Supplemental Digital Content is available in the text
Abstract
Preclinical researches indicated a potential synergistic effect of taxanes-containing chemotherapy (TCC) and antiangiogenic agents (AAs) on the treatment of advanced nonsmall-cell lung cancer (NSCLC). The advantage of adding AA to TCC in the real world remains confusing. We summarized the current evidences from relevant phase II/III randomized controlled trials (RCTs) by performing this meta-analyses.
Electronic databases were searched for eligible literatures. The primary endpoint was overall survival (OS). Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes were calculated using RevMan 5.2.
A total of 14 phase II/III RCTs involving 9703 participants were included. Compared to standard TCC, the addition of AA was associated with the significant better OS (HR 0.92, 95% CI 0.87–0.97, P = 0.002), prolonged progression-free survival (HR 0.79, 95% CI 0.71–0.87, P < 0.00001), superior response rate (risk ratio [RR] 1.69, 95% CI 1.47–1.95, P < 0.0001), and disease control rate (RR 1.19, 95% CI 1.08–1.32, P < 0.00001). Subgroup analyses indicated that patient treated with monoclonal antibodies (HR 0.89, 95% CI 0.82–0.96, P = 0.02) as well as application in second-line (HR 0.91, 95% CI 0.85–0.96, P = 0.02) acquired significant OS improvement. Other clinical factors directing significant OS improvement by the combination strategy included nonsquamous cancer (P = 0.002), nonsmokers (P = 0.0005), and female (P = 0.02). Toxicities were greater but generally mild or moderate in the combination group, and were mostly manageable.
In summary, the addition of AAs to TCC could improve prognosis of advanced NSCLC. Furthermore, proper selection of patient population and AAs is crucial for clinical trials design and clinical practice in the future.
INTRODUCTION
Lung cancer is still the leading cause of cancer-related mortality around the world.1 Nonsmall-cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases, and most patients are diagnosed as advanced or metastatic disease.2 Although several targeted therapies against driver mutations have been developed and led to extraordinary clinical benefit for NSCLC patients, the prognosis of patients without known driver mutations remains poor.3,4 Therefore, novel treatment strategy for this patient population is urgently warranted.
The important role of angiogenesis in tumor development, growth, and metastasis has been well established.5 Several antiangiogenic agents (AAs), including small-molecule multiple receptor tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, have been developed. To date, more than 20 randomized placebo-controlled clinical trials were conducted to test the hypothesis that combining standard therapies and AAs might confer additional clinical benefit in advanced NSCLC patients. However, only very few studies (ECOG 4599, REVEL) revealed overall survival improvement by additional antiangiogenic treatment.6,7
Preclinical studies demonstrated that taxanes (paclitaxel and docetaxel) would induce endothelial progenitor cells (EPCs) mobilization, which contributed to drug resistance and regrowth of tumor cells.8,9 In addition, antiangiogenic drugs could block the mobilization of EPC and increase antitumor efficacy.10 These results indicated a potential synergistic effect of taxanes-containing chemotherapy (TCC) and AAs on the treatment of NSCLC. Furthermore, our previous meta-analyses found that the combination of AAs and TCC could confer overall survival (OS) improvement in NSCLC patients who failed from first-line treatment when compared with TCC alone. However, the advantage of adding AA to TCC in the overall population of NSCLC remains confusing. Therefore, we performed this meta-analyses to compare the efficacy of angiogenesis inhibitors plus TCC versus TCC alone for patients with advanced NSCLC.
METHODS
Meta-analyses were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.11 The study protocol was approved by the Ethics Committee of Sun Yat-sen University Cancer Center, China.
Literature Research
A systematic review of eligible randomized controlled trials (RCTs) was performed by searching the electronic databases, including Cochrane Central Register of Controlled Trials, PubMed, EMBASE, MEDLINE, ASCO abstracts, and ESMO abstracts. All the RCTs on combination of AA with TCC for advanced NSCLC were collected and identified.
The AA was defined as agent blocking various angiogenesis relevant targets, primarily vascular endothelial growth factor (VEGF). Small-molecule TKIs or monoclonal antibodies were defined as 2 types of AA. Any dosage and schedules of AA as first- or second-line therapy were included for analyses. TCC referred to conventional cytotoxic regimens including taxanes, such as docetaxel, paclitaxel plus carboplatin, and doctaxel plus carboplatin.
The reference lists of identified articles or meta-analyses were searched manually to find other relevant articles.
Inclusion Criteria
Eligibility criteria were as follows: type of participants: adults patient with pathologically confirmed, squamous or nonsquamous, recurrent or metastatic NSCLC that untreated before or progressed after a single platinum-based chemotherapy regimen. Type of study: studies had to be phase II or III RCTs comparing the efficacy and safety profile of adding AA to TCC with TCC alone in patients with advanced NSCLC. Type of publication: full papers on original data were included if original data about OS were reported. Abstracts with sufficient information on study design, characteristics of participants, interventions, and outcomes were also eligible for analyses.
Trials were excluded if they fail to meet the including criteria. In cases of duplicate trials, the most complete reports were included. The deadline of this search was February 28, 2015. The articles were limited to those in English language.
Data Extraction and Quality Assessment
The data collection and assessment of methodological quality followed the Cochrane Collaboration guidelines (http://www.cochrane.de). Researcher evaluated the quality of each eligible study with Jadad score according to aforementioned criteria.12
OS was set as the primary outcome. Other outcome measures included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and toxicity profile. The hazard ratios (HRs) of OS and PFS were extracted from the original studies or accounted from the reported data. Other important information included the characteristic of each RCT and the efficacy of each group. Two reviewers independently extracted all above data from the finally included studies. Disagreements were resolved by consensus or by the third investigators. The name of the first author and the year of publication of the article were used for identification.
Statistical Analyses
The experimental arm was defined as AA plus TCC, whereas the control arm was TCC alone. The heterogeneity across studies was assessed with the inconsistency statistic (I2) and forest plot. A fixed-effect model was applied if no existence of significant heterogeneity (I2 ≤ 50%); otherwise, the random-effect model was used. Pooled HRs for survival outcomes (PFS and OS) and pooled risk ratios (RRs) for ORR and DCR with 95% confidence interval (CI) were calculated using Review Manager (version 5.2 for Windows; Cochrane Collaboration, Oxford, UK). An estimate of the potential publication bias was carried out by funnel plot. An asymmetric plot suggested a possible publication bias. Subgroup analyses were conducted to define potential groups that may potentially benefit from combining antiangiogenesis with TCC. P < 0.05 was considered statistically significant for all analyses.
RESULTS
Characteristics of Included Studies
Figure 1 was the flow chart reflecting the selection process for eligible RCTs. Twenty-nine potentially eligible trials were rigorously identified by full-text review, 14 studies with 9703 patients met the inclusion criteria and were finally included for OS analyses. Other potential eligible studies were excluded for reasons of single-armed or without chemotherapy combination. Among the included studies, there were nine phase III RCTs.6,7,13–19 Furthermore, nine agents (vandetanib, sunitinib, cediranib, nintedanib, sorafenib, motesanib, aflibercept, bevacizumab, and ramucirumab) with comparable data were identified. Eight studies were set as first-line investigational clinical trials.13–15,17,20–23 One study explored the efficacy and safety of 2 dosages of vandetanib.24 There were 6 studies designed with OS as the primary outcome.6,7,13–16 All studies were scored 3 to 5, and evaluated as high quality except 1 phase III trial15 with 4 arms, which evaluated the efficacy and toxicity of the combination of docetaxel/carboplatin with bevacizumab or erlotinib as first-line treatment for advanced NSCLC.
FIGURE 1.

The flowchart of the process for selecting relevant articles.
Table 1 summarized the important baseline characteristics and the quality assessment results of selected trials. Apparently, all of the trials were qualified enough with the Jadad score ≥3. For each study, no high risk of bias was detected for all key domains, including sequence generation, allocation concealment, blinding of participants or outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias.
TABLE 1.
Characteristics of Included Studies
Publication Bias
The highly sensitive search strategy was applied to minimize the potential of publication bias. Furthermore, we reviewed the articles strictly according to the inclusion criteria. Publication bias was detected by funnel plot. No apparent publication bias was found in the analyses for our primary measure (S1_Figure, http://links.lww.com/MD/A352).
Primary Measure: Overall Survival
According to the original data, 2 trials reported statistically significant improvement on OS.6,7 The pooled result showed that the combination with AA was associated with the significant improved OS (HR 0.92, 95% CI 0.87–0.97, P = 0.002) compared with standard TCC. No apparent heterogeneity was detected among the recruited studies (P = 0.34, I2 = 11%) (Figure 2). As shown in Figure 2, study LUME-Lung 1 occupied 20.1% relative weight. However, when we performed additional analyses with the subtraction of this trial, the overall results remained similar (HR 0.92, 95% CI 0.87–0.97, P = 0.004, I2 = 17%). Therefore, the weight of study LUME-Lung 1 did not impact the overall results.
FIGURE 2.

Forest plot and pooled HR and 95% CI for OS: antiangionesis therapy plus TCC versus TCC alone. CI = confidence interval, HR = hazard ratio, OS = overall survival, TCC = taxanes-containing chemotherapy.
Subgroup analyses indicated that slightly OS improvement was observed in first-line application (HR 0.96, 95% CI 0.87–1.06, P = 0.39). However, the practice in second-line application was associated with the significant prolonged OS (HR 0.91, 95% CI 0.85–0.96, P = 0.002). Other clinical factors directing significant OS improvement by the combination strategy included histologically nonsquamous cancer (HR 0.90, 95% CI 0.84–0.96, P = 0.002), nonsmokers (HR 0.81, 95% CI 0.70–0.94, P = 0.0005), or female (HR 0.87, 95% CI 0.77–0.98, P = 0.02). Only monoclonal antibodies (HR 0.89, 95% CI 0.82–0.96, P = 0.004) were proved efficient in combination with TCC.
However, indirect analyses failed to validate the superiority of monoclonal antibodies (HR 0.94, 95% CI 0.84–1.04, P = 0.22). The results of subgroup analyses were summarized in Table 2.
TABLE 2.
Summary of the Subgroup Results: Pooled HRs and 95% CIs for OS

Secondary Measure: PFS, ORR, DCR, and Toxicity
Thirteen studies reported the original data of PFS and ORR. Compared with TCC alone, the combination of AA and TCC resulted in significant improvement on PFS (HR 0.79, 95% CI 0.71–0.87, P < 0.0001) and high response rate (RR 1.69, 95% CI 1.47–1.95, P < 0.0001) (Figures 3 and 4). The DCR was also improved by this combination strategy (RR 1.19, 95% CI 1.08–1.32, P < 0.00001) (Figure 5).
FIGURE 3.

Forest plot and pooled HR and 95% CI for PFS: antiangionesis therapy plus TCC versus TCC alone. CI = confidence interval, HR = hazard ratio, PFS = progression-free survival, TCC = taxanes-containing chemotherapy.
FIGURE 4.
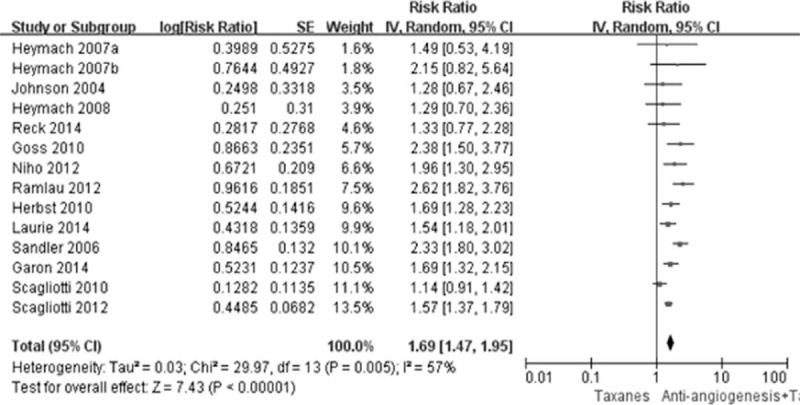
Forest plot and pooled RR and 95% CI for ORR: antiangionesis therapy plus TCC versus TCC alone. CI = confidence interval, ORR = objective response rate, RR = risk ratio, TCC = taxanes-containing chemotherapy.
FIGURE 5.
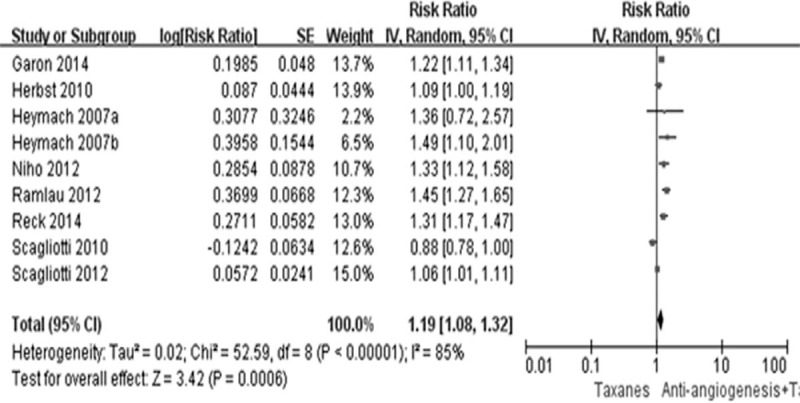
Forest plot and pooled RR and 95% CI for DCR: antiangionesis therapy plus TCC versus TCC alone. CI = confidence interval, DCR = disease control rate, RR = risk ratio, TCC = taxanes-containing chemotherapy.
In general, grade ≥3 adverse events occurring more frequently in the combination arms versus the TCC arms, such as hypertension, hemorrhage, proteinuria, thromboembolic events and diarrhea for anti-VEGF-induced events and neutropenia, leukopenia, and fatigue for chemotherapy-induced events. Moreover, it had been reported that addition of AA to chemotherapy lead to more treatment-induced death.6,25 However, the combination therapy had a safety profile compared with that of AA such as bevacizumab taken individually.15 In addition, various AAs had their own toxicity profiles. On the whole, the toxicities were greater but generally mild or moderate in severity and manageable in the combination group.
DISCUSSION
To our knowledge, this is the first systemic review and meta-analyses assessing the efficacy of AA plus TCC versus standard TCC for patients with advanced NSCLC. Our study revealed the addition of AA was associated with the significant longer OS (HR 0.92, 95% CI 0.87–0.97, P = 0.002), prolonged PFS (HR 0.79, 95% CI 0.71–0.87, P < 0.00001), superior ORR (RR 1.69, 95% CI 1.47–1.95, P < 0.0001), and DCR (RR 1.19, 95% CI 1.08–1.32, P < 0.00001). Subgroup analyses indicated that nonsquamous, nonsmoker, and female lung cancer patients as well as patients in second-line might be the potential target population. Furthermore, monoclonal antibodies might be the preferable AA in combination with TCC.
Several preclinical researches indicated that TCC could promote angiogenesis by various mechanisms and induce drug resistance. As previously mentioned, taxanes could cause EPC elevations within 24 hours of a single bolus injection, whereas other agents (gemcitabine, cisplatin, doxorubicin, irinotecan, and cyclophosphamide) failed to induce mobilization of EPC.8 In addition, TCC treatment would result in a significant increase in the influx of angio-supportive bone marrow-derived cells and lead to enhanced angiogenesis.26 Moreover, a recent study found that paclitaxel therapy might activate systemic inflammatory circuits which promoted angiogenesis.27 These findings implied the addition of AA could enhance the anticancer activity of TCC. The efficiency of this strategy had been confirmed by this meta-analyses. Therefore, it is reasonable to apply this strategy to NSCLC patients in the real world.
As NSCLC is no longer seen as one disease but as a cluster of different disease variants that can be classified by histological subtypes or genetic mutations, AA in combination with chemotherapy may not provide significant clinical benefit to unselected NSCLC patients.28,29 Unfortunately, although several markers (VEGF-A, VEGFR, placental growth factor (PLGF), neuropilin-1 [NRP-1]) have been evaluated as predictive factors for antiangiogenic therapy, no validated biomarker has been identified.30–32 Subgroup analyses of this study presented that only nonsquamous (HR 0.90, 95% CI 0.84–0.96, P = 0.002), nonsmoker (HR 0.81, 95% CI 0.70–0.94, P = 0.0005), and female (HR 0.87, 95% CI 0.77–0.98, P = 0.02) NSCLC patients could gain significant survival benefit from the combination regimens. The underlying biological mechanism is still unknown. Notably, these characteristics are associated with the high EGFR mutation frequency.33,34 However, as EGFR mutation test was not routinely performed in these included trials, we could not analyze the relationship between clinical efficacy of this combination strategy and the mutation status of EGFR. Further studies are warranted to explore this objective in future.
Our study also found that application of monoclonal antibodies rather than multitargeted antiangiogenic TKIs (MATKIs) could prolong overall survival of NSCLC patients. This was consistent with the results of previous studies.35,36 These 2 meta-analyses both revealed that MATKIs-containing regimens were superior to MATKIs-free regimens in terms of tumor response and PFS in patients with advanced NSCLC. However, no significant benefits in OS were observed. One possible explanation for this observation is that MATKIs tested to date have inhibitory activity over a range of receptors and are lacking of specificity to a particular one. Thus, they may not be able to completely inhibit angiogenesis signaling.37 It is possible that MATKIs with preferable specificities could provide more clinical benefit to NSCLC patients.
The present meta-analyses are limited by the heterogeneity of various agents used in the individual trials. In addition, the analyses were not based on individual patient data, which might provide further insight into the efficacy of the combination strategy. Other limitations include publication status as ongoing studies were ineligible for inclusion. In addition, only a small number of trials met the subgroup analyses criteria, thus reducing the power of the analyses. These factors may have a potential impact on our results. Moreover, we are unable to assess the effects of this combination strategy on other meaningful endpoints, such as quality of life, details of toxicity profile, because of sparse and inconsistent reporting across studies.
CONCLUSIONS
In summary, the addition of AAs to TCC could improve prognosis of NSCLC patients. Furthermore, proper selection of patient population and AAs is crucial for clinical trials design and clinical practice in the future.
Acknowledgments
The authors would like to thank the authors who kindly provided the data necessary for this study.
Footnotes
Abbreviations: AAs = antiangiogenic agents, CIs = confidence intervals, DCR = disease control rate, EPCs = endothelial progenitor cells, HRs = hazard ratios, MATKIs = multitargeted antiangiogenic TKIs, NSCLC = nonsmall-cell lung cancer, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, RCTs = randomized controlled trials, RRs = risk ratios, TCC = taxanes-containing chemotherapy, TKIs = tyrosine kinase inhibitors, VEGF = vascular endothelial growth factor.
The authors of this article have declared that no competing interests exist.
This work was funded by National High Technology Research and Development Program of China (Grant No. 2012AA02A502), Innovative drug R&D center based on real-time high-throughput cell-based screening platform and large capacity compound library (Grant No. 2013ZX09401003-002), National Natural Science Funds of China (Grant No. 81372502), and Wu Jieping Medical Foundation Project (Grant No. 320.6750.131). All the funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
JS, Y-PY, B-JY, Y-YZ, and Y-XM contributed equally to this work as first authors.
JS and Y-PY conceived and designed the experiments. Y-XM and Y-XZ contributed analyses tools. B-JY and S-DH performed the experiments. Y-YZ conducted the statistical analyses. The manuscript was written by JS and Y-PY. Proofreading was provided by H-YZ and YH. The covering letter was from LZ.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353:123–132. [DOI] [PubMed] [Google Scholar]
- 4.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol 2012; 19:S52–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005; 23:1011–1027. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 7.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384:665–673. [DOI] [PubMed] [Google Scholar]
- 8.Bertolini F, Paul S, Mancuso P, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003; 63:4342–4346. [PubMed] [Google Scholar]
- 9.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 2008; 14:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 2006; 313:1785–1787. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Sun XJ, Jiang TH, et al. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: a meta-analyses. World J Gastroenterol 2013; 19:7461–7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:1835–1842. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol 2012; 30:2829–2836. [DOI] [PubMed] [Google Scholar]
- 15.Boutsikou E, Kontakiotis T, Zarogoulidis P, et al. Docetaxel-carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther 2013; 6:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012; 30:3640–3647. [DOI] [PubMed] [Google Scholar]
- 17.Laurie SA, Solomon BJ, Seymour L, et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC Clinical Trials Group study BR29. Eur J Cancer 2014; 50:706–712. [DOI] [PubMed] [Google Scholar]
- 18.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15:143–155. [DOI] [PubMed] [Google Scholar]
- 19.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 2010; 11:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004; 22:2184–2191. [DOI] [PubMed] [Google Scholar]
- 21.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2008; 26:5407–5415. [DOI] [PubMed] [Google Scholar]
- 22.Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol 2010; 28:49–55. [DOI] [PubMed] [Google Scholar]
- 23.Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012; 76:362–367. [DOI] [PubMed] [Google Scholar]
- 24.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol 2007; 25:4270–4277. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Fang W, Liang W, et al. Risk of treatment-related deaths with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a meta-analyses of 41 randomized controlled trials. Onco Targets Ther 2014; 7:1851–1867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Roodhart JM, He H, Daenen LG, et al. Notch1 regulates angio-supportive bone marrow-derived cells in mice: relevance to chemoresistance. Blood 2013; 122:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volk-Draper L, Hall K, Griggs C, et al. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res 2014; 74:5421–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo JS, Ju YS, Lee WC, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res 2012; 22:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brauer MJ, Zhuang G, Schmidt M, et al. Identification and analyses of in vivo VEGF downstream markers link VEGF pathway activity with efficacy of anti-VEGF therapies. Clin Cancer Res 2013; 19:3681–3692. [DOI] [PubMed] [Google Scholar]
- 31.Mok T, Gorbunova V, Juhasz E, et al. A correlative biomarker analyses of the combination of bevacizumab and carboplatin-based chemotherapy for advanced nonsquamous non-small-cell lung cancer: results of the phase II randomized ABIGAIL study (BO21015). J Thorac Oncol 2014; 9:848–855. [DOI] [PubMed] [Google Scholar]
- 32.Bass MB, Yao B, Hei YJ, et al. Challenges in developing a validated biomarker for angiogenesis inhibitors: the motesanib experience. PLoS One 2014; 9:e108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361:947–957. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12:735–742. [DOI] [PubMed] [Google Scholar]
- 35.Liang W, Wu X, Hong S, et al. Multi-targeted antiangiogenic tyrosine kinase inhibitors in advanced non-small cell lung cancer: meta-analyses of 20 randomized controlled trials and subgroup analyses. PLoS One 2014; 9:e109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao YY, Zhan P, Yuan DM, et al. Chemotherapy plus multitargeted antiangiogenic tyrosine kinase inhibitors or chemotherapy alone in advanced NSCLC: a meta-analyses of randomized controlled trials. Eur J Clin Pharmacol 2013; 69:151–159. [DOI] [PubMed] [Google Scholar]
- 37.Crino L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev 2014; 23:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


