Supplemental Digital Content is available in the text
Abstract
Gastric cancer (GC) is one of the most common upper gastrointestinal malignancies. Surgical resection remains the mainstay of curative treatment for GC. Enteral immunonutrition (EIN) has been increasingly used to enhance host immunity and relieve inflammatory response of patients undergoing surgery for GC; however, conclusions across studies still remain unclear. We aimed to evaluate the effects of EIN for such patients.
We searched some electronic databases including PubMed, EBSCO-Medline, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE to identify any latent studies which investigated the effects of EIN compared with standard EN on GC patients who undergoing surgery until the end of December 30, 2014. Relative risk (RR), mean difference (MD), or standard mean difference (SMD) with 95% confidence interval (CI) were calculated and we also assessed heterogeneity by using Cochrane Q and I2 statistic combined with corresponding P-value.
We included 9 eligible studies which included 785 patients eventually. The meta-analysis results shown that EIN increased level of IgA (MD, 0.31; 95% CI, 0.12–0.51), IgG (MD, 1.5; 95% CI, 0.73–2.28), IgM (MD, 0.22; 95% CI, 0.06–0.39), CD4+ (SMD, 0.81; 95% CI, 0.53–1.09), CD3+ (SMD, 0.68; 95% CI, 0.21–1.15), CD4+/CD8+ ratio (MD, 0.56; 95% CI, 0.12–1.01), and NK cell (MD, 2.35; 95% CI, 0.66–4.05); decreased IL-6 (MD, −98.22; 95% CI, −156.16 to −40.28) and TNF-α (MD, −118.29; 95% CI, −162.00 to −74.58), but not improve remained outcomes of interest involving postoperative complications, length of hospitalization, serum total protein, and CD8+. Descriptive analysis suggested that EIN also increased the concentration of IL-2 but not CRP. Impact on lymphocytes remains inconsistent.
EIN is effective for enhancing host immunity and relieving the inflammatory response in GC patients undergoing gastrectomy, but clinical outcomes cannot be benefit from it. Heterogeneity caused by different compositions and timing of administration of EIN regimes and not enough sample size and number of eligible studies in most of sensitive analyses with subgroup analysis may impaired the power of our study, and thus some large-scale and well-designed studies are warranted to further establish effects.
INTRODUCTION
Gastric cancer (GC) is one of the most common upper gastrointestinal malignant tumors. It negatively affects the patient's health and quality of life and seriously increases the financial burden to the family and the whole society.1 Surgical resection is still the mainstay of curative treatment although effective alternatives has been developed.2 It is noted that, however, patients undergoing surgery for GC will suffer from various serious postoperative complications, such as malnutrition and immune function suppression. Importantly, these given postoperative complications are well known factors capable of impairing the immunological functions3–5 and causing vicious circle eventually because of the catabolism and changes in the metabolic, endocrine, neuroendocrine, as well as immune function suppression resulted from stress effect caused by surgery, absolute diet, and infection.2–4
Proper nutrition regimes have been an important issue for clinicians to facilitate recovery of the patients undergoing the gastrectomy.6 Studies previously published suggested that enteral nutrition (EN) support is more economical and effective and have lower complications than parenteral nutrition (PN).7–10 However, standard EN product has insufficient essential immune strengthening ingredients which effectively improve the immune function of GC patients after gastrectomy.10 In contrast, enteral immunonutrition (EIN), a immune product which enriched with arginine (Arg), glutamine (Gln), omega-3 fatty acids (α-3-FAs), and ribonucleic acid (RNA), just remedy those weakness existed in standard EN product. Gln and Arg are conditionally essential amino acid (AA). Secretion of various hormones (eg, growth hormone, glucagon, and insulin) which can profoundly modulate the immune response will be initiated under the stimulation of Arg.11 Polyamines and nucleic acids which are all essential substances guarantee that cell successfully finish the process of proliferation and differentiation.12 In addition, Arg is also associated with increase of lymphocyte mitogenic, allogenic responses, and cytotoxicity of natural killer cell.13 Gln is a precursor for synthesis of nucleotide and glutathione, and what's more is that the glutathione is at the heart of antioxidative defense.14 The structural and functional integrity of the cell membrane, intercellular signal transduction, and eicosanoids synthesis is the basis on which fatty acids modulate the immune response.15 The flexibility of membrane that is essential for phagocytosis and expression of IL-2 receptors will be enhanced if other fatty acids were replaced by α-3-FAs.16
Hence, the investigators increasingly have shifted their attention away from standard EN, and are now focused on EIN.13,17,18 Although a wealth of studies have been completed in order to determine the effects on EIN compared to standard EN in GC patients who underwent the gastrectomy; however, whether the EIN is effective for improving the immunologic and clinical status compared with standard EN remains uncertainty.2,14,21–25 Therefore, we performed this systematic review and meta-analysis of randomized controlled trials (RCTs) to systematically evaluate the impact of EIN on clinical and immunological outcomes of patients requiring selective surgery for GC.
MATERIALS AND METHODS
We planned and performed this systematic review and meta-analysis in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement26 and Cochrane Handbook for Systematic Reviews of Intervention.27 The prospective protocol of this study has registered on PROSPERO database (available at: http://www.crd.york.ac.uk/prospero/) and a registration number has been approved (CRD42015017893). We critically assessed the reporting quality of the study according to the PRISMA 2009 checklists (Supplemental Table 1 http://links.lww.com/MD/A360).26 This study did not require the ethic approval and informed consent due to all analyses were carried out based on the data extracted from previous published trials.
Citations Capture
We searched target electronic databases which included PubMed, EBSCO-Medline, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE to identify all latent randomized controlled trials (RCTs) which investigated effects of EIN relative to standard EN for GC patients who were submitted to gastrectomy until the end of December 30, 2014. The computerized search procedure was conducted by using following search terms based on the strategy of combination medical subject heading (MeSH) and free text: “Stomach Neoplasms,” “Gastric Cancer∗,” “Gastric Carcinoma∗,” “Gastric Neoplasm∗,” “Stomach Cancer∗,” “Stomach Carcinoma∗,” “Gastric Tumor∗,” “Enteral Immune Nutrition, ” “Enteral Immunonutrition,” “Immunoenhanced Enteral Nutrition,” “Immune-Enhancing Enteral Nutrition,” “Immune Enhanced Enteral Nutrition,” “Randomized Controlled Trial,” “Randomized Controlled Trials as Topic,” “Random∗.” Lists of references of eligible studies and topic-related reviews were also manually searched to guarantee the precision and recall ratio. Only article published in English- or Chinese language met the inclusion criteria. The PubMed and EBSCO-Medline search terms and string were presented in Appendixes 1 and 2, respectively.
Entry Criteria
We defined following eligibility criteria according to the PCIOS acronym (population, intervention, comparison, outcome, and study design): Population (P): All the patients diagnosed with GC with histological techniques scheduled for gastrectomy were included in the systematic review and meta-analysis. Intervention (I): EIN. Comparison (C): standard EN. Outcomes of interest (O): we assessed the following outcome measures of interest: clinical outcomes including infectious complications which was categorized into surgical site infection (SSI) and other infectious complications (eg, respiratory infection, urinary tract infection, abdominal abscess, etc.) defined according to the criteria issued by the American College of Chest Physician, Centers for Diseases Control guidelines, or others established by authors and length of hospitalization; immune indices which consisted of immunoglobulin (including IgA, IgG, and IgM), T cell subsets (included CD3+, CD4+, CD8+, CD4+/CD8+ ratio), cytokines (interlukin-2 [IL-6], IL-6, tumor necrosis factor-alpha [TNF-α]), and natural killer cell (NK cell); and biochemical indices (refers to total protein, albumin, peoalbumin, transferrin). Study design (S): only RCTs were included into our study.
The references will be excluded if it met following one of the items: patients have unresectable neoplasm, underlying cardiovascular pathology, previous abdominal radiotherapy, active preoperative infection, administration of corticosteroids or immunosuppressive agents, and renal or hepatic function impairment; experimental data; lack of essential information and cannot acquire primary data from authors; we only incorporate one with the most strict methodology and most complete data of articles, in which the same data were reported by 1 author or a medical center, into our study; nonoriginal research, such as review, letter and specialist comments and non-RCTs.
Data Extraction
The following basic information and essential continuous and dichotomous data for specific outcome were extracted independently from each original eligible study by 2 authors (Guo-Min Song and Xu Tian) by using the predesigned data extraction table (Supplemental Table 2 http://links.lww.com/MD/A360): study ID (including surname of the first author and publication year), country, diagnosis, age of participants, sample size, nutrition status, study setting and interventions, and reporting outcome measures of interest. The author would be contacted to acquire the complete data when necessary. Any divergences between authors concerning the eligibility of a study were resolved by consensus or consulting a third author (Hui Liang or Li-Juan Yi).
Assessment of Risk of Bias
We assigned independent 2 investigators (Ting Shuai and Zi Zeng) to critically assess the risk of bias of all eligible studies according to the Cochrane Collaboration's tool for assessing the risk of bias.27 The following evaluation domains were assessed accordingly: randomization sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other biases.27 The risk of each domain was rated as “high risk,” “unclear risk,” or “low risk” according to the match level between information extracted and evaluation criteria.27
Statistical Analysis
All analyses were conducted by using Review Manager (RevMan) 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013). We calculated the relative risk (RR) with corresponding 95 % confidence interval (CI) to estimate the dichotomous outcomes. For continuous data, the pooled results were expressed as the mean difference (MD) or standard mean difference (SMD) with 95% CI. The end-point value was extracted to calculate the effect size.27 We evaluated the heterogeneity across studies of each outcome measure by using the Chi-square test and associated with P-value, Moreover, substantial level of heterogeneity was estimated by I2 statistic proposed by Higgins et al. If I2 was <50%, the eligible studies were thought to be heterogeneity; in contrast, if I2 was ≥50%, the pooled results will be affected by heterogeneity. We performed each meta-analysis via selected a random-effects model or fixed-effects model based on Mantel–Haenszel (MH) or inverse variance (IV) statistical approach. Assessment of the clinical characteristic and methodology of eligible studies pooled is the premise to determine the selection stated above. A qualitative analysis will be used to describe the studies, in which data are incomplete; heterogeneity can affect the pooled results or lack of a number of studies to pool. We did not produce the funnel plot to test the publication bias due to the limited number (below 10) of studies included in each analysis.28 Separate sensitive analyses were also conducted with subgroup analysis through changed inclusion criteria to assess the robustness of summarized effect sizes.
RESULTS
Study Selection and Trial Characteristics
We used the PRISMA 2009 flow diagram (see Supplemental Table 3 http://links.lww.com/MD/A360) to guide the process of study selection. A total of 82 citations were captured at the initial searched stage. Forty-two citations were duplicated by using EndNote 7.2.1 literature manager software. Twenty-four studies were excluded according to following reasons when the investigators independently reviewed the title and abstract of remained latent citations: 4 studies were performed based on ineligible intervention regimes which included PN alone group versus standard EN alone group versus (Bazhen Decoction + standard EN) group (3 groups were established) and (rhubarb + Sijunzi decoction + standard EN group vs. standard EN alone group (2 research groups), and (Sijunzi decoction + standard EN) group versus standard EN alone group (2 study groups); 1 study was published in Turkish; 16 studies enrolled ineligible participants who included patients undergoing chemotherapy, patients with esophageal cancer, and patients with various cancers; and 3 irrelevant studies. Eventually, 9 eligible studies2,14,19–25 met our inclusion criteria and 7 studies were excluded due to following reasons: ineligible interventions (n = 4) which included standard EN alone group versus (HIIC + standard EN) group versus (HIIC + PN) group (3 groups were divided) and standard EN alone versus conventional postoperative care (2 groups were identified); ineligible participants (n = 1) which included patients with various cancers such as esophageal cancer and pancreatic carcinoma, etc.; ineligible intervention regimes (n = 2) refer to perioperative versus postoperative immunonutrition and preoperative EN versus postoperative EN (2 research group); lack of essential data (n = 1). A flow chart of literature retrieval and selection is shown in Figure 1. The basic characteristics of each eligible study included in the systematic review and meta-analysis are summarized in Table 1 .
FIGURE 1.
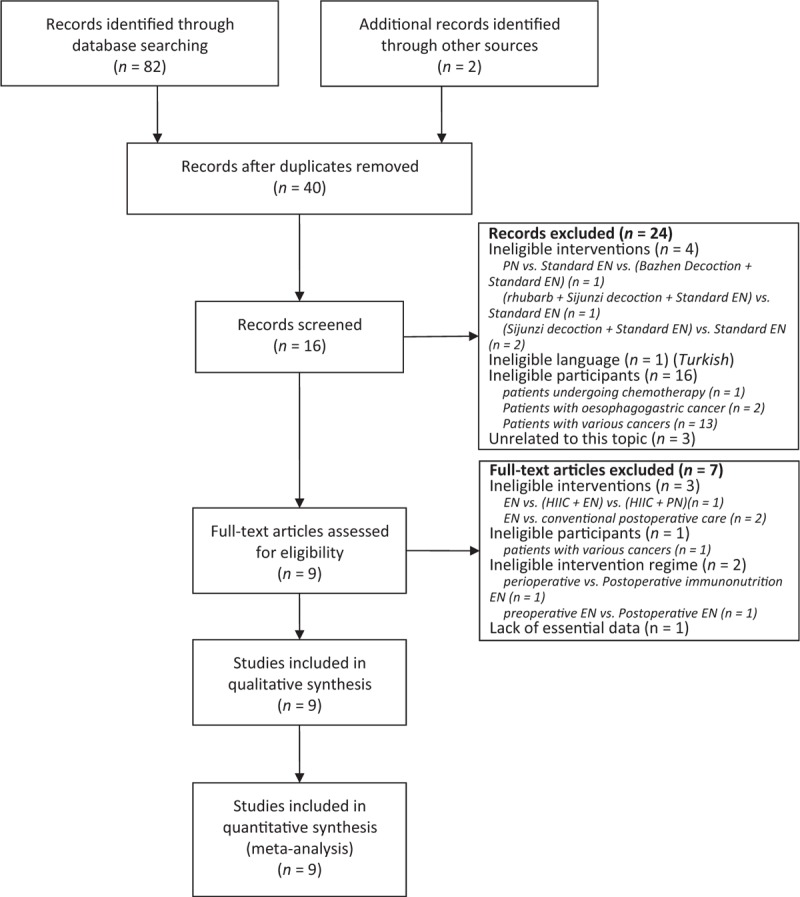
Flowchart of computerized searched and included eligible studies into this systematic review and meta-analysis.
TABLE 1.
Basic Characteristics of Each Study Included in This Systematic Review and Meta-Analysis
Assessing Risk of Bias
We identified 9 eligible RCTs2,14,19–25 and of all incorporated into the study to estimate corresponding pooled effect size. Six studies2,14,19,22–24 reported the methods which generated the randomization sequence, three2,19,24 of all studies appropriately performed the allocation concealment and blinding of personnel and personnel, only one23 masked the outcome assessor, 3 studies2,14,19 reported the drop-out before conducting the EIN and remained reported the expected outcome measures of interest; therefore, corresponding domain was graded as “low risk,” of all reported the expected outcome measures of interest, and no other bias sources were detected. The assessment of risk of bias outcome of each study is summarized in Figure 2A and B.
FIGURE 2.
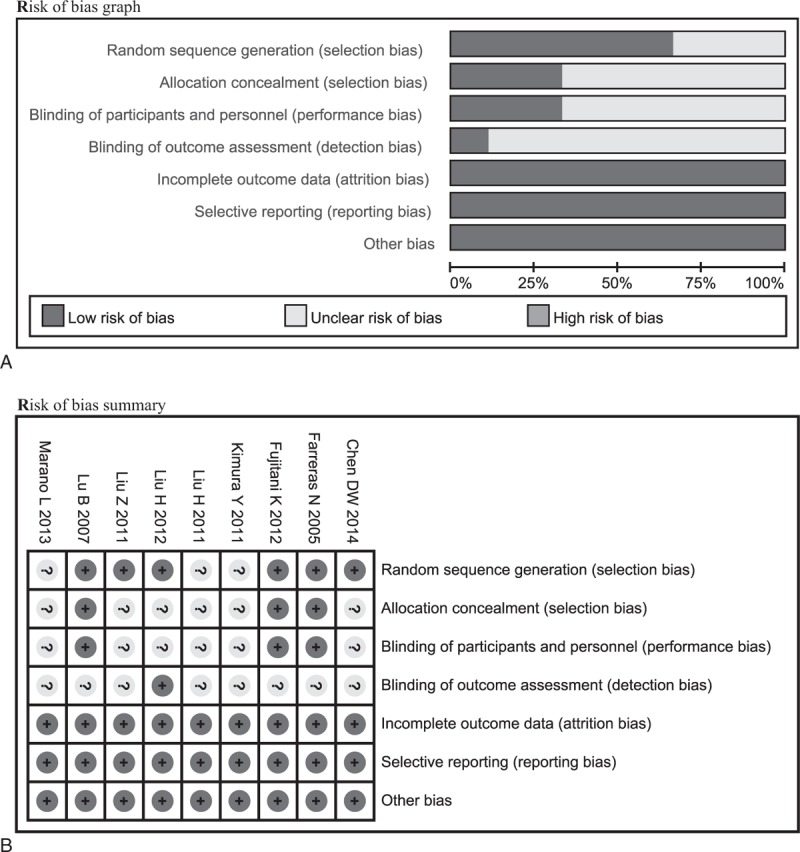
Assessment of risk of bias based on the evaluation domains listed in the Cochrane Collaboration's Risk of Bias Tool: risk of bias graph (A), risk of bias summary (B).
Meta-Analysis on Clinical Outcomes
Depending on the difference in reporting on the index, we performed 2 independent meta-analyses for postoperative infectious complications which included SSI and other infectious complications (refer to respiratory infection, urinary tract infection, abdominal sepsis, etc.). Five studies2,19,20,23,25 which included 671 participants reported the SSI indicator, and all were pooled to examine the effect of EIN for patients scheduled for gastrectomy. All were considered to be homogeneity (χ2 = 3.10, P = 0.54, I2 = 0%); therefore, a fixed-effect model was selected to calculate the effect size. The pooled results revealed that there was no significant difference in terms of SSI between groups (RR, 1.04; 95% CI, 0.75–1.44; P = 0.82) (see Supplemental Figure 1a http://links.lww.com/MD/A360). Other infectious complications were presented in 4 studies2,19,23,25 which included 232 and 220 patients between groups, respectively. No obvious statistical heterogeneity were identified (χ2 = 4.26, P = 0.23, I2 = 30%); therefore, we adopted a fixed-effects model to estimate the effect size. The meta-analysis indicated no significant difference between groups in terms of the given index (RR, 0.79; 95% CI, 0.54–1.16; P = 0.23) (see Supplemental Figure 1a http://links.lww.com/MD/A360).
The data of length of hospitalization were reported in 2 studies2,19 which enrolled 291 participants. We estimated the associated mean and standard deviation according to the median and range by using the equation proposed by Hozo et al.29 There was statistical heterogeneity (χ2 = 2.64, P = 0.10, I2 = 62%) though no significant differences in clinical and methodology were identified; so a random-effect model was used. The estimated effect size indicated no significant difference between groups (MD, −0.88; 95% CI, −3.73 to 1.96; P = 0.54) (see Supplemental Figure 1b http://links.lww.com/MD/A360).
Meta-Analysis on Immune Indices
Data of CD4+ can be extracted from 6 studies,14,21–25 and corresponding effect size was expressed as SMD with 95% CI due to different units were used across studies. However, one25 performed by Marano et al was not included to calculate estimate of interest due to significant variance in magnitude and unit; hence a descriptive analysis was selected to present corresponding value. Eventually, 5 eligible studies were incorporated into the given synthesis. These studies were deemed to be homogeneity (χ2 = 3.92, P = 0.42, I2 = 0%) and a random-effect model was selected to calculate the result. The meta-analysis indicated a significant difference between groups (SMD, 0.81; 95% CI, 0.53–1.09; P = 0.00) (see Figure 3A). Moreover, the same studies14,21–25 reported the CD8+ and statistical heterogeneity was identified (χ2 = 17.72, P = 0.00, I2 = 77%); therefore, a random-effect model was used and result indicated no significant difference between groups in terms of CD8+ (SMD, 0.07; 95% CI, −0.50 to 0.64; P = 0.81) (see Figure 3B). Two studies22,24 reported the CD3+, and they were deemed to be homogeneity. The meta-analysis indicated a significant difference between (SMD, 0.68; 95% CI, 0.21–1.15; P = 0.00) (see Figure 3C). Three studies14,22,24 presented the information of CD4+/CD8+ ratio and all were deemed to be heterogeneity (χ2 = 24.42, P = 0.00, I2 = 88%), so we used random-effect model to pool them. The meta-analysis suggested that EIN effectively increased the level of CD4+/CD8+ ratio (MD, 0.56; 95% CI, 0.12–1.01; P = 0.01) (see Figure 3D).
FIGURE 3.
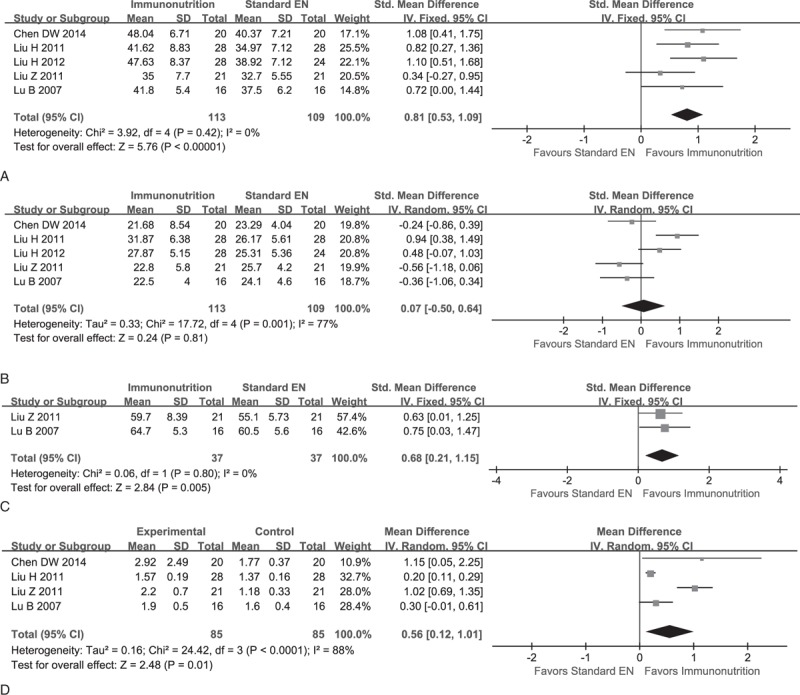
Meta-analysis on immune indices. (A) Pooled result of CD4+ between EIN and standard EN: fixed-effect model. (B) Change of CD8+ when EIM compared to standard EN: random-effect model. (C): change of CD3+ when compared EIN with standard EN: fixed-effect model. (D) Change of CD4+/CD8+ ratio when EIN relative to standard EN: random-effect model.
Three studies which included 114 patients,14,22,24 5 studies including 222 participants,14,21–24 and 5 studies14,21–24 which enrolled 222 clients reported the IgA, IgG, and IgM, respectively. No statistical heterogeneity was detected of IgA (χ2 = 1.74, P = 0.42, I2 = 0%); however, meta-analysis on IgG (χ2 = 10.39, P = 0.03, I2 = 62%) and IgM (χ2 = 14.75, P = 0.01, I2 = 73%) have statistical heterogeneity; therefore, we adopted a random-effect model to perform all analyses. The meta-analyses revealed that EIN effectively increased the level of IgA (SMD, 0.31; 95% CI, 0.12–0.51; P = 0.00), IgG (SMD, 1.50; 95% CI, 0.73–2.28; P = 0.00), and IgM (SMD, 0.22; 95% CI, 0.06–0.39; P = 0.00) (see Figure 4A–C).
FIGURE 4.
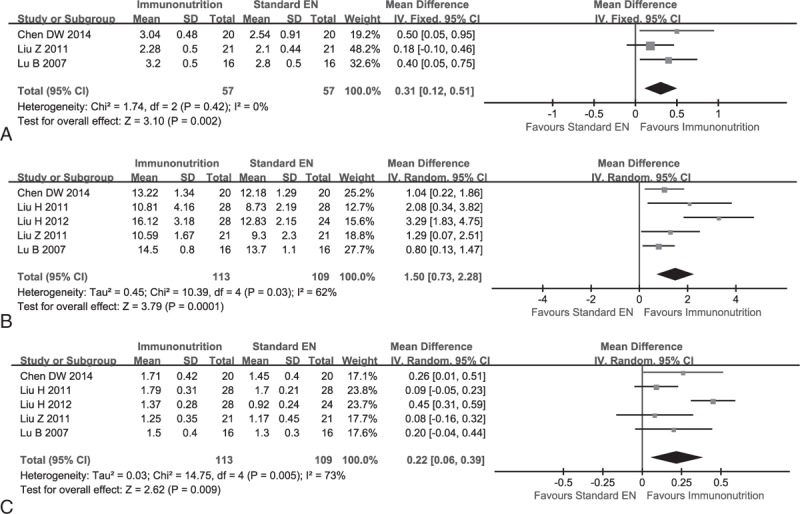
Meta-analysis on immunoglobulin (IG). (A) Change of IgA between EIN and standard EN: fixed-effect model. (B) Change of IgG when EIN versus standard EN: random-effect model. (C) Change of IgM when EIN relative to standard EN: random-effect model.
Two studies14,22 reported the interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and they were deemed to be homogeneity in terms of IL-6 (χ2 = 0.39, P = 0.53, I2 = 0%) and TNF-α (χ2 = 0.33, P = 0.57, I2 = 0%); and then we used a fixed-effect model to carried out meta-analyses. The pooled results revealed that the level of IL-6 (MD, −0.71; 95% CI, −1.15 to −0.26; P = 0.00) and TNF-α (MD, −1.14; 95% CI, −1.61 to −0.66; P = 0.00) significantly decreased in EIN group (see Figure 5A and B).
FIGURE 5.
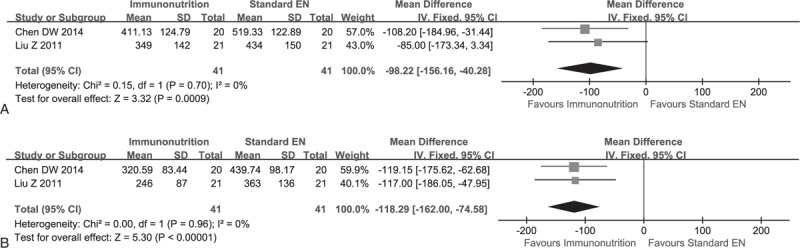
Meta-analysis on cytokine. (A) Change of IL-6 between EIN and standard EN: fixed-effect model. (B) Change of TNF-α Between EIN and standard EN: fixed-effect model.
Data of counts of NK cell were extracted from 2 studies,21,23 which included 49 and 45 patients between groups, respectively. They were deemed to be homogeneity (χ2 = 0.02, P = 0.90, I2 = 0%); and thus a fixed-effect model was used to perform the meta-analysis. The pooled result suggested that the count of the NK cell in EIN group were superior to that of standard EN group (MD, 2.35; 95% CI, 0.66–4.05; P = 0.00) (see Figure 6).
FIGURE 6.
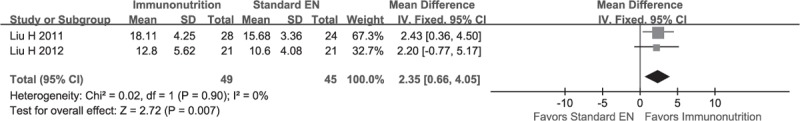
Meta-analysis on NK cell and the result indicate that EIN is superior to standard EN in terms of this given outcomes.
Meta-Analysis on Biochemical Indices
The data of serum total protein, albumin, proalbumin, and transferrin were extracted from 5 studies which included 289 patients,14,21,23–25 4 trials enrolling 180 patients,14,21,23,24 5 studies recruiting 289 participants,14,21,23–25 and 4 studies which included 270 patients.14,21,23,24 The meta-analysis on proalbumin and transferrin indicated statistical heterogeneity; so a random-effect model was adopted to conduct these meta-analyses. The summarized results indicated no significant differences between groups in terms of all serum indexes (see Supplemental Figure 2a–d http://links.lww.com/MD/A360).
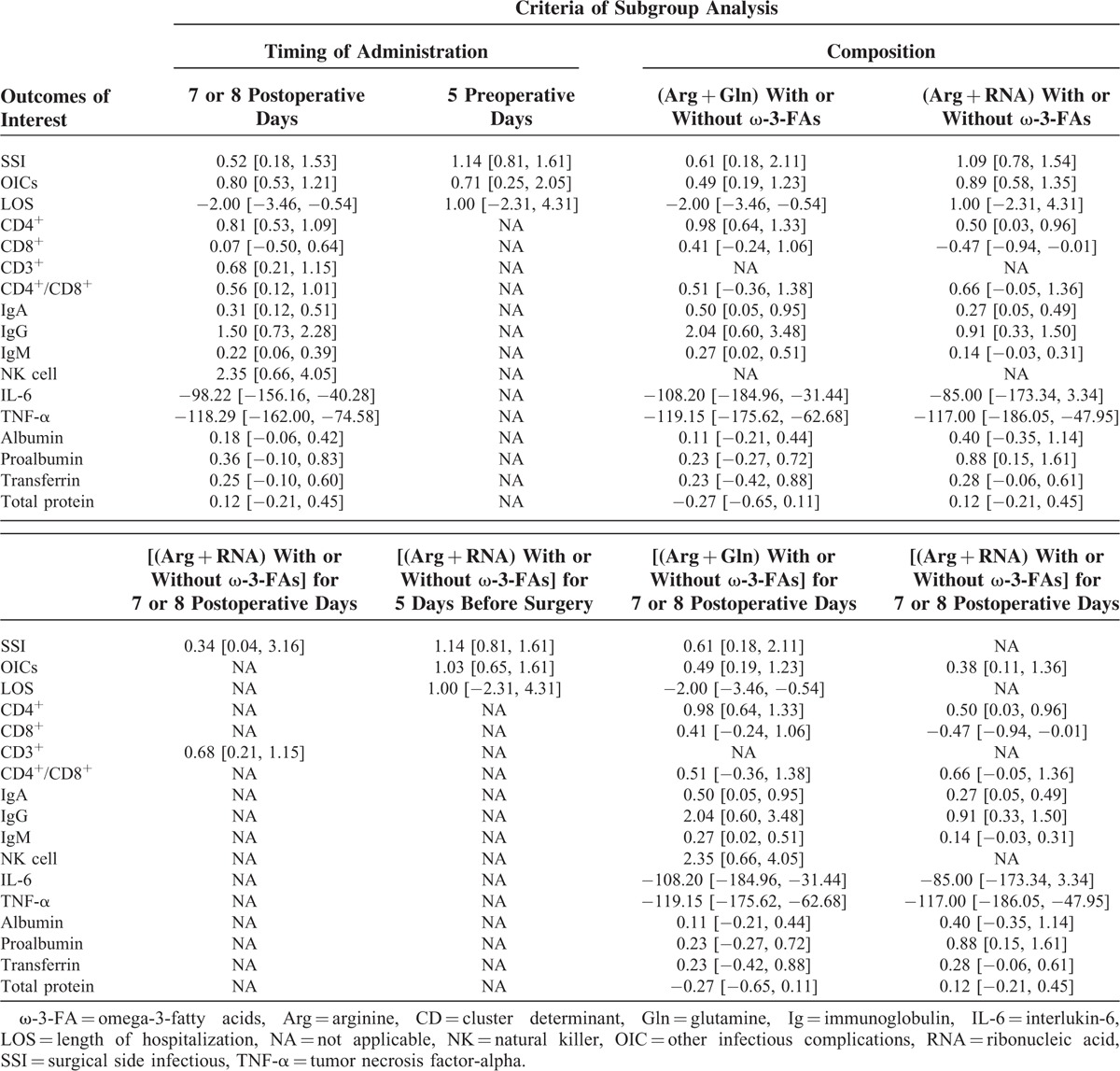
Sensitive Analysis
Sensitive analysis is a statistical method which was adopted to test the reliability of pooled result through omitting study with low-quality, changing effect size, inclusion criteria, or analysis model. To assess the robustness of summarized results in our study, we conducted also separate sensitive analyses based on various inclusion criteria. These estimated effect sizes are summarized in Table 2.
TABLE 1 (Continued).
Basic Characteristics of Each Study Included in This Systematic Review and Meta-Analysis
TABLE 2.
Sensitive Analysis With Subgroup Analysis Based on Various Inclusion Criteria
Descriptive Analysis
Only 1 study14 reported the IL-2, and thus we did not perform a meta-analysis. The study indicated that EIN increased the level of IL-2, with significant statistical difference compared with standard EN (P < 0.01). Two studies19,25 reported the lymphocytes. However, the results are inconsistent and one15 indicated that EIN significantly increased the counts of lymphocyte, in contrast, another24 suggested no significant difference between group. One study24 reported the leukocytes and indicated that corresponding counts in EIN group is higher than that of standard EN. One study14 investigated the C-response protein (CRP) and indicated no significant difference between group (P = 0.11). Most importantly, one24 which was excluded from synthesis analysis on CD4+ and CD8+ suggested that the level of CD4+ and CD8+ in EIN group was lower than that of standard EN group. The result was different from the pooled result.
DISCUSSION
Despite its incidence have decreased substantially over the past few decades, GC is currently one of the major health problem worldwide and the fifth most common type of cancer.30 Issued data indicated that 989 thousands new cases and 738 thousands cancer deaths caused by GC occurred annually worldwide.31 Most patients with GC are subjected to poor prognosis and published data have illustrated that corresponding 5-year overall survival rates (OS) is approximately 20%.32 The gastrectomy remains the primary cure.33 However, surgical resection is a potential contributor to further impair the host immune defense function and altered inflammatory responses in GC patients who have underlying malnutrition and immune function suppression.10,34 Therefore, investigators have increasingly focused on the host defense of the gastrointestinal tract in recent decades,35 and evidence suggest that the EN is superior to PN route in terms of effect for reducing the incidence of postoperative complications.36 However, the EIN product has been gaining increasing attention for the purpose to improve the status of nutrition and immune function of patients underwent surgery for GC.37
To our knowledge, this is the first systematic review and meta-analysis to evaluate the clinical and immunological impact of EIN in GC patients undergoing surgery. The findings of our meta-analysis suggested that EIN effectively increased the level of IgA, IgG, IgM, CD4+, CD3+, CD4+/CD8+ ratio, and the count of NK cell. A wealth of cells such as NK cells, lymphocytes, and macrophage will be involved into modulating its immune function due to complexity of immune system in gastrointestinal tract. Because of EIN significantly improved the levels of CD3+, CD4+ and CD4+/CD8+, so bacterial translocation (BT) which was closely association with reduction of adenosine triphosphate was suppressed, and eventually, the intestinal immune barrier and resistance of microbe were enhanced. The pooled results also indicated that the EIN is a contributor to enhance the humoral and cellular immune function14 due to the concentrations of associated immunoglobulin were significantly increased and may be an alternative for enhancing the host immunity and relieving the inflammatory response. For cytokines, EIN significantly decreased the concentration of IL-6 and TNF-α and one14 indicated that the degree of IL-2 in EIN group is higher than that of conventional EN group. IL-2 is an essential substance to modulate cellular immune response and is associated with proliferation and differentiation of T cell and eventually enhanced the humoral immune function.16 IL-6, belongs to inflammatory mediator, is a core substance which relieve the stress response. TNF-α which is an inflammatory cytokine mainly originated from immune cell (eg, T cell), endotheliocyte, macrophage, etc. It can effectively modulate the immune function and resist infection.38 Achieved these profound results was reasonable due to the basic components which included Arg, Gln, ω-3 fatty acids, and RNA play an important role in modulating the secretion of various hormones, cytokines, and so on. However, EIN did not decrease the incidence of postoperative complications, shorten the length of hospitalization, and improve the level of serum indexes (including total protein, albumin, proalbumin, transferring), and CD8+. For lymphocytes, the results remain inconsistent. The pooled results are contrary to that of meta-analysis which analyzed the effects of all Chinese RCTs.39 Nutrition status is the essential to promote the healing process of surgical site and enhance the disease resistance. Our study proved that EIN cannot effectively promote protein synthesis, and thus it cannot also improve the nutrition status of GC patients who underwent gastrectomy compared to standard EN. So the postoperative complications and length of hospitalization will not be improved. The pooled results of postoperative complications and length of hospitalization further confirmed the relation.
Due to various compositions of EIN regimes and timing of administration were designed across studies, we conducted separate sensitive analyses with subgroup analysis through changing inclusion criteria to further assess the effects of EIN versus standard EN. Although various pooled results were generated from different sensitive analyses with subgroup analysis, the insufficient required sample size and small number of eligible studies impaired the power of most of summarized estimate effect sizes. In addition, heterogeneous existed in these eligible studies and it may impair the power of our study. Consequently, well-designed and large-scale RCTs are urgently warranted to further establish associated effects.
Our systematic review and meta-analysis included more RCTs via extensive literature retrieval. Meanwhile, the statistical power and precision ratio were increased by enlarged the sample size based on this study, and thus more accurate estimates of effects were generated. However, there are several limitations of our study that need to be acknowledged. Firstly, and perhaps most notably, the power of all meta-analyses which were performed based on the end-point value were impaired due to the durations of interventions are different across studies. Secondly, only a small number of studies which included large sample size met inclusion criteria of the study, and thus reduced the power of these pooled results. Thirdly, these studies in other language except for English and Chinese language were ineligible for our criteria, so it is possible that additional relevant studies may have not been identified if the search had been extended to literature in other languages. A search of 4 electronic databases including PubMed, EMBASE, CENTRAL, and EBSCO was performed; however, ISI Web of Science, SpringerLink, ScienceDirect, and China Biomedical Literature Database (CBM) were not been included, so the risk of incompletely retrieved information also impaired the power of the meta-analysis. All trials incorporated into the meta-analysis were deemed to be homogeneity in clinical and methodological aspects; however, significant statistical heterogeneity which impaired the power of all meta-analysis was detected for meta-analyses in terms of certain outcome measures. In addition, the test on publication bias for studies was not conducted due to the small number of eligible studies, and it may have a negative effect on the pooled results of current meta-analysis. Finally, the unpublished and missing data might lead bias to the pooled effect. More importantly, some descriptive analysis presented associated results which were different from estimates of effects generated from our synthesis analysis.
CONCLUSIONS
EIN is effective for improving the nutritional and immunological status of GC patients undergoing gastrectomy because it can effectively enhance the host immunity and relieve the inflammatory response through significantly increasing the level of IgG, relevant T cell subsets and NK cell, and obviously decreasing the concentration of cytokines such as IL-6 and TNF-α. However, more well-designed and large-scale RCTs are urgently warranted to further establish the effects because of insufficient evidences in present study and the differences in incidence postoperative complications, length of hospitalization, level of biochemical indices (refer to total protein, albumin, proalbumin, transferring), and CD8+ compared with standard EN were not identified. One point should be noted is that various compositions and timing of administration of EIN regime were included in individual studies. We conducted separate sensitive analyses with subgroup analysis to reassess the estimated effects of EIN relative to standard EN, but not enough sample size and number of eligible studies impaired these pooled results generated from different subgroups. Consequently, more RCTs concerning comparative effects of EIN regime with similar compositions and timing of administration compared to standard EN were needed. Moreover, it is necessary to develop more RCTs with high-quality to verify the comparative effects of preoperative with postoperative EIN in the treatment of GC patients undergoing gastrectomy.
ACKNOWLEDGMENTS
We would like to appreciate the work of editors and anonymous referees. Dr. Gita Priya and Ms. Li Ma critically edited language.
Appendix 1: PubMed search terms
#1 Search “Stomach Neoplasms”[Mesh] #2 Search ((((((gastric cancer∗[Title/Abstract]) OR gastric carcinoma∗[Title/Abstract]) OR gastric neoplasm∗[Title/Abstract]) OR stomach neoplasm∗[Title/Abstract]) OR stomach cancer∗[Title/Abstract]) OR stomach carcinoma∗[Title/Abstract]) OR gastric tumor∗[Title/Abstract] #3: #1 OR #2 #4 Search ((((enteral immune nutrition[Title/Abstract]) OR enteral immunonutrition[Title/Abstract]) OR immunoenhanced enteral nutrition[Title/Abstract]) OR immune-enhancing enteral nutrition[Title/Abstract]) OR immune enhanced enteral nutrition[Title/Abstract] #5 Search (“Randomized Controlled Trial” [Publication Type]) OR “Randomized Controlled Trials as Topic”[Mesh] #6 Search random∗[Title/Abstract] #7: #5 OR #6 #8: #3 AND #4 AND #7
Appendix 2: EBSCO-Medline search terms
S1 (MH “Stomach Neoplasms”)
S2 TX Stomach Neoplasm∗ OR TX stomach cancer∗ OR TX stomach carcinoma∗ OR TX stomach tumor∗ OR TX gastric cancer∗ OR TX gastric carcinoma∗ OR gastric tumor∗
S3 S1 OR S2
S4 TX enteral immune nutrition OR TX enteral immunonutrition OR TX immunoenhanced enteral nutrition OR TX immune-enhancing enteral nutrition OR TX immune enhanced enteral nutrition
S5 (MH “Randomized Controlled Trial”) OR (MH “Randomized Controlled Trials as Topic”)
S6 TX random∗
S7 S5 OR S6
S8 S3 AND S4 AND S7
Footnotes
Abbreviations: ω-3-fatty acids = omega-3-fatty acids, AA = amino acid, Arg = arginine, CD = cluster determinant, EIN = enteral immunonutrition, EN = enteral nutrition, GC = gastric cancer, Gln = glutamine, Ig = immunoglobulin, IL = interlukin, IV = inverse variance, MeSH = medical subject heading, M-H = Mantel–Haenszel, NK = natural killer, OS = overall survival, PN = parenteral nutrition, RNA = ribonucleic acid, SSI = surgical site infection, TNF-α = tumor necrosis factor-α.
G-MS, XT, HL, and L-JY have contributed equally to this work as first author.
Author contributions: Guo-Min Song, Xu Tian, Hui Liang and Li-Juan Yi conceived the study and participated in the preparation of protocol. Guo-Min Song, Xu Tian, and Hui Liang collected the data and performed statistical analyses. Zi Zeng and Ting Shuai helped to collect the data and assess the methodological quality of included studies. Guo-Min song, Xu Tian, Hui Liang, and Li-Juan Yi analyzed all data and drawn the conclusion. Guo-Min Song, Xu Tian, Hui Liang, and Yang-Xiang Ou have written the manuscript draft. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Anderson WF, Camargo MC, Fraumeni JF, Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010; 303:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujitani K, Tsujinaka T, Fujita J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg 2012; 99:621–629. [DOI] [PubMed] [Google Scholar]
- 3.Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995; 345:745–748. [DOI] [PubMed] [Google Scholar]
- 4.Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996; 347:995–999. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Zhong Y, Jing D, et al. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg 2006; 30:1284–1289. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zhang Z, Xiong M, et al. Early enteral nutrition after total gastrectomy for gastric cancer. Asia Pac J Clin Nutr 2014; 23:607–611. [DOI] [PubMed] [Google Scholar]
- 7.Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: surgery including organ transplantation. Clin Nutr 2006; 25:224–244. [DOI] [PubMed] [Google Scholar]
- 8.Klek S, Kulig J, Sierzega M, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg 2008; 248:212–220. [DOI] [PubMed] [Google Scholar]
- 9.Jiang XH, Li N, Li JS. Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol 2003; 9:1878–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braga M, Vignali A, Gianotti L, et al. Immune and nutritional effects of early enteral nutrition after major abdominal operations. Eur J Surg 1996; 162:105–112. [PubMed] [Google Scholar]
- 11.Merimee TJ, Rabinowtitz D, Fineberg SE. Arginine-initiated release of human growth hormone. Factors modifying the response in normal man. N Eng J Med 1969; 280:1434–1438. [DOI] [PubMed] [Google Scholar]
- 12.Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr 1986; 10:227–238. [DOI] [PubMed] [Google Scholar]
- 13.Heyland DK, Novak F, Drover JW, et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001; 286:944–953. [DOI] [PubMed] [Google Scholar]
- 14.Chen DW, Wei Fei Z, Zhang YC, et al. Role of enteral immunonutrition in patients with gastric carcinoma undergoing major surgery. Asian J Surg 2005; 28:121–124. [DOI] [PubMed] [Google Scholar]
- 15.Daly JM, Reynolds J, Thom A, et al. Immune and metabolic effects of arginine in the surgical patient. Ann Surg 1988; 208:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billiar T, Bankey P, Svingen B, et al. Fatty acid intake and Kupffer cell function: fish oil alters eicosanoid and monokine production to endotoxin stimulation. Surgery 1988; 104:343–349. [PubMed] [Google Scholar]
- 17.Galban C, Montejo JC, Mesejo A, et al. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med 2000; 28:643–648. [DOI] [PubMed] [Google Scholar]
- 18.Montejo JC, Zarazaga A, Lopez-Martinez J, et al. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr 2003; 22:221–233. [DOI] [PubMed] [Google Scholar]
- 19.Farreras N, Artigas V, Cardona D, et al. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr (Edinburgh, Scotland) 2005; 24:55–65. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Tsujinaka T, Fujitani K, et al. A randomized controlled phase III trial to evaluate the effect of preoperative enteral immunonutrition on the surgical site infection after total gastrectomy (OGSG0507). J Clin Oncol 2011; 29: [Google Scholar]
- 21.Liu H, Ling W, Cao H. Effects of immune-enhanced enteral nutrition and parenteral nutrition on immune and nutritional function in elderly patients with gastric cancer after total gastrectomy. J Shanghai Jiaotong Univ (Medical Science) 2011; 31:1000–1004. [Google Scholar]
- 22.Liu Z, Yu J. Effect of immune enhanced enteral nutrition on postoperative immune function and inflammatory responses in gastric cancer patients with radical gastrectomy. Chin J Cancer Prevent Treat 2011; 18:66–67.+69. [Google Scholar]
- 23.Liu H, Ling W, Shen ZY, et al. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J Digest Dis 2012; 13:401–406. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Cai Y, Feng G-H, et al. Prospective study of early application of immune-enhanced enteral nutrition and recombined human growth hormone on patients with gastric neoplasms after total gastrectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2007; 10:550–554. [PubMed] [Google Scholar]
- 25.Marano L, Porfidia R, Pezzella M, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol 2013; 20:3912–3918. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269.W264. [DOI] [PubMed] [Google Scholar]
- 27.Wiley Online Library, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5. 2008. [Google Scholar]
- 28.Tian X, Zhou JG, Zeng Z, et al. Cetuximab in patients with esophageal cancer: a systematic review and meta-analysis of randomized controlled trials. Med Oncol 2015; 32:127. [DOI] [PubMed] [Google Scholar]
- 29.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharm Ther 2014; 40:250–260. [DOI] [PubMed] [Google Scholar]
- 31.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 32.Basaran H, Koca T, Cerkesli AK, et al. Treatment outcomes and survival study of gastric cancer patients: a retrospective analysis in an endemic region. Asian Pacific J Cancer Prevent 2015; 16:2055–2060. [DOI] [PubMed] [Google Scholar]
- 33.Miao RL, Wu AW. Towards personalized perioperative treatment for advanced gastric cancer. World J Gastroenterol 2014; 20:11586–11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decker D, Schondorf M, Bidlingmaier F, et al. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996; 119:316–325. [DOI] [PubMed] [Google Scholar]
- 35.Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr 2003; 27:1–9. [DOI] [PubMed] [Google Scholar]
- 36.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992; 216:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin MT, Saito H, Fukushima R, et al. Route of nutritional supply influences local, systemic, and remote organ responses to intraperitoneal bacterial challenge. Ann Surg 1996; 223:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatolo Rep 2002; 4:208–217. [DOI] [PubMed] [Google Scholar]
- 39.Wu Jia ZY, Zhang L, Han W, et al. Postoperative enteral immunonutrition for patients undergoing surgery for gastric cancer: a meta analysis (in Chinese). J Evid Based Med 2012; 12:291–298. [Google Scholar]


