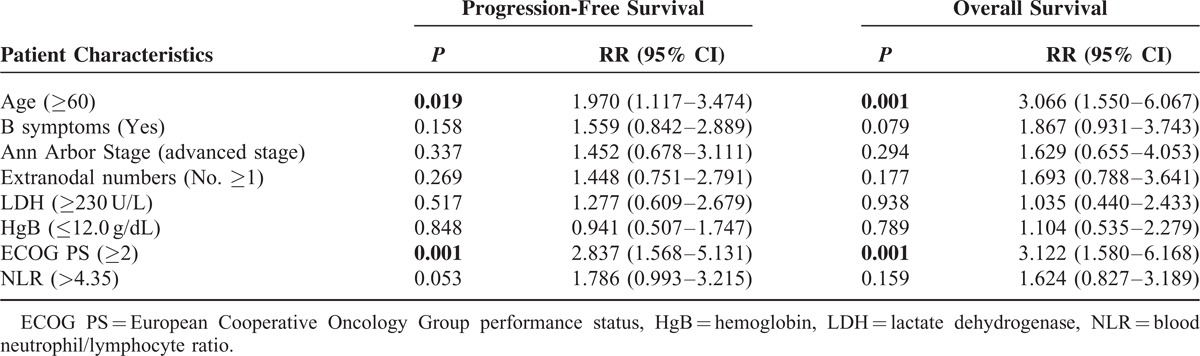
TABLE 8.
Multivariate Cox Regression Analysis of 5-Year Progression-Free Survival and Overall Survival in Relation to ALC/AMC PS
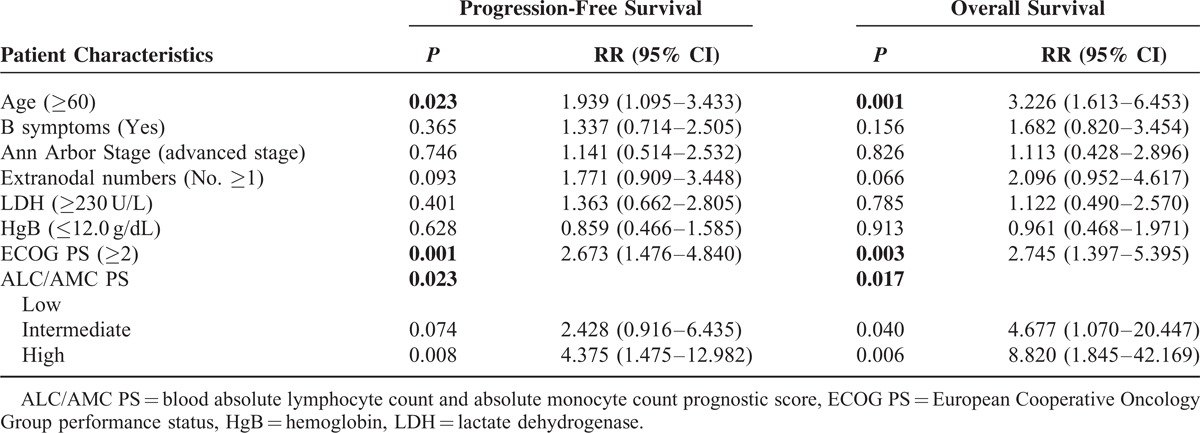
DISCUSSION
Prognostic factors in cancer patients provide information regarding possible clinical outcomes and help in the classification of patients into different risk groups. The IPI, which is based on 5 clinical factors, is a simple prognostic model for aggressive non-Hodgkin lymphoma and has been widely used for more than 20 years. However, the addition of R to conventional chemotherapy for DLBCLs has resulted in a dramatic improvement in survival across all risk groups, and the predictive capacity of the IPI has gradually declined. Advanced techniques have provided crucial information regarding several new prognostic parameters; however, these are expensive, technically challenging, and are not standardized for clinical practice. Some studies have recently reported using the ALC, AMC, NLR, LMR, and ALC/AMC PS as simple tools for predicting outcome for DLBCLs. We validated these tools in our patients and confirmed their prognostic significance.
In our study, the ALC and AMC respectively had low or no correlation with the other clinical parameters but reached statistical significance in the survival analysis. These findings suggested that the ALC and AMC were independent factors for DLBCLs outcomes. The patients in our cohort with an ALC ≤1162/mm3 or AMC >555/mm3 had a poor outcome.
The rationale for the NLR is to compare the host's inflammatory response (neutrophils) to cancer with the host's immune response (lymphocytes). Recently, the NLR at diagnosis was shown to be a prognostic factor for patients with DLBCL undergoing R-chemotherapy.20 Consistent with this finding, we also confirmed the predictive capacity of the NLR in our univariate analysis; however, no statistical significance was found in the subsequent multivariate analysis. This result in the multivariate analysis may be associated with prognostic factors of tumor burden such as the stage and LDH, in addition to biomarkers of inflammation such as B symptoms. Furthermore, the cut-off value of the absolute neutrophil count, which was determined using the ROC method, was not statistically significant (P = 0.952), and the impact of the NLR may have therefore resulted only from the ALC. Hence, the NLR should be applied more carefully and the interaction with other clinical factors such as the stage, LDH, and B symptoms should also be taken into account.
LMR at diagnosis is a simple tool that assesses the host's immune homeostasis and the tumor microenvironment. LMR at diagnosis was recently shown to be an independent prognostic indicator in DLBCL.21,22 In our study, LMR played a significant role in predicting the outcome; however, this was not statistically significant in the multivariate analysis. There may have been interactions with other parameters. Although there was a low degree of correlation between the ALC (or AMC) and the other factors, the synergistic effects may have amplified the associated interactions. Moreover, the LMR always represents the immune homeostasis of the host; however, extreme differences in the distribution of blood cells are usually present when the patients experience leukocytosis or leukopenia. Thus, the problems may affect the predictive capacity of the LMR. Further studies are necessary to clarify these issues.
The ALC/AMC PS is generated by combining the dichotomized ALC and AMC. The tool retains the predictive capacity of the ALC or AMC, reflects the immune status of the host, and divides patients into 3 risk groups. Consistent with previous reports, the ALC/AMC PS was a useful tool in our study, and there was less interaction with other parameters in the multivariate analysis. Therefore, the AMC/ALC PS may provide additional prognostic information when used in conjunction with the IPI.
In our AIC analysis, the ALC/AMC PS had a lower score and appeared to be a better prognostic tool than the NLR or LMR. However, the ideal cut-off points of the ALC and AMC require further investigation. The ALC/AMC PS also needs to be validated in other patient groups.
This study had some limitations. First, the correlation of age and circulating white blood cell (WBC) counts has been reported in human blood samples,28,29 and the cut-off values for the ALC and AMC may differ according to the age of the patient. This problem may cause deviation when the NLR, LMR, and ALC/AMC PS are applied in the survival analysis. Second, we did not analyze the relationships between the WBC counts and the duration of therapy regimens, the different treatment circles of R-CHOP-like regimens, or even second-line salvage therapies. The major causes were relative too small sample sizes in different study groups, and the therapeutic regimens could be revised based on different performance status, like R-CHOP would be revised to R-mini-CEOP (cyclophosphamide, epirubicin, vinblastine, and prednisone) in a patient with poor performance status or cardiac dysfunction. The variation in WBC counts at diagnosis may also affect therapeutic strategy decision-making by both providers and patients. Additionally, an adequate number of lymphocytes (including natural killer [NK] cells) may be required for the rituximab-mediated cytotoxicity-dependent destruction of malignant B cells,30 and lymphopenia is an adverse prognostic factor in DLBCLs. Thus, further studies are warranted to investigate the relationship between these simple prognostic tools and therapeutic strategies. Finally, our study was a retrospective, nonrandomized analysis, and only included small patient numbers in a single institution. Nevertheless, we found these interpreted tools to be of clinical value. The NLR, LMR, and ALC/AMC PS are low-cost and easily obtainable prognostic tools. The ALC/AMC PS appears to be more reliable than the NLR or LMR, and this parameter may provide additional prognostic information when used in conjunction with the IPI.
CONCLUSION
In summary, the NLR, LMR, and ALC/AMC PS are inexpensive tools that are useful for predicting the outcome in DLBCL patients undergoing R-chemotherapy. The ALC/AMC PS, which has less interaction with other clinical parameters, may improve the insufficient IPI prognostic model and provide an additional ability to identify high-risk patients. However, there were no ideal cut-off values in the prognostic analysis for the ALC and AMC. Therefore, the ideal parameters for the ALC and AMC warrant further discussion prior to wider use in DLBCL patients. Further validation of the NLR, LMR, and ALC/AMC PS in other groups is encouraged, and studies encompassing a larger patient population are also needed to investigate the highest predictive values of these simple tools in this era of targeted therapy.
Acknowledgments
The authors thank the Cancer Registry Group, Tri-Service General Hospital, Taipei, Taiwan for kindly collecting the patient information.
Footnotes
Abbreviations: AIC = akaike information criterion, ALC = absolute lymphocyte count, ALC/AMC PS = absolute lymphocyte count/absolute monocyte count prognostic score, AMC = absolute monocyte count, CIs = confidence intervals, DLBCL = diffuse large B-cell lymphoma, ECOG PS = Eastern Cooperative Oncology Group performance status, IPI = International Prognostic Index, LMR = lymphocyte/monocyte ratio, NLR = neutrophil/lymphocyte ratio, OS = overall survival, PFS = progression-free survival.
C-LH designed the research study, wrote and edited the paper. C-SL performed data analysis and wrote the first draft. J-HC, Y-GC, T-CH, and Y-YW integrated clinical data and updated the database.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005; 23:4117–4126. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7:379–391. [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007; 109:1857–1861. [DOI] [PubMed] [Google Scholar]
- 4.Tarella C, Zanni M, Di Nicola M, et al. Prolonged survival in poor-risk diffuse large B-cell lymphoma following front-line treatment with rituximabsupplemented, early-intensified chemotherapy with multiple autologous hematopoietic stem cell support: a multicenter study by GITIL (Gruppo Italiano Terapie Innovative nei Linfomi). Leukemia 2007; 21:1802–1811. [DOI] [PubMed] [Google Scholar]
- 5.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329:987–994. [DOI] [PubMed] [Google Scholar]
- 6.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse largeB-cell lymphoma identified by gene expression profiling. Nature 2000; 403:503–511. [DOI] [PubMed] [Google Scholar]
- 7.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2012; 120:3986–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood 2007; 109:4930–4935. [DOI] [PubMed] [Google Scholar]
- 9.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, et al. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol 2009; 22:1094–1101. [DOI] [PubMed] [Google Scholar]
- 10.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103:275–282. [DOI] [PubMed] [Google Scholar]
- 11.Tzankov A, Meier C, Hirschmann P, et al. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica 2008; 93:193–200. [DOI] [PubMed] [Google Scholar]
- 12.Mikhaeel NG. Interim fluorodeoxyglucose positron emission tomography for early response assessment in diffuse large B cell lymphoma: where are we now? Leuk Lymphoma 2009; 50:1931–1936. [DOI] [PubMed] [Google Scholar]
- 13.Duhrsen U, Huttmann A, Jockel KH, et al. Positron emission tomography guided therapy of aggressive non-Hodgkin lymphomas Fthe PETAL trial. Leuk Lymphoma 2009; 50:1757–1760. [DOI] [PubMed] [Google Scholar]
- 14.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 15.Moore MM, Chua W, Charles KA, et al. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther 2010; 87:504–508. [DOI] [PubMed] [Google Scholar]
- 16.Cox MC, Nofroni I, Laverde G, et al. Absolute lymphocyte count is a prognostic factor for diffuse large B-cell lymphoma. Br J Haematol 2008; 191:265–268. [DOI] [PubMed] [Google Scholar]
- 17.Cox MC, Nofroni I, Rucco I, et al. Low absolute lymphocyte count is a poor prognostic factor in diffuse large B-cell lymphoma. Leuk Lymphoma 2008; 49:745–751. [DOI] [PubMed] [Google Scholar]
- 18.Oki Y, Yamamoto K, Kato H, et al. Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients survival benefit from rituximab. Eur J Haematol 2008; 81:448–453. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia 2011; 25:1502–1509. [DOI] [PubMed] [Google Scholar]
- 20.Porrata LF, Ristow K, Habermann T, et al. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol 2010; 85:896–899. [DOI] [PubMed] [Google Scholar]
- 21.Rambaldi A, Boschini C, Gritti G, et al. The lymphocyte to monocyte ratio improves the IPI-risk definition of diffuse large B-cell lymphoma when rituximab is added to chemotherapy. Am J Hematol 2013; 88:1062–1067. [DOI] [PubMed] [Google Scholar]
- 22.Li ZM, Huang JJ, Xia Y, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One 2012; 7:e41658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC; 2008. [Google Scholar]
- 24.Zelenetz AD, Abramson JS, Advani RH, et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin's lymphomas. J Natl Compr Canc Netw 2010; 8:288–334. [DOI] [PubMed] [Google Scholar]
- 25.Lu CS, Chen JH, Huang TC, et al. Diffuse large B-cell lymphoma: the sites of extranodal involvement are the stronger predictor than the number of extranodal sites in the Rituximab era. Leuk Lymphoma 2015. 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Hui D, Proctor B, Donaldson J, et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma 2010; 51:1658–1667. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Zhou, Laurie H, Sehn, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014; 123:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Jauanen E, Strickland RG, Williams RC. Studies of human lymphocytes in the newborn and the aged. Am J Med 1975; 58:620–628. [DOI] [PubMed] [Google Scholar]
- 29.Wiener D, Shah S, Malone J, et al. Multiparametric analysis of peripheral blood in the normal pediatric population by flow cytometry. J Clin Lab Anal 1990; 4:175–179. [DOI] [PubMed] [Google Scholar]
- 30.Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010; 47:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]


