Abstract
The aim of this study was to investigate the evolution of sternocleidomastoid muscle (SCM) atrophy in nasopharyngeal carcinoma (NPC) patients following intensity-modulated radiotherapy (IMRT), and the relationship between SCM atrophy and neck weakness.
Data were retrospectively analyzed from 223 biopsy-proven NPC patients with no distant metastasis who underwent IMRT with or without chemotherapy. The volume of SCM was measured on pretreatment magnetic resonance imaging (MRI), and MRIs were conducted 1, 2, and 3 years after the completion of IMRT. Change in SCM volume was calculated and classified using the late effects of normal tissues–subjective, objective, management, and analytic system. The grade of neck muscle weakness, classified by the Common Terminology Criteria for Adverse Events V 3.0, was measured 3 years after the completion of IMRT.
The average SCM atrophy ratio was −10.97%, −18.65%, and −22.25% at 1, 2, and 3 years postirradiation, respectively. Multivariate analysis indicated N stage and the length of time after IMRT were independent prognostic variables. There were significant associations between the degree of SCM atrophy and neck weakness.
Radical IMRT can cause significant SCM atrophy in NPC patients. A more advanced N stage was associated with more severe SCM atrophy, but no difference was observed between N2 and N3. SCM atrophy progresses over time during the 3 years following IMRT. Grade of SCM atrophy is significantly associated with neck weakness.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is endemic in Southern part of China with annual incidence of 15 to 50 cases per 100,000 population.1 Radiotherapy is the primary therapeutic modality for NPC patients with no distant metastasis. Owing to a high nodal metastasis rate in NPC, most treatment protocols require irradiation of the entire neck lymphatic drainage area to the supraclavicular fossa,2 which may cause muscle atrophy. Although it does not present as prominently as other late toxicities, muscle atrophy is of particular concern because of its clinical presentation, irreversible nature, and complexity. Inconsistent with the traditional concept that muscle tissue is insensitive to conventional fractionated radiotherapy,3,4 much recent research documents that radiotherapy can cause significant muscular atrophy.5–7
In recent years, intensity-modulated radiotherapy (IMRT) has gradually become the most important means of radiotherapy.8 Our own clinical experiences showed that many NPC patients undergoing IMRT have required clinical follow-up for neck muscle atrophy, injury, or weakness, particularly affecting the sternocleidomastoid muscle (SCM). As the major superficial cervical flexor muscle, SCM is one of the prime movers of the neck,9 making the SCM atrophy typical for radiation-induced neck muscle atrophy in NPC patients. Acquiring information on incidence and severity of SCM atrophy after IMRT is important for improving understanding and predicting the development of neck weakness.
Muscle morphology can be quantified using medical imaging methods such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound, by assessment of the muscular cross-sectional area or volume.5,10 MRI provides higher resolution for soft tissue and is nonionizing compared with CT, and, subsequently, MRI is increasingly used in studies concerning human muscle.10–12
This retrospective study was conducted to investigate the evolution of radiation-induced SCM muscle atrophy and neck weakness in NPC patients treated with IMRT, and to explore their risk factors, therefore providing clinical clues for reducing the incidence of muscle atrophy and weakness.
MATERIALS AND METHODS
Patient Selection and Staging Evaluation
This retrospective cohort study was approved by the ethics committee. Informed consents were also obtained from subjects. In total, 945 newly diagnosed NPC patients with no distant metastasis that were consecutively diagnosed and had completed treatment using IMRT with or without chemotherapy between January 2010 and January 2012 were recruited. Among these 945 patients, the time interval between the completion of the IMRT and this study ranged from 36 to 61 months. The selection criteria were as follows: the patient had a pretreatment MRI scan and regular follow-up MRI scans performed 1, 2, and 3 years after the completion of radiotherapy and confirming no NPC recurrence; patients returned to the hospital for follow-up appointments for 3 years after the completion of radiotherapy. Patients were excluded if they had any condition that may result in muscle atrophy and affect the measurement of radiation-induced muscle atrophy,13,14 including connective tissue disorders, hypertension, or diabetes mellitus, or if they had a history of neck surgery. Neck surgery was defined as any type of surgery with the potential to damage the SCM, such as neck dissection. In total, 223 cases met these requirements and were recruited. The following parameters of clinical characteristics and patient treatments were collected to analyze their effect on the muscle atrophy ratio: age, sex, T stage, N stage, the weight change ratio, the presence or absence of chemotherapy, and the length of time after IMRT.
All patients selected had undergone pretreatment baseline evaluations that included complete medical history, thorough physical examinations, hematology and biochemistry testing, MRI of the neck and nasopharynx, chest radiography, abdominal sonography, and bone scans using single-photon emission CT. All patients had previously been staged before they were treated according to the Seventh Edition of the International Union against Cancer/American Joint Committee on Cancer system for NPC.15
Investigation and Treatment Procedures
Radiotherapy and Chemotherapy
All patients in this study were immobilized in the supine position using a thermoplastic head–neck–shoulder mask. For the purpose of treatment planning, 3 mm slices of contrast-enhanced CT imagings from head to 2 cm below the sternoclavicular joint were obtained. The prescribed dose was defined as follows: a total dose of 68 to 70 Gy/30 to 33 fractions and 60 to 68 Gy/30 to 33 fractions to the planning target volume (PTV) of gross tumor volume of the primary (GTV-P) and nodal gross tumor volume (GTV-N), respectively, and the total prescribed dose to PTV of clinical target volume (CTV)-1 (high-risk region), CTV-2 (low-risk region), and CTV-N (neck nodal regions) was 60, 54, and 54 Gy, respectively. All patients were treated with 1 fraction every day >5 d/wk.
In the study period, the chemotherapy guidelines in our institutional protocol for NPC were implemented as follows: no chemotherapy for stage I, and concurrent chemoradiotherapy ± neoadjuvant chemotherapy for stages II to IV A–C. Neoadjuvant cisplatin combined with taxanes or cisplatin combined with 5-fluorouracil chemotherapy was given every 2 or 3 weeks as a cycle for 1 to 4 cycles. Concurrent cisplatin chemotherapy was given weekly or on weeks 1, 4, and 7 of radiotherapy. Patients with documented residual or relapse disease received treatments, including chemotherapy, neck dissection, and brachytherapy.
MRI Technique
Each subject underwent 1.5-Tesla (Signa CV/i; General Electric Healthcare, Chalfont St. Giles, UK) MRI. The region from the suprasellar cistern to the inferior margin of the sternal end of the clavicle was examined. For the axial plane, the section thickness was 5 mm with a 1 mm interslice spacing; and for the coronal and sagittal planes, the section thickness was 6 mm with a 1 mm interslice spacing. T1-weighted images in the axial, sagittal, and coronal planes, and T2-weighted images in the axial plane were obtained before injection of contrast material. Contrast-enhanced T1-weighted images in the axial and sagittal planes were obtained after injection of contrast material.
Muscle Volume Measurement
Measurements of muscle volume were performed after we selected the cohort of 223. Owing to a discrepancy of the muscle atrophy ratio between the left and right SCM in NPC patients, both sides were examined for this study. The pretreatment MRI and the MRIs taken 1, 2, and 3 years after radiotherapy completion were examined for each study subject. The MRI scanning images were conveyed to Advantage Workstation 4.4 (General Electric Company, Fairfield, CT). T2-weighted axial images were selected, and the outline of the SCM muscle was traced on each axial image manually, beginning at manubrium and collarbone and ending at mastoid. Muscle volume was calculated by volume rendering. Two observers (1 radiologist and 1 other clinician), trained for using the software and delineating the SCM muscles, contoured each muscle independently. The average of the 2 measurements was defined as the final muscular volume.
The grading of muscle atrophy was classified using the Late Effects of Normal Tissues–Subjective, Objective, Management, and Analytic) system as follows: Grade 0: no atrophy; Grade 1: −10% ≤ SCM atrophy ratio <0; Grade 2: −20% ≤ SCM atrophy ratio <−10%; Grade 3:−50% ≤ SCM atrophy ratio <−20%; and Grade 4: SCM atrophy ratio <−50%.16 In order to calculate the amount of contrast material, patient's weight was measured and recorded. The weight at pretreatment 1, 2, and 3 years after radiotherapy completion was used.
Follow-Up
As part of our routine clinical practice, we documented all patients who complained of neck weakness 3 years after radiation therapy. Neck weakness is graded according to the Common Terminology Criteria for Adverse Events V3.0 as follows: Grade 0: none; Grade 1 (mild): asymptomatic, weakness on physical examination; Grade 2 (moderate): symptomatic and interfering with function, but not interfering with activities of daily living; Grade 3 (severe): symptomatic and interfering with activities of daily living; Grade 4: life-threatening, disabling; and Grade 5: death. We retrospectively reviewed these records to collect the required data. Patients returned to the hospital for follow-up examination at least every 3 months over the first 24 months, and thereafter, at least every 6 months until death. All patients underwent follow-up MRI examinations of the nasopharynx and neck at least every 6 to 12 months. The grading of neck weakness was recorded when the patient returned for follow-up appointments, 3 years after the completion of radiotherapy. The follow-up of this cohort of patients was therefore 36 months.
Statistical Analysis
The data were analyzed using SAS statistical software version 9.1 (SAS Institute Inc, Cary, NC). Calculations were based on the average value measured by 2 observers. The ratio of atrophy was calculated using the following formulae:
SCM atrophy ratio (%) = [(volume of 1/2/3 years − volume of pretreatment)/volume of pretreatment] × 100.
Weight change ratio (%) = [(weight value of 1/2/3 years − weight value of pretreatment)/weight value of pretreatment] × 100.
Univariate and multivariate analysis was carried out using generalized linear mixed effects model.17 Spearman rank correlation analysis18 was used to calculate the ratio of muscle atrophy and the degree of SCM weakness. We further used the repeated-measures single factor analysis of variance and Bonferroni statistical tests to compare the SCM atrophy ratio of all N stages with each other.19,20 A P value of 0.05 was chosen as the criteria for statistical significance.
RESULTS
Clinical Characteristics of the Selected Cohort
Of the 223 selected patients, the ratio of male to female was 2.67:1 (162 men and 61 women), and the median age was 42 years (range: 14–71 years). Histological analysis showed that 0.9% of cases were classified as World Health Organization (WHO) Grade I and 99.1% were WHO Grade II/III. Out of 176 (stage III and IV), 165 had received chemotherapy. The characteristics of the 223 cases are shown in Table 1.
TABLE 1.
Clinical Characteristics and Treatment Parameters of the Study Cohort
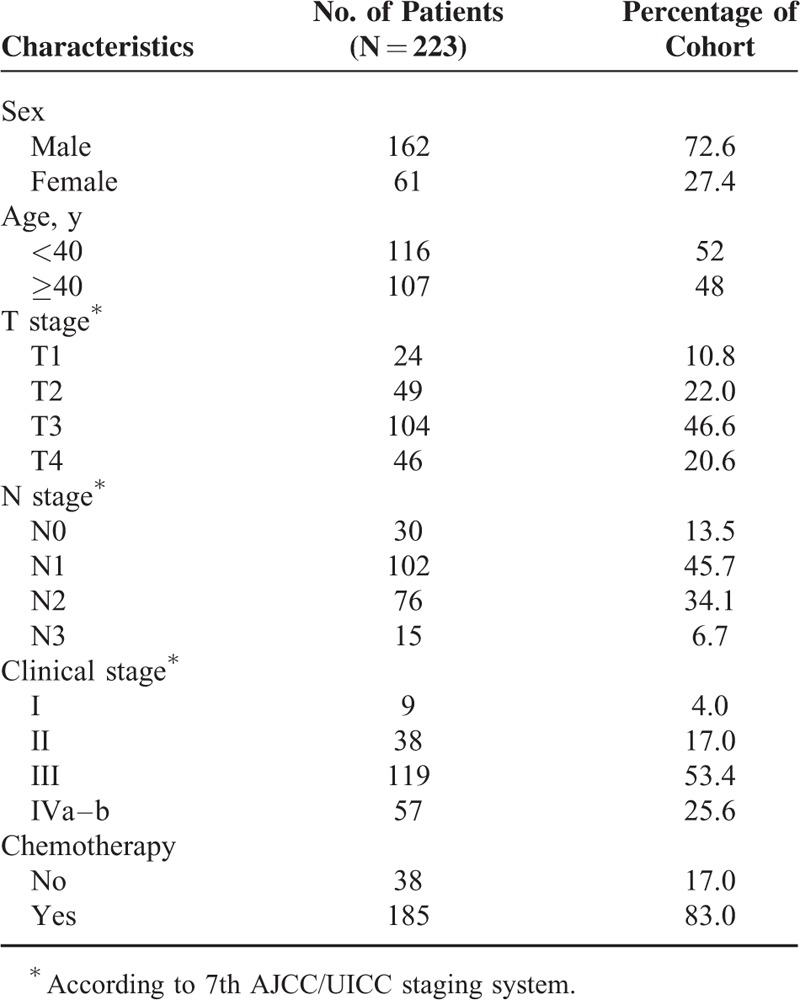
Incidence and Severity of Muscle Atrophy
The average ratios of muscle atrophy at 1, 2, and 3 years postirradiation were −10.97% (95% confidence interval [CI]: −11.89% to −10.06%), −18.65% (95% CI: −19.57 to −17.73%), −22.25% (95% CI: −23.31% to −21.19%), respectively. Initially after IMRT, the degree of SCM atrophy increases at a considerable rate; later, the rate slows down (Figure 1). The ratio of SCM atrophy for each category of N stage and T stage at 1, 2, and 3 years postirradiation is shown in Table 2.
FIGURE 1.
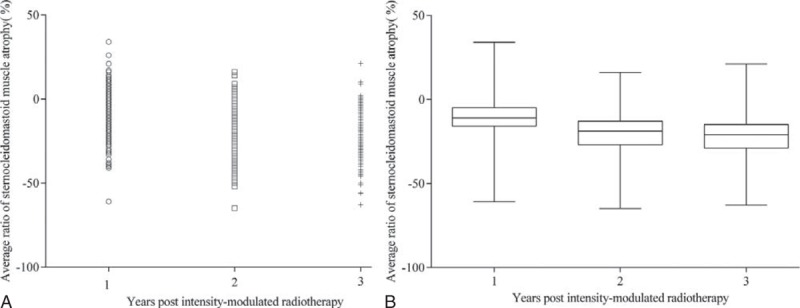
Dot density plot (A) and box and whisker plot (B) depicting the evolution of intensity-modulated radiotherapy-induced sternocleidomastoid muscle atrophy ratio in 223 nasopharyngeal carcinoma patients during the 3 years following intensity-modulated radiotherapy. In plot (B), the boxes, horizontal lines located in the box, and whiskers represent the 25th to 75th percentiles, median values, and the maximum and minimum sternocleidomastoid muscle atrophy ratio, respectively.
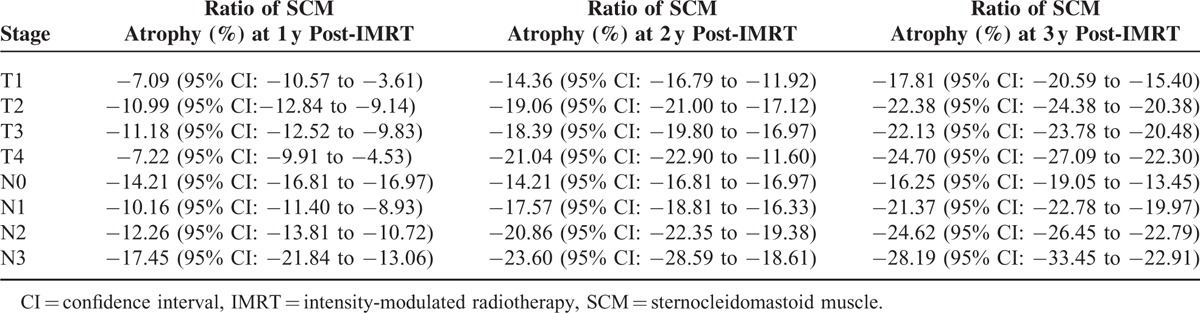
TABLE 2.
Ratio of Sternocleidomastoid Atrophy for Each Category of T Stage and N Stage
For grade of SCM atrophy from 1 to 3 years after treatment, the overall percentage of subjects scoring Grade 0 to 2 decreased from 85.6% to 42.6%, whereas the overall percentage scoring Grade 3 and Grade 4 increased from 14.3% to 57.4% (Table 3). The higher the N stage, the more severe the SCM atrophy (Figure 2).
TABLE 3.
Degree of Sternocleidomastoid Atrophy of Study Cohort After Intensity-Modulated Radiotherapy
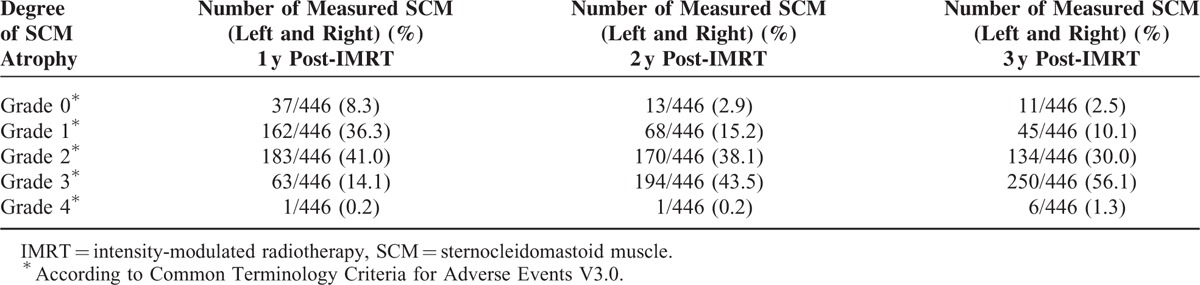
FIGURE 2.
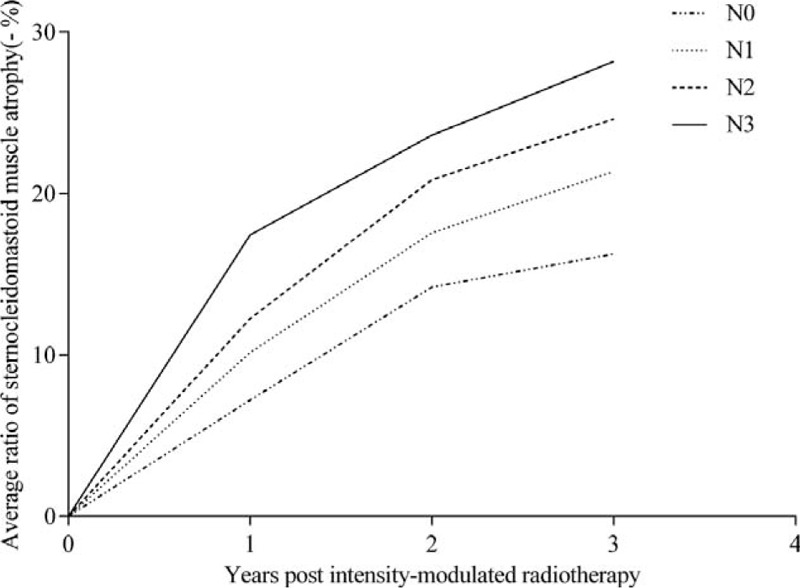
Time line chart depicting N staging variation and the evolution of intensity-modulated radiotherapy-induced sternocleidomastoid muscle atrophy in nasopharyngeal carcinoma patients during the 3 years following intensity-modulated radiotherapy.
The difference in SCM atrophy ratio for each N stage is significant between N0 and N1 (P = 0.025), N0 and N2 (P = 0.000), N0 and N3 (P = 0.000), N1 and N2 (P = 0.018), N1 and N3 (P = 0.001), but insignificant between N2 and N3 (P = 0.203).
Variables Affecting the SCM Atrophy Ratio
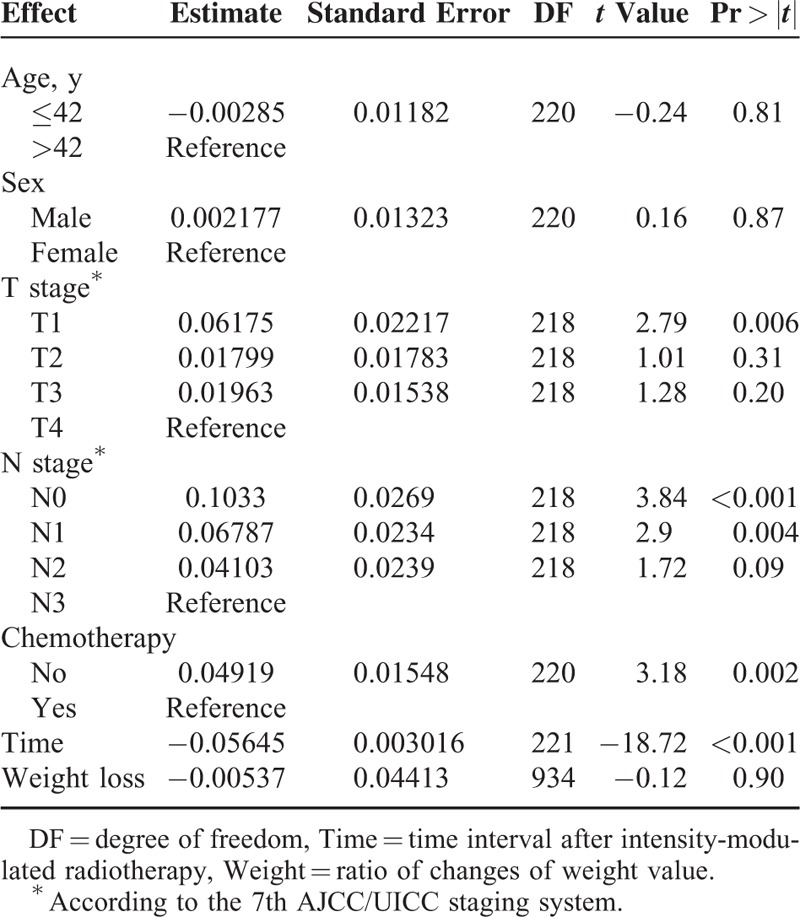
Parameters of clinical characteristics and treatment of patients were subject to univariate analysis. Age (≤42 years, >42 years), sex (male, female), and weight change ratio had no correlation with SCM atrophy ratio (P = 0.87, P = 0.81, P = 0.90). The presence or absence of chemotherapy and the length of time after IMRT was significant (P = 0.002, P < 0.001). T stage and N stage demonstrated a significant correlation with muscle atrophy ratio (Table 4).
TABLE 4.
Univariate Analysis of Prognostic Factors for Ratio of Sternocleidomastoid Atrophy in Study Cohort After Intensity-Modulated Radiotherapy
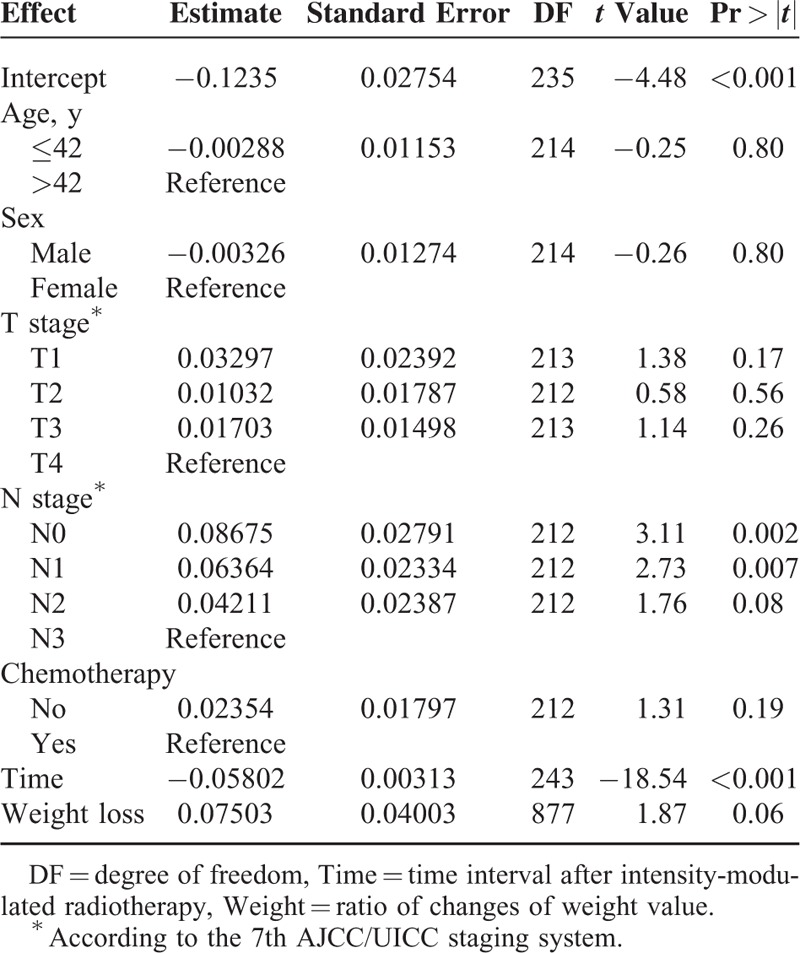
To test independent significance, multivariate analysis included the following parameters: age (≤42 years, >42 years), sex (male, female), T stage (T1, T2, T3, T4), N classification (N0, N1, N2, N3), chemotherapy (no, yes), the length of time after IMRT, and weight change ratio. N stage and the time after IMRT were significant predictive variables for muscular atrophy (Table 5).
TABLE 5.
Multivariate Analysis of Prognostic Factors for Ratio of Sternocleidomastoid Atrophy in Study Cohort After Intensity-Modulated Radiotherapy
The Relationship Between SCM Atrophy and Neck Weakness
The grade of neck weakness 3 years after IMRT was as follows: Grade 0: 115/223 (51.6%); Grade 1: 57/223 (25.6%); Grade 2: 51/223 (22.9%); Grade 3: 0/223 (0%); Grade 4: 0/223 (0%); and Grade 5: 0/223 (0%). Spearman rank correlation test showed significant associations between the grade of SCM atrophy and the grade of neck weakness (R = 0.392, P < 0.001). There were no missing patients in this study.
DISCUSSION
Muscle Tissue is Sensitive to Fraction Radiotherapy
Radiation-induced muscle atrophy is characterized by derangement in size and number of muscle fibers.3 Rubin and Casarett21 first reported in 1968 that muscles were relatively radio-resistant based on histopathologic observations within 2 months of treatment. Then, Powers et al3 reported in 1991 that obvious muscle atrophy was observed after a single high-dose irradiation delivered to muscles, but muscles were considered to be insensitive to conventional fractionated radiotherapy; the process of muscle atrophy may progress for many years.4 Therefore, traditionally, there has been no dose limitation when muscles are within the radiation field.
However, some research, based on various radiotherapy techniques, demonstrates that conventional fractionated radiotherapy can cause significant muscle atrophy after a variable period of time. In 12 patients who received mantle field radiotherapy between 1969 and 1998,7 8 patients had severe atrophy of the SCM between 10 and 39 years later, which showed that muscles injuries could be a long-term effect. However, Nichol et al5 demonstrated a significant decrease in the masticatory muscle area after radiotherapy with a median follow-up of 2.9 years. Aron et al22 reported that the average thickness of the SCM decreases by 7.89% 3 months after the chemoirradiation of stages III to IV head and neck cancer. These 2 papers showed that muscle atrophy is a relatively short-term effect. In accordance with the findings of these papers, our report showed an average SCM atrophy ratio of −10.97%, −18.65%, and −22.25% at 1, 2, and 3 years after radiotherapy, respectively. These findings demonstrated that significant SCM atrophy occurred in NPC patients during the first 3 years after radical IMRT. These results demonstrate that muscles are not as radio-resistant as was traditionally considered.
Possible Reasons for Significant SCM Atrophy
Although mature muscle fibers were considered relatively radio-resistant based on histopathologic observations within a few months postirradiation,21,23 this is not reasonable because muscle injury belongs to the group of late effects, which only become obvious years after radiation therapy.4
High doses of radiation may influence the proliferation of muscular precursor cells, resulting in inhibition of muscle spontaneous regeneration.24 Fajardo and Berthrong25 hypothesized in 1988 that the high susceptibility of capillaries and vessels to high-dose radiation leads to vascular damage causing delayed muscle ischemia and muscle atrophy. Although the reasons for differences in the severity of muscle atrophy are not clear, we speculate that varying degrees of muscle atrophy may be due to exposure dose differences or the differential damaging effects on capillaries and other vascular structures.
The total percentage of cervical node metastasis in NPC patients was 79.8% in the current study. Ho et al26 reported that the percentage of cervical node metastasis in NPC patients was 74.0%. As a result of the high ratio of cervical node metastasis,26 irradiation of the neck nodes and entire draining lymphatic regions down to the supraclavicular fossa has been traditional practice in treating NPC, without an established neck muscle dose limit. SCM is usually adjacent to the irradiation target volume of cervical lymph nodes. In some patients with lymph nodes directly filtrating the SCM, the CTV is enlarged to include part of SCM in the corresponding infiltrated levels,27 potentially resulting in unavoidably large doses of radiation to SCM. Thus, we hypothesize that high-dose radiation-induced damage to capillaries and vascular structures located in the SCM, which results in significant SCM atrophy within the first 3 years after IMRT in patients with NPC.
N Staging and Time Were Significant Predictive Variables for SCM Atrophy
It has been shown that atrophy of SCM is dose dependent.22 Multivariate analysis in our study demonstrated that the volume of SCM decreased the more advanced the N stage. In general, at more advanced N stage, the prescribed dose to the GTV-N will be higher; therefore, the irradiated volume of SCM exposed to high radiation (a dose causing muscle atrophy) may be larger. Although we did not investigate the dose distribution of the SCM, it can be speculated that the effect of N stage on muscular atrophy ratio is primarily attributable to dose distribution. Given its irreversible nature, and the difficulties with management, prevention of muscle atrophy is the primary strategy. This is probably most effectively done by limiting the radiation volume and dose in the neck muscles.
This study also demonstrated that the volume of SCM decreased in a time-dependent manner postradiotherapy (P < 0.001). Previous research has shown that radiation-induced muscle atrophy and weakness can progress for many years in Hodgkin lymphoma patients.7 In addition, the increase of degree of SCM atrophy slows down year by year indicating that atrophy becomes slower over time, suggesting there may be a plateau for muscular atrophy over time.
Chemotherapy and Weight Loss had No Correlation With SCM Atrophy Ratio
The study data showed that SCM atrophy was not always accompanied by weight loss (P = 0.06), indicating that SCM atrophy is attributed to treatment rather than loss of weight; this conclusion is consistent with the results reported by Aron.22
Although chemotherapy may increase the incidence of acute side effects in patients receiving radiotherapy28 and doxorubicin-based chemotherapy can be a cause of muscle atrophy,29 there have been no systematic studies into the effect of chemotherapy on muscle atrophy. Multivariate analysis of the study data revealed that chemotherapy was not a significant predictive variable for muscular atrophy.
The Association Between SCM Atrophy and Neck Weakness
Elena et al reported that 83% of Hodgkin lymphoma patients experienced neck pain and muscle weakness several decades after field radiotherapy. However, these patients had no muscle weakness in the first 10 years after radiotherapy.7 In our observation, 51.1% of NPC patients had experienced neck weakness by 3 years post-IMRT. This difference may be attributable to the radiation dose of lymphoma being far lower than that of NPC.
The actual causal relationship between muscle atrophy and symptoms is unknown, but previous research has shown that low muscle mass, which leads to muscle atrophy, is a potential factor in the development of weakness.30 Consistent with this, the study results demonstrated that SCM atrophy ratio correlates significantly with the grade of muscle weakness.
Bcl-2 Provides Further Possibilities for Limiting the Radiation Dose to Neck Muscles
The most well-known apoptosis-related B-cell CLL/lymphoma 2 (Bcl-2) family comprises pro-apoptotic proteins such as Bax (Bcl-associated X) and antiapoptotic proteins such as Bcl-2.31 Previous studies have demonstrated that a Bcl-2 inhibitor and Bax overexpression may induce the radiosensitization of NPC cells.32 Recently, small-molecule Bcl-2 inhibitors or/and Bax agonists have been proposed as promising strategies for cancer therapy.33
High-dose radiation is associated with a high probability of treatment-induced toxicities. If radiotherapy with a reduced dose is combined with a Bcl-2 inhibitor and/or Bax activator, and this strategy is shown to be equally effective for the treatment of patients with NPC, lower radiation doses might be given in the future. The aim would be to control tumors effectively while reducing the risk of long-term side effects. Undoubtedly, future studies are needed to confirm this hypothesis.
Limitations
One limitation of this study is that the length of follow-up, at 3 years, is inadequate. However, the first few years after IMRT do appear critical to understanding the evolution of IMRT-induced muscle atrophy and further investigating how to reduce late adverse reactions.
There is no evidence that measurement of muscular volume is more effective than measuring transversal muscular diameter or area; however, measuring the SCM volume provides more comprehensive information about the muscle as a whole, especially when it is unclear which level of SCM muscle is the most representative for muscle atrophy.
CONCLUSIONS
A review of the literature suggests that this is a relatively large study with a lengthy follow-up period evaluating IMRT-induced neck muscle atrophy and neck weakness of NPC patients. Understanding the evolution of IMRT-induced muscle atrophy may assist in informing treatment choices. The study results indicate that radical IMRT can cause significant SCM atrophy in NPC patients. A more advanced N stage is associated with more severe SCM atrophy, but no difference was observed in this study between N2 and N3. SCM atrophy progresses most rapidly in the earlier period of the first 3 years after IMRT. The grade of SCM atrophy is significantly associated with neck weakness.
ACKNOWLEDGMENTS
The authors would like to thank anonymous native English speakers for their linguistic assistance in improving this manuscript.
Footnotes
Abbreviations: CT = computed tomography, CTV = clinical target volume, IMRT = intensity-modulated radiotherapy, MRI = magnetic resonance imaging, NPC = nasopharyngeal carcinoma, SCM = sternocleidomastoid muscle.
L-LZ and Y-PM contributed equally to this work.
This work was supported by grants from the National Natural Science Foundation of China (No. 8172409), the Sun Yat-sen University Clinical Research 5010 Program (No. 2012011), the National Natural Science Foundation of China (No. 81201746), and the Natural Science Foundation of Guangdong Province (No. S2013010012220).
The authors can confirm that this manuscript has not been submitted to any other journal for publication and this study has no conflicts of interest to report.
REFERENCES
- 1.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol 2002; 13:1007–1015. [DOI] [PubMed] [Google Scholar]
- 2.Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol 2003; 69:227–236. [DOI] [PubMed] [Google Scholar]
- 3.Powers BE, Gillette EL, Gillette SL, et al. Muscle injury following experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys 1991; 20:463–471. [DOI] [PubMed] [Google Scholar]
- 4.Gillette BL, Mahler PA, Powers BE, et al. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys 1995; 31:1309–1318. [DOI] [PubMed] [Google Scholar]
- 5.Nichol AM, Smith SL, D’yachkova Y, et al. Quantification of masticatory muscle atrophy after high-dose radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56:1170–1179. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh PS, Milone M. Clinical and laboratory findings of 21 patients with radiation-induced myopathy. J Neurol Neurosurg Psychiatry 2015; 86:152–158. [DOI] [PubMed] [Google Scholar]
- 7.Van Leeuwen-Segarceanu EM, Dorresteijn LD, Pillen S, et al. Progressive muscle atrophy and weakness after treatment by mantle field radiotherapy in Hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys 2012; 82:612–618. [DOI] [PubMed] [Google Scholar]
- 8.Saarilahti K, Kouri M, Collan J, et al. Intensity modulated radiotherapy for head and neck cancer: evidence for preserved salivary gland function. Radiother Oncol 2005; 74:251–258. [DOI] [PubMed] [Google Scholar]
- 9.Blouin JS, Siegmund GP, Carpenter MG, et al. Neural control of superficial and deep neck muscles in humans. J Neurophysiol 2007; 98:920–928. [DOI] [PubMed] [Google Scholar]
- 10.Lim HK, Hong SH, Yoo HJ, et al. Visual MRI grading system to evaluate atrophy of the supraspinatus muscle. Korean J Radiol 2014; 15:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tingart MJ, Apreleva M, Lehtinen JT, et al. Magnetic resonance imaging in quantitative analysis of rotator cuff muscle volume. Clin Orthop Relat Res 2003; 415:104–110. [DOI] [PubMed] [Google Scholar]
- 12.Al-Saleh MA, Jaremko JL, Saltaji H, et al. MRI findings of radiation-induced changes of masticatory muscles: a systematic review. J Otolaryngol Head Neck Surg 2013; 42:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuccia G, Shelley OP, d’Alcontres FS, et al. Evidence of significant sternocleidomastoid atrophy following modified radical neck dissection type III. Plast Reconstr Surg 2006; 117:227–232. [DOI] [PubMed] [Google Scholar]
- 14.Delanian S1, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 2004; 73:119–131. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 16.Pavy JJ, Denekamp J, Letschert J, et al. EORTC Late Effects Working Group. Late effects toxicity scoring: the SOMA scale. Radiother Oncol 1995; 35:11–15. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Lu N, Feng C, et al. On fitting generalized linear mixed-effects models for binary responses using different statistical packages. Stat Med 2011; 30:2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilke DR, Krahn M, Tomlinson G, et al. Sex or survival: short-term versus long-term androgen deprivation in patients with locally advanced prostate cancer treated with radiotherapy. Cancer 2010; 116:1909–1917. [DOI] [PubMed] [Google Scholar]
- 19.Panjabi M, Malcolmson G, Teng E, et al. Hybrid testing of lumbar CHARITE discs versus fusions. Spine (Phila Pa 1976) 2007; 32:959–966. [DOI] [PubMed] [Google Scholar]
- 20.Xin M, Li R, Xie M, et al. Small-molecule Bax agonists for cancer therapy. Nat Commun 2014; 5:4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin P, Casarett GW. Clinical radiation pathology. Cancer 1968; 22:767–778. [DOI] [PubMed] [Google Scholar]
- 22.Popovtzer A, Cao Y, Feng FY, et al. Anatomical changes in the pharyngeal constrictors after chemo-irradiation of head and neck cancer and their dose-effect relationships: MRI-based study. Radiother Oncol 2009; 93:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh JS, Torre TG, DeWeese TL, et al. Radiation myositis. Ann Oncol 1999; 10:1105–1108. [DOI] [PubMed] [Google Scholar]
- 24.Alameddine HS, Dehaupas M, Fardeau M. Regeneration of skeletal muscle fibers from autologous satellite cells multiplied in vitro. An experimental model for testing cultured cell myogenicity. Muscle Nerve 1989; 12:544–555. [DOI] [PubMed] [Google Scholar]
- 25.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu 1988; 23 (pt 1):297–330. [PubMed] [Google Scholar]
- 26.Ho FC, Tham IW, Earnest A, et al. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer 2012; 12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014; 110:172–181. [DOI] [PubMed] [Google Scholar]
- 28.Adelstein DJ, Lavertu P, Saxton JP, et al. Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer 2000; 88:876–883. [DOI] [PubMed] [Google Scholar]
- 29.Bonifati DM, Ori C, Rossi CR, et al. Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol 2000; 46:517–522. [DOI] [PubMed] [Google Scholar]
- 30.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69:547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kontos CK, Fendri A, Khabir A, et al. Quantitative expression analysis and prognostic significance of the BCL2-associated X gene in nasopharyngeal carcinoma: a retrospective cohort study. BMC Cancer 2013; 13:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He JH, Liao XL, Wang W, et al. Apogossypolone, a small-molecule inhibitor of Bcl-2, induces radiosensitization of nasopharyngeal carcinoma cells by stimulating autophagy. Int J Oncol 2014; 45:1099–1108. [DOI] [PubMed] [Google Scholar]
- 33.Vaillant F, Merino D, Lee L, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 2013; 24:120–129. [DOI] [PubMed] [Google Scholar]


