Supplemental Digital Content is available in the text
Abstract
The current retrospective study aimed to investigate the relationship between prognostic factors and overall survival (OS) in patients with advanced pancreatic head cancers who initially presented with obstructive jaundice. Furthermore, the impact of age and comorbidities on therapeutic strategies in such patients was evaluated.
A total of 79 advanced pancreatic head cancer patients who were treated at our institution between January 2006 and November 2013 were reviewed. We analyzed OS risk factors including sex, age, laboratory characteristics, Eastern Cooperative Oncology Group performance status, Charlson Comorbidity Index Scores (CCIS), and therapeutic strategies using Cox proportional hazards regression models.
There was no difference in the OS of patients according to the type biliary drainage procedure they underwent. Other related factors, such as better performance status, lower CCIS, and receiving chemotherapy significantly correlated with survival in multivariate analyses. There was a significant survival benefit in systemic chemotherapy compared to best supportive care (BSC) or local radiotherapy. However, no survival benefit was found in elderly patients (age >70 years) undergoing systemic therapy compared to younger patients, except in those elderly patients with CCIS ≤ 1.
In advanced pancreatic head cancer patients with obstructive jaundice, systemic therapy and adequate biliary drainage were still the most effective procedures for improving OS in the general population. However, in elderly patients with relatively higher CCIS, BSC with adequate biliary drainage was palliative and no less effective than systemic/local therapies.
INTRODUCTION
Pancreatic cancer is a major gastrointestinal tract cancer with a relatively high mortality rate and extremely poor prognosis regardless of stage. In 2013, pancreatic cancer was the fourth and eighth leading cause of cancer-related deaths in the United States and Taiwan, respectively.1 Only 10% to 20% of patients are diagnosed at a relatively early stage and can undergo potentially curative complete surgical resection and have a better prognosis.2 In a previous study, curative resection was a major factor influencing improved survival and outcome whether in the general population or in elderly patients.3,4 Most cases in our daily practice were locally advanced, unresectable, or metastatic pancreatic cancer cases. According to previous studies, advanced pancreatic cancer patients had worse prognoses compared to patients with early stage disease who could undergo surgical resection.5
Tumors in the head of the pancreas have distinct initial symptoms compared to tumors located on the body or tail, such as obstructive jaundice and duodenal obstruction. Most chemotherapy strategies are deferred until an improvement of hyperbilirubinemia by biliary drainage is achieved.6 Severe complications such as repeated biliary tract infections, ascending cholangitis, and septic shock are common in pancreatic head cancer patients, resulting in early death.
A previous study demonstrated that advanced age is not an absolute contraindication for pancreatic cancer patients, and there was no increased mortality after surgery in elderly patients, even in those over age 80 years of age.7 Generally, chemotherapy is still a therapeutic strategy in advanced or unresectable disease patients, whether with concurrent radiotherapy or not.8 However, only a few studies have focused on advanced pancreatic head cancer patients and analyzed potential prognostic factors in this particular population. Additionally, not many studies have been conducted to evaluate the therapeutic strategies in elderly patients (>70 years). Thus, we conducted this retrospective study to investigate and compare characteristics, treatment outcome, biliary drainage procedures, and prognostics factors in advanced pancreatic head cancer patients with obstructive jaundice.
METHODS
Patients and Study Design
This retrospective analysis included 79 patients with locally advanced, unresectable, or metastatic pancreatic cancer treated at Tri-Service General Hospital between January 2006 and November 2013. All patients were evaluated at the biweekly gastrointestinal tract and pancreatic malignancy conference. Patients with advanced pancreatic cancers were defined as patients not eligible for surgical intervention, patients with locally advanced pancreatic cancer, and patients with metastatic disease status. Those with locally advanced disease were diagnosed based on original diagnostic computed tomography (CT) scans, magnetic resonance imaging (MRI) scans, endoscopic ultrasound, or aborted surgical resection. Patients were deemed to have metastasis based on either of the following criteria: pathologically proven by aspiration biopsy, cytological evaluation, or bone/bone marrow biopsy; or in those who did not receive biopsy, the presence of classical clinical symptoms or signs of bone or visceral organs metastasis plus definitive evidence by serial imaging studies. These studies included CT, MRI, or positron emission tomography scanning.
The clinical data collected from medical records included age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), laboratory data, treatment patterns, survival duration, and causes of death. The comorbidity disease severity at diagnosis was determined by Charlson Comorbidity Index scores (CCIS).9 The final score was calculated and determined for each patient by 2 experienced reviewers. All CCIS were calculated without consideration for the pancreatic cancer. Additional auxiliary diagnostic tests included the serum tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). All patients had received standard therapies including chemotherapy, radiotherapy, targeted therapy, and best supportive care (BSC) as appropriate. Furthermore, relevant imaging studies and tumor marker evaluations were performed regularly as part of the follow-up. Patients with active or unresolved infections were excluded from study. Pretreatment biliary drainage was stratified into 3 groups based on pretreatment conditions, including percutaneous transhepatic biliary drainage (PTBD), intrabiliary plastic stent, or percutaneous transhepatic gallbladder drainage (PTGBD). All procedures were performed within 1 week of diagnosis. Intrabiliary plastic stenting was considered first in patients with obstructive jaundice; this procedure was available after January 2008. If a patient did not tolerate or otherwise failed to complete the whole procedure, alternative management with PTBD or PTGBD was considered. Complications among the 3 groups were also noted. Overall survival (OS) was measured from the date of observed clinical diagnosis until death. Surviving patients and those who stopped follow-up screening but were known to be alive were excluded from OS analysis. The Institutional Review Board and Cancer Registry Group of the Tri-Service General Hospital approved the use and analysis of the clinical characteristics (IRB: 1-103-05-170.)
Statistical Analysis
All statistical analyses were performed using SPSS version 18.0 statistical software (Chicago, IL). All descriptive data are expressed as median or mean ± standard deviation (SD). The differences in categorical variables were analyzed using the Chi-square test, and differences of continuous variables were estimated using t tests, as were comparisons between more than 2 groups. A P-value <0.05 was considered statistically significant. Univariate and multivariate Cox proportional hazard regression models were performed to assess the associated factor of OS. Hazard ratios (HR) and 95% confidence intervals (CIs) were calculated using the Cox model to estimate the effect of each factor on OS. Multivariate models were run for all predictors that were considered statistically significant in the univariate Cox proportional hazard regression models. The Kaplan–Meier method was used to determine the time-to-disease end points, and the log-rank test was used to calculate the differences between each group.
RESULTS
Patient Characteristics and Treatment
A total of 79 locally advanced or metastatic pancreatic cancer patients were recruited for this study. Seventy-six patients (96.2%) had died by the date of the most recently scheduled follow-up; only 3 patients (3.8%) were alive at the time of this analysis. The median and mean OS rates were 5.5 and 6.18 months, respectively. Table 1 summarizes the baseline characteristics. The mean age was 67.86 years with an SD of 15.0. There were more male patients than in our group than female patients, with a male/female ratio of 1.47. The ECOG PS scores for all patients were as follows: PS 1, 28 patients (35.4%); PS 2, 20 patients (25.3%); PS 3, 22 patients (27.8%); and PS 4, 9 patients (11.4%). The median and mean CCIS were 1 and 1.24 points, respectively. American Joint Committee on Cancer (AJCC) staging (stages III–IV), pre- and postdrainage procedure 1 week total bilirubin (TB), CA19-9, CEA, liver metastases status, and biliary drainage procedure are also listed. Table 2 shows treatment types in our patients. The biliary drainage procedure such as PTBD, intrabiliary plastic stent, and PTGBD groups were compared in terms of efficacy of serum bilirubin drainage (as determined by total and direct bilirubin (TB/DB) levels 1- and 2 weeks postprocedure), and TB 1-week clearance (defined as postprocedure TB − preprocedure TB). There was a statistically significant difference in postprocedure 1- and 2-week TB drainage and clearance among patients who underwent different biliary drainage procedure (Supplemental Table 1, http://links.lww.com/MD/A357). Among procedure-related complications, obstruction, stent migration, PTGBD dislodging, or infection were the most frequently reported adverse effects, which are summarized in Supplemental Table 2, http://links.lww.com/MD/A357. Additionally, various infectious pathogens are also listed. Most patients received chemotherapy (33 patients) or concurrent chemoradiotherapy (CCRT) (24 patients), and 46 patients received gemcitabine-based combination therapy as the first line treatment. The OS was significantly different for patients with gemcitabine-based combination therapy (median: 6.93 months) and fluoropyrimidine-based combination therapy (median: 6.20 months) compared to BSC (median: 3.20 months) (Figure 1A). There was a survival benefit for patients treated with chemotherapy or CCRT compared to those who received BSC (median survival for chemotherapy vs. BSC: 6.37 months vs. 2.50 months, respectively; and for CCRT vs. BSC: 6.83 months vs. 2.50 months, respectively). However, there was no significant therapeutic benefit for CCRT compared to chemotherapy alone (Figure 1B).
TABLE 1.
Clinical Characteristics of the Patients With Advanced Pancreatic Head Cancer (n = 79)
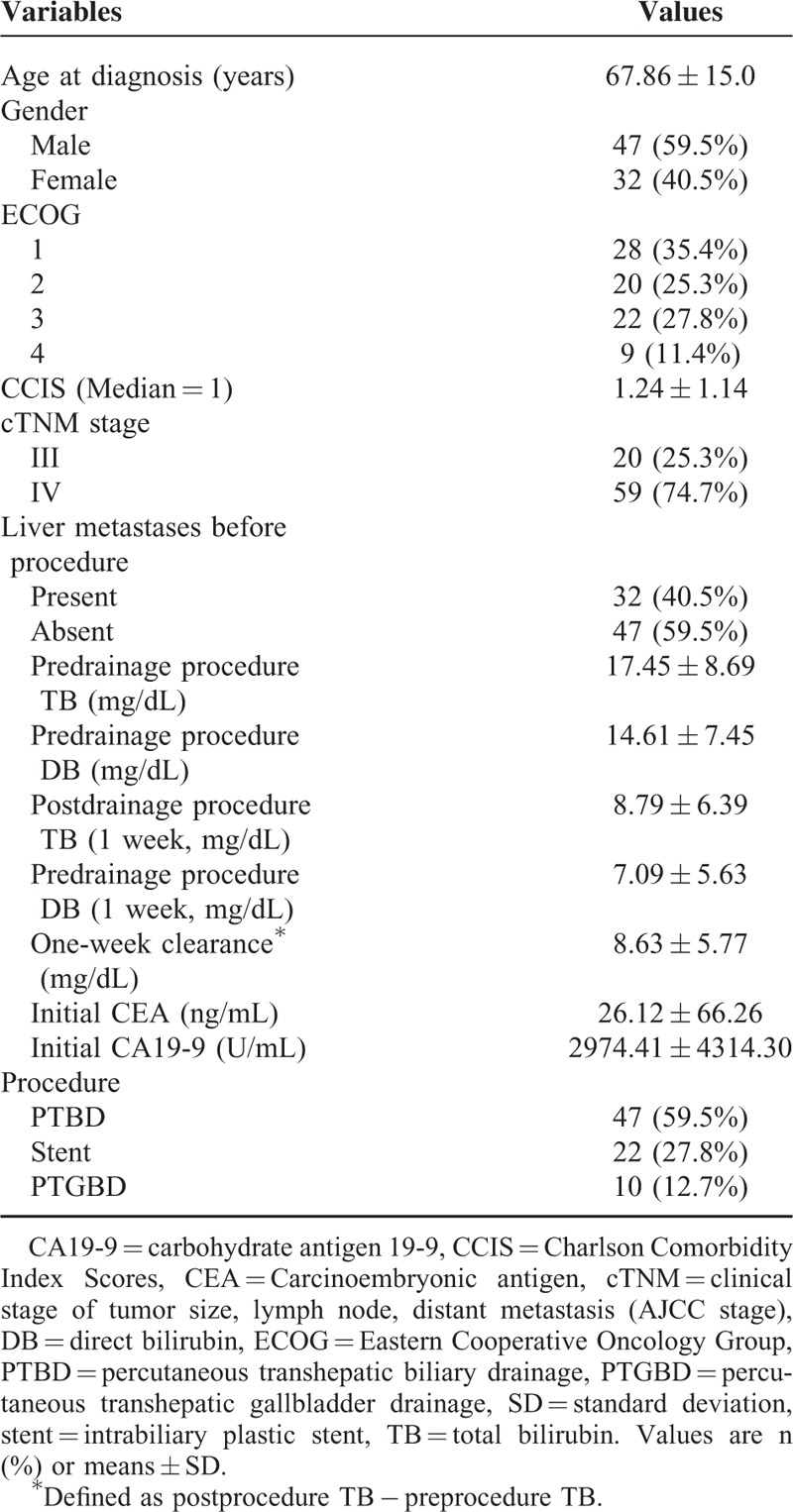
TABLE 2.
Treatment Regimens of the Patients With Advanced Pancreatic Head Cancer (n = 79)
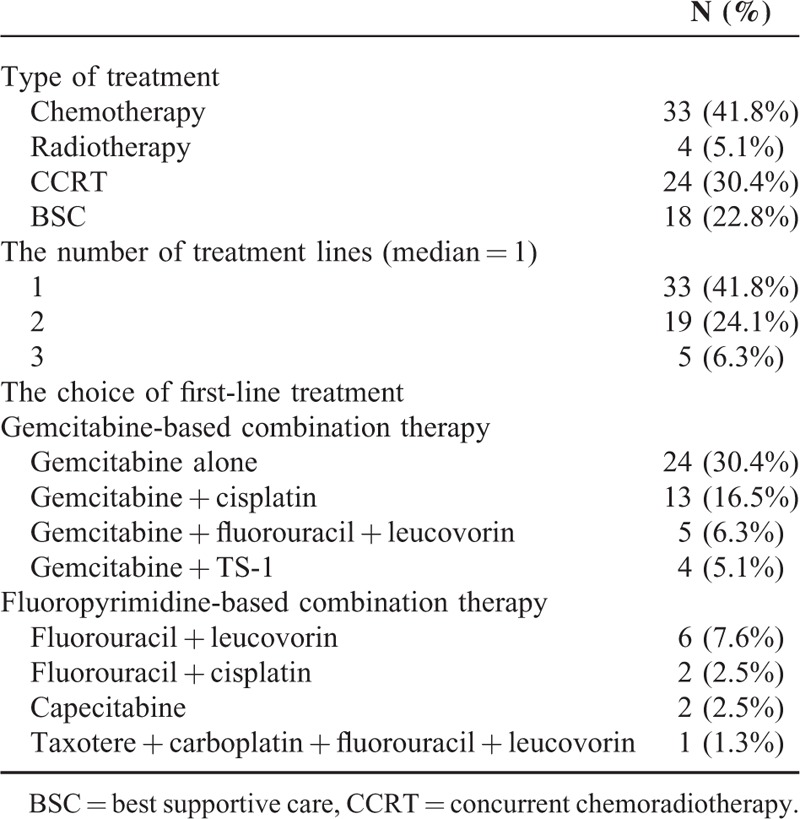
FIGURE 1.
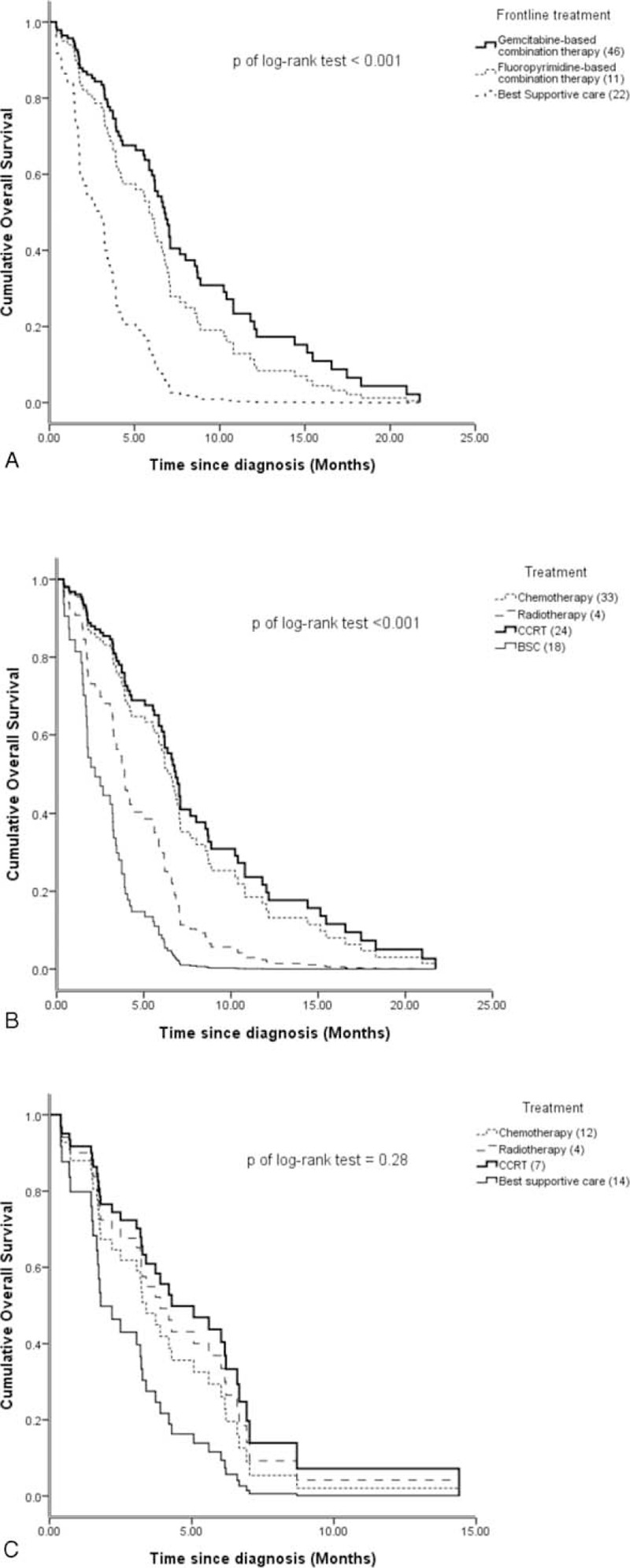
(A) There is statistically significant difference in the overall survival (OS) of patients depending on the type of frontline therapeutic regimen (P < 0.001). However, no difference in OS was detected in patients receiving gemcitabine-based versus fluoropyrimidine-based combination therapy. (B) The impact of different therapies on OS in advanced pancreatic patients. Patients treated by chemotherapy or concurrent chemoradiotherapy had significantly longer lifespans than those who received best supportive care (P < 0.001). (C) In elderly patients (>70 years old), there is no significant difference in the OS with respect to different therapeutic strategies.
The Impact of Variable Contributing Factors on Survival Outcome
Univariate Cox regression analysis revealed that the following factors were associated with OS: age, ECOG PS, CCIS, initial CEA values, postprocedure serum TB/DB, and subsequent therapeutic management. We also performed multivariate analyses of the predictors determined by univariate Cox regression. The results showed that ECOG PS (an increased risk for each additional ECOG PS score, HR = 1.40, 95% CI: 1.05–1.85), CCIS (an increased risk for each additional CCIS score, HR = 1.46, 95% CI: 1.12–1.91), initial CEA values (HR = 1.01, 95% CI: 1.00–1.01), and chemotherapy (HR = 0.39, 95% CI: 0.17–0.87) were independently associated with OS (Table 3).
TABLE 3.
Multivariate Analysis of Factors Associated With Overall Survival in the Patients With Advanced Pancreatic Head Cancer (n = 79)

In our study, the median age of the patients was 68 years old. However, we stratified age according to the more common cutoff of 70 years and divided the patient population into 2 ago groups accordingly. We then performed a subgroup analysis that included 42 patients (53.2%) ≤70 years and 37 patients (46.8%) >70 years. Univariate Cox regression was performed as previously described on the variables listed in Table 3. We also performed multivariate analysis on the positive correlation predictors determined by univariate Cox regression in these 2 subgroups. Multivariate analysis in patients ≤70 years showed that initial serum CEA and chemotherapy/CCRT were independently associated with OS. However, in patients >70 years, CCIS played a more important role in predicting longer OS. An increased risk for each respective CCIS was observed (HR = 2.20, 95% CI: 1.41–3.41) (Table 4). Although there was a trend toward increased survival in patients >70 years who received therapy, the difference was not statistically significant in terms of the type of therapeutic management (Figure 1C). In patients >70 years and relatively lower CCIS (≤1), systemic therapy (chemotherapy or CCRT) or local therapy (radiotherapy) produced a survival benefit compared to patients receiving BSC alone (median survival for systemic/local therapy vs. BSC: 6.03 months vs. 2.50 months, respectively, P = 0.01; Figure 2A). Nevertheless, this therapeutic effect was not observed in patients >70 years with CCIS >1 (median survival for systemic/local therapy vs. BSC: 1.70 months vs. 1.73 months, respectively, P = 0.23; Figure 2B).
TABLE 4.
Univariate and Multivariate Analyses of Predictive Factors Associated With Overall Survival Between Different Age Groups of Patients With Advanced Pancreatic Head Cancer
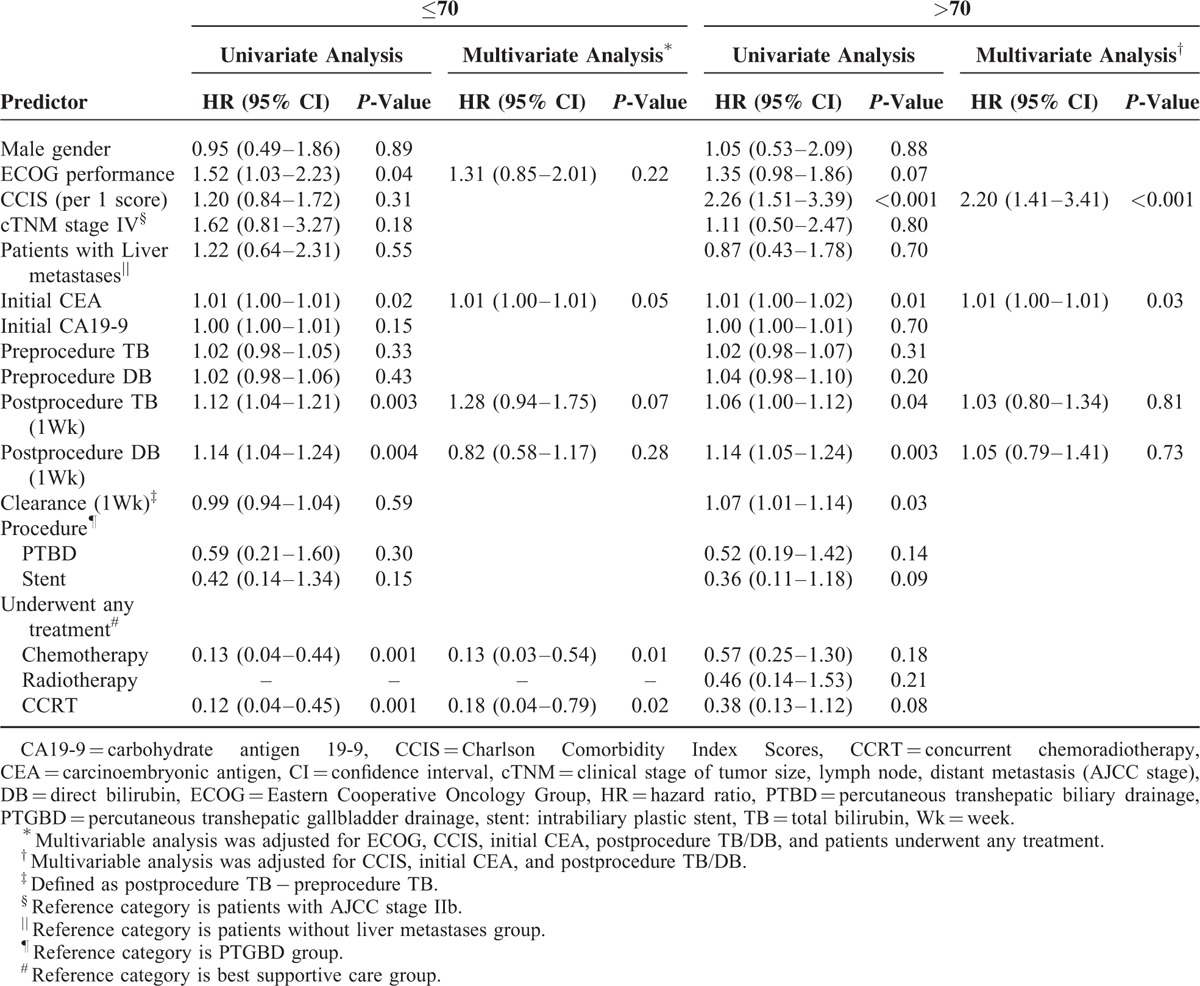
FIGURE 2.
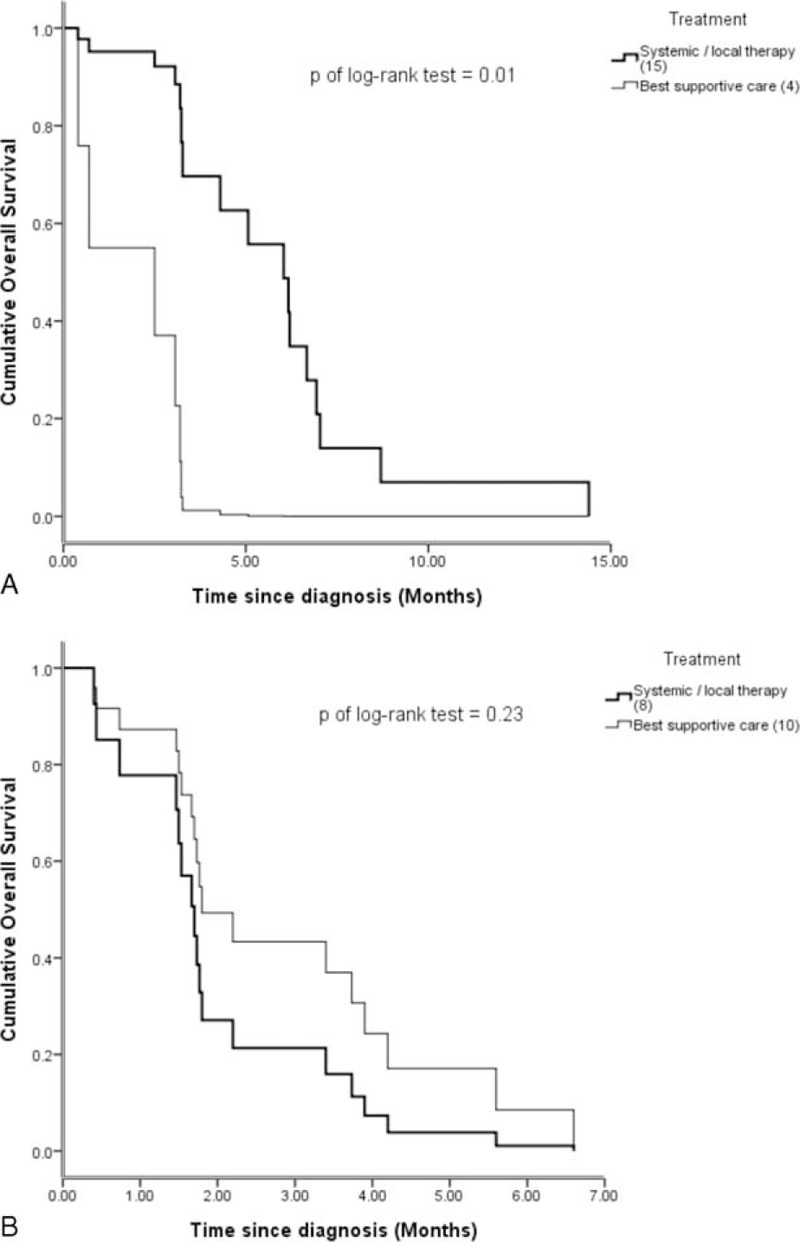
(A) In elderly patients (>70 years old) with less comorbidities (Charlson Comorbidity Index score ≤1 group), patients treated with systemic therapies had significantly longer lifespans than those who underwent best supportive care (P = 0.01). (B) In another group of elderly patients who had relatively more comorbidities (Charlson Comorbidity Index score >1), whether patients received systemic therapies or not had no statistically significant effect on their OS.
DISCUSSION
This study retrospectively evaluated the therapeutic outcomes in advanced pancreatic head cancer patients who initially presented with obstructive jaundice. The major strength of our study was that we used the simple parameters of patients’ age and comorbidities to evaluate therapeutic strategies. Our data suggested that the treatment modality should be modified according to patient status. In younger patients, aggressive chemotherapy has an important survival benefit; however, there was no survival benefit in elderly patients (>70 years) with more comorbidities (CCIS >1) who received systemic or local therapy.
In previous studies, chemotherapy treatment combined with FOLFIRINOX demonstrated obvious survival advantages in metastatic pancreatic cancer patients. However, increased toxicities in bone marrow and the gastrointestinal tract limited this regimen only to good performance patients.10 A plethora of additional studies have been conducted to explore better alternatives that can provide similar survival benefits with less toxicities than traditional chemotherapy agents.11,12 The results of several recent studies demonstrate that gemcitabine based combination therapy is the most suitable for patients with adequate performance status. Additionally, gemcitabine monotherapy, which has less treatment-related toxicities, is not inferior to these combination therapies, particularly in elderly patients or patients with poor performance status.13 A recent study was conducted to evaluate the role of systemic therapies in 237 metastatic pancreatic cancer patients, and showed that advanced age is not an absolute contraindication to receive systemic therapy and that there was still a survival benefit in elderly patients (>70 years) who underwent such therapies.14 However, in our present study group, we demonstrated that there was no survival benefit in patients >70 years old who received systemic therapy such as chemotherapy or CCRT.
With regard to therapeutic management, we found that underlying morbidities have a crucial role in predicting the survival benefit of treatment in elderly patients. The Charlson Comorbidity Index is a well-known scoring system that has been used to evaluate underlying comorbidity statuses in patients. Previous nationwide population-based cohort studies demonstrated that the CCIS could influence mortality in colorectal cancer patients. Successful management of underlying comorbidities may reduces mortality and avoid the deleterious effects of having the combined ailments of cancer and comorbidities.15 Another study also demonstrated that comorbidities are an independent risk factor in long-term survival of elderly pancreatic cancer patients who received gemcitabine-based chemotherapy.16 However, a phase III clinical trial revealed no prognostic role of age or comorbidity in pancreatic cancer patients receiving gemcitabine plus erlotinib therapy.17 In our present study, there was no significant difference in the survival of the elderly patient group (>70 years) with CCIS >1, but there was such a difference in elderly patients with CCIS ≤1. The CCIS may play a more important role in elderly pancreatic head cancer patients than in younger (≤70 years) patients.
There were 2 possible explanations of our results. First, all our cohort comprised of pancreatic head cancer patients who initially presented with hyperbilirubinemia. Despite the fact that our patients underwent biliary drainage procedures like PTBD or stenting, complications such as biliary tract infections (ascending cholangitis or catheter associated infection) could result in severe septic events more readily once chemotherapy-related neutropenia manifests. There was relative higher risk to get these infectious events or complications in elderly patients with more comorbidities. Second, although previous clinical trials showed that there was no increase in toxicity in elderly patients compared to younger patients, increasing studies have focused on low grade toxicities in elderly patients who received palliative or adjuvant chemotherapy.18,19 A retrospective study demonstrated that discontinuation of early treatment in elderly patients was generally because of intolerable lower grade toxicities; these patients had no major adverse effects due to chemotherapy, that is, >grade III chemotoxicities.20 These low grade toxicities could lead those elderly patients with more comorbidities readily to exhaust or die from their comorbidities but not from malignancy.
Furthermore, our data (Supplemental Tables 1 and 2, http://links.lww.com/MD/A357) revealed no difference in patients’ OS with respect to the type of biliary drainage procedure they underwent. However, more intervention-related complications, like obstruction and infection, were observed in patients in the PTBD and PTGBD groups compared to the endoscopic stent group. In patients with relatively more comorbidities or poor performance statuses, endoscopic stent implantation appears to be a better choice to avoid biliary tract infection and repeated obstruction-related hyperbilirubinemia.
There were several limitations in our present study. First, this was a retrospective study with several shortcomings, such as uneven case numbers, heterogeneity of patients, and different treatment protocols. Nevertheless, the study did reflect a real-life situation in a clinical practice, and determining influences due to specific treatments was not our primary objective. Second, this was a proof-of-concept inquiry using small cohorts of patients at a single institute. More valuable scoring systems including “activities of daily living” and “instrumental activities of daily living” scores could be more relevant than the ECOG PS system to assess general conditions.21–23 Third, there were no chemotherapy-related toxicities recorded for the duration of the systemic therapies in our study. Our data did not help determine an optimal management regimen that balances between treatment toxicity and efficacy.24 In elderly patients, the clinical efficacy of the geriatric scoring system must be assessed to avoid more obscure treatment-related adverse effect and lower grade toxicities. Our own studies did not included these scoring data.25 Fourth, although our results may provide useful information that assists physicians in making appropriate therapeutic decisions, there were some differences between the population examined in our study and other epidemiological distributions. Fifth, the fact that our results were derived from a retrospective single center study means that our data have lower statistical power than that derived from randomized trials.
CONCLUSION
This is the first study to investigate age and CCIS as predictive factors with which to determine therapeutic strategies in advanced pancreatic head cancer patients. In the general population as well as in elderly patients with relative lower CCIS, systemic therapy with chemotherapy is still the gold standard and has a greater survival benefit than local therapy or BSC. However, this therapeutic benefit is absent in elderly patients with more comorbidities.
Footnotes
Abbreviations: BSC = best supportive care, CA 19-9 = carbohydrate antigen 19-9, CCIS = Charlson Comorbidity Index Scores, CCRT = concurrent chemoradiotherapy, CEA = carcinoembryonic antigen, CI = confidence interval, ECOG PS = Eastern Cooperative Oncology Group performance status, HR = hazard ratio, OS = overall survival, PTBD = percutaneous transhepatic biliary drainage, PTGBD = percutaneous transhepatic gallbladder drainage, SD = standard deviation, Stent = intrabiliary plastic stent.
Author contributions: These authors’ individual contributions were as follows. These authors’ individual contributions were as follows. Conception and design: Yu-Guang Chen, Ching-Liang Ho; collection and assembly of data: all authors; data analysis and interpretation: Yu-Guang Chen and Hsueh-Hsing Pan; manuscript writing: all authors; final approval of manuscript: all authors.
Sources of funding: None.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012; 118:2674–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol 2014; 20:10802–10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004; 91:586–594. [DOI] [PubMed] [Google Scholar]
- 5.Gurusamy KS, Kumar S, Davidson BR, et al. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev 2014; 2:CD010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toki MI, Syrigos KN, Saif MW. The role of biliary drainage in patients with pancreatic adenocarcinoma. JOP 2014; 15:128–131. [DOI] [PubMed] [Google Scholar]
- 7.Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol 2015; 7:68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huguet F, Mukherjee S, Javle M. Locally advanced pancreatic cancer: the role of definitive chemoradiotherapy. Clin Oncol (R Coll Radiol) 2014; 26:560–568. [DOI] [PubMed] [Google Scholar]
- 9.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 11.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31:1640–1648. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann L, Maute L, Heil G, et al. A prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research—EWIV. Eur J Cancer 2015; 51:27–36. [DOI] [PubMed] [Google Scholar]
- 13.Mian OY, Ram AN, Tuli R, et al. Management options in locally advanced pancreatic cancer. Curr Oncol Rep 2014; 16:388. [DOI] [PubMed] [Google Scholar]
- 14.Kougioumtzopoulou AS, Syrigos KN, Saif MW. Elderly patients with pancreatic cancer. JOP 2014; 15:322–325. [DOI] [PubMed] [Google Scholar]
- 15.Erichsen R, Horvath-Puho E, Iversen LH, et al. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer 2013; 109:2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakai Y, Isayama H, Sasaki T, et al. Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapy. Crit Rev Oncol Hematol 2011; 78:252–259. [DOI] [PubMed] [Google Scholar]
- 17.Vickers MM, Powell ED, Asmis TR, et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer—results from NCIC CTG PA.3: a phase III trial of gemcitabine plus erlotinib or placebo. Eur J Cancer 2012; 48:1434–1442. [DOI] [PubMed] [Google Scholar]
- 18.Giovanazzi-Bannon S, Rademaker A, Lai G, et al. Treatment tolerance of elderly cancer patients entered onto phase II clinical trials: an Illinois Cancer Center study. J Clin Oncol 1994; 12:2447–2452. [DOI] [PubMed] [Google Scholar]
- 19.Droz JP, Aapro M, Balducci L. Overcoming challenges associated with chemotherapy treatment in the senior adult population. Crit Rev Oncol Hematol 2008; 68 Suppl 1:S1–S8. [DOI] [PubMed] [Google Scholar]
- 20.Kalsi T, Babic-Illman G, Fields P, et al. The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. Br J Cancer 2014; 111:2224–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschollek M, Nemitz G, Gietzelt M, et al. Predicting in-patient falls in a geriatric clinic: a clinical study combining assessment data and simple sensory gait measurements. Z Gerontol Geriatr 2009; 42:317–321. [DOI] [PubMed] [Google Scholar]
- 22.Ramjaun A, Nassif MO, Krotneva S, et al. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol 2013; 4:271–281. [DOI] [PubMed] [Google Scholar]
- 23.Leo S, Accettura C, Gnoni A, et al. Systemic treatment of gastrointestinal cancer in elderly patients. J Gastrointest Cancer 2013; 44:22–32. [DOI] [PubMed] [Google Scholar]
- 24.Galic S, Glavic Z, Cesarik M. Stress and quality of life in patients with gastrointestinal cancer. Acta Clin Croat 2014; 53:279–290. [PubMed] [Google Scholar]
- 25.Hamaker ME, Vos AG, Smorenburg CH, et al. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist 2012; 17:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]


