Abstract
Serum gamma-glutamyltransferase (GGT) level has been considered marker of oxidative stress as well as liver function. Serum GGT level has been reported to be associated with the mortality in hemodialysis patients. However, it is not well established whether serum GGT level is associated with all-cause mortality in peritoneal dialysis (PD) patients. The aim of this study was to determine the association between serum GGT levels and all-cause mortality in PD patients.
PD patients were included from the Clinical Research Center registry for end-stage renal disease cohort, a multicenter prospective observational cohort study in Korea. Patients were categorized into 3 groups by tertile of serum GGT levels as follows: tertile 1, GGT < 16 IU/L; tertile 2, GGT = 16 to 27 IU/L; and tertile 3, GGT > 27 IU/L. Primary outcome was all-cause mortality.
A total of 820 PD patients were included. The median follow-up period was 34 months. Kaplan–Meier analysis showed that the all-cause mortality rate was significantly different according to tertiles of GGT (P = 0.001, log-rank). The multivariate Cox regression analysis showed that higher tertiles significantly associated with higher risk for all-cause mortality (tertile 2: hazard ratio [HR] 2.08, 95% confidence interval [CI], 1.17–3.72, P = 0.013; tertile 3: HR 1.83, 95% CI, 1.04–3.22, P = 0.035) in using tertile 1 as the reference group after adjusting for clinical variables.
Our study demonstrated that high serum GGT levels were an independent risk factor for all-cause mortality in PD patients. Our findings suggest that serum GGT levels might be a useful biomarker to predict all-cause mortality in PD patients.
INTRODUCTION
Gamma-glutamyltransferase (GGT) is a commonly used diagnostic test for liver function and alcohol consumption in clinical practice. Furthermore, GGT has been considered a marker of oxidative stress because it plays an important role in the extracellular catabolism of glutathione, the main thiol intracellular antioxidant agent in mammalian cells.1–3 An increasing number of epidemiological studies have demonstrated that serum GGT level is associated with morbidity and mortality, including cardiovascular disease, irrespective of liver disease, or alcohol consumption.4,5
End-stage renal disease (ESRD) patients have high mortality, especially cardiovascular and infectious mortality, compared with the general population. Furthermore, increased oxidative stress has been observed in patients with ESRD.6 Increased pro-oxidant activity such as old age, high prevalence of diabetes mellitus and hypertension, inflammation, incompatibility of dialysis membranes, and composite of dialysis solutions in ESRD patients with undergoing dialysis therapy may cause an imbalance between the generation of free radicals and endogenous antioxidant defense, and it may contribute to the accelerated development of oxidative nucleic acid damage.6–8 Increased oxidative stress is associated with mortality in ESRD patients with undergoing dialysis.9 Considering that serum GGT levels is a marker of oxidative stress, it may be postulated that serum GGT levels may predict mortality in ESRD patients. In hemodialysis (HD) patients, a previous study reported that high serum GGT level is associated with all-cause mortality and cardiovascular mortality.10 Oxidative stress varies with dialysis modality, and the impact of oxidative stress on mortality is different between HD and peritoneal dialysis (PD) patients.9,11 However, there are only few studies for the association between serum GGT levels and clinical outcomes in ESRD patients with undergoing PD therapy.
In this study, we performed a prospective observational study to investigate the association between serum GGT levels and all-cause mortality in PD patients.
METHODS
Study Population
All patients included in this study were enrolled in from the Clinical Research Center (CRC) registry for ESRD cohort in Korea. This is an ongoing observational prospective cohort study of patients with ESRD from 31 medical centers in Korea. The cohort started in April 2009 and included adult (>18 years of age) dialysis patients. A total of 1772 patients with PD were enrolled in this cohort. For the present study, we excluded patients for whom information about serum GGT levels was not available (n = 952); therefore, 820 patients were included in the final analysis. Demographic and clinical data were collected at the time of enrollment. Assessment of dialysis characteristics and measurements of health were performed every 6 months until follow-up was complete. Dates and causes of mortality were reported throughout the follow-up period. The study was approved by the medical ethics committees of all of the participating hospitals and performed in accordance to the Declaration of Helsinki. Written informed consent was obtained from all patients before inclusion.
Data Collection
Demographic and clinical data, including age, sex, height, weight, body mass index (BMI), comorbidities, laboratory investigations, and therapeutic characteristics, were collected at baseline. We defined cardiovascular disease as the presence of coronary artery disease, congestive heart failure, peripheral vascular disease, or cerebrovascular disease. For the assessment of comorbity, Davies comorbidity score and Modified Charlson comorbidity score were used.12–14 According to Davies comorbidity score, the low-risk group had a score of 0, the moderate-risk group a score of 1 to 2, and the high-risk group a score of ≥3.12,13 The modified Charlson comorbidity index combines information from 14 medical conditions designed to predict 1-year mortality among patients with ESRD.14 Medication use, including aspirin, angiotensin converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), β-blocker, and vitamin D, was investigated. Serum hemoglobin, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), calcium, phosphorus, intact parathyroid hormone, total cholesterol, triglyceride, uric acid, and high-sensitivity C-reactive protein were measured at baseline from blood samples. Serum GGT levels were measured at 37°C using fasting blood samples and were analyzed with an enzyme kinetic assay (Hitachi 7600-210, Tokyo, Japan) by a standard method of the International Federation of Clinical Chemistry. Patients were categorized into 3 groups by tertiles of serum GGT levels as follows: tertile 1, GGT < 16 IU/L; tertile 2, GGT = 16 to 27 IU/L; and tertile 3, GGT > 27 IU/L.
Outcomes
The primary outcome was all-cause mortality. All patients were followed until death or the end of the study, with censoring of data at the time that a patient underwent renal transplantation or was lost to follow-up because of patient's refusal of further participation or transfer to a nonparticipating hospital. For each death, the clinical center's principal investigators completed a form that included the cause of death according to the CRC for ESRD study classification.
Statistical Analysis
Continuous variables with normal distributions were expressed as mean ± standard deviation, and those without normal distribution were expressed as the median with interquartile ranges as appropriate for the type of variable. Comparisons between groups were performed by Student t test, Mann–Whitney U test, one-way analysis of variances test, or Kruskal–Wallis test, as appropriate, to determine differences in continuous variables. Categorical variables were expressed as numbers and percentages. The Pearson χ2 test or Fisher exact test was performed to determine the differences in categorical variables.
The primary outcome was all-cause mortality. Absolute mortality rates were calculated per 100 person-years of follow-up. Cumulative survival curves were generated using the Kaplan–Meier method with log-rank test. We used the Cox proportional hazard regression model to estimate the hazard ratio (HR) with 95% confidence interval (CI) for all-cause mortality, using tertile 1 as the reference category. The proportional hazards assumption over time was assessed by plotting the log-minus-log survival. Analyses were adjusted for potential confounders using 3 models. Model 1 was adjusted for age and sex, and model 2 was adjusted for age, sex, diabetes mellitus, previous cardiovascular diseases, hemoglobin levels, serum albumin level, serum total cholesterol level, serum AST level, serum ALT level, hepatitis B surface antigen (HBS Ag), antihepatitis C virus antibody, and comorbidity score. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 software (SPSS Inc, Chicago, IL).
RESULTS
Patient Characteristics
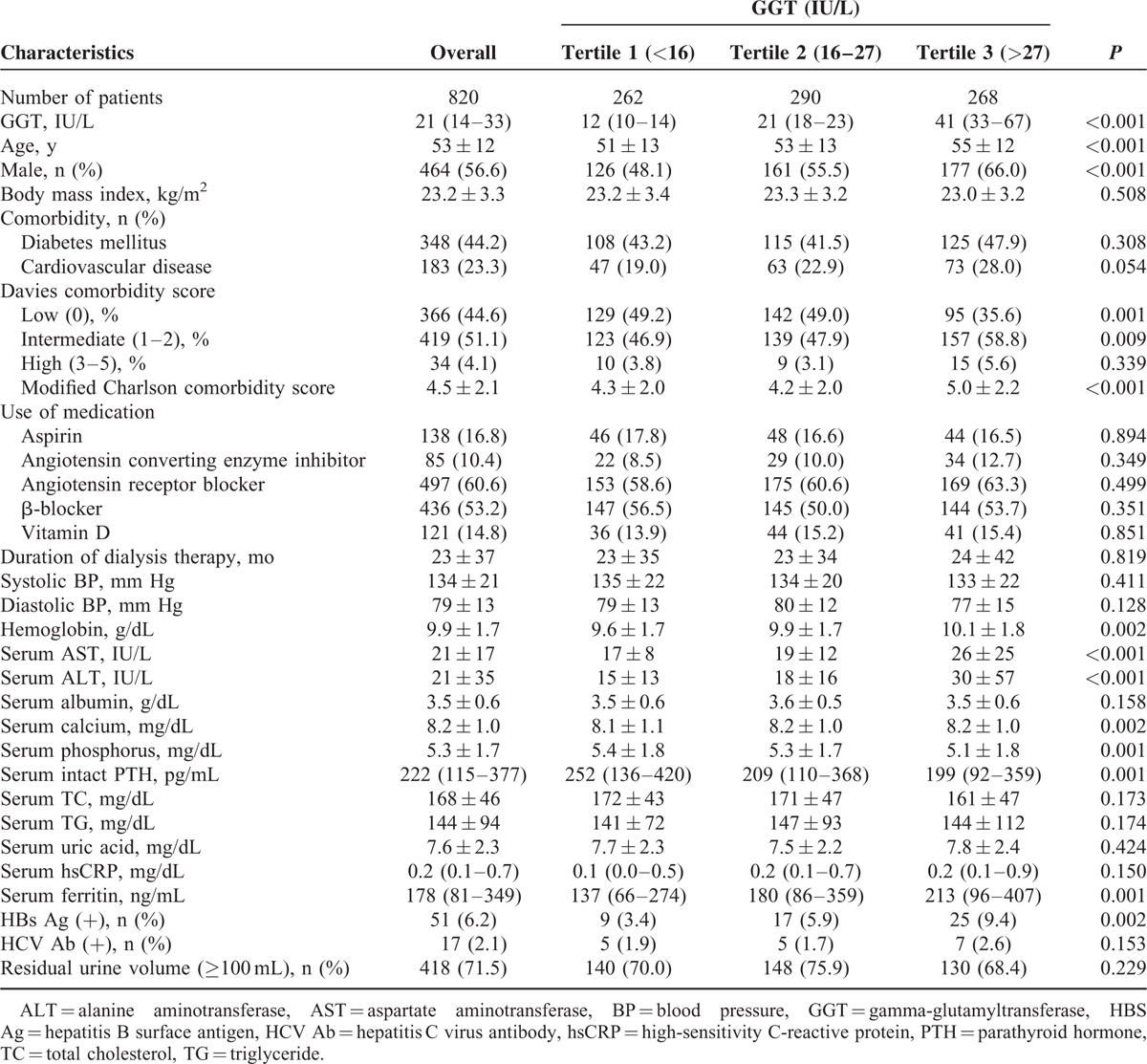
The median GGT level was 21 IU/L (interquartile range, 14–33 IU/L). Baseline characteristics of the study population by tertiles of serum GGT levels are shown in Table 1. Patients with higher GGT levels were older, and a higher proportion of patients were male. There was no significant difference for prevalence of diabetes mellitus, cardiovascular diseases as GGT levels. In Davies comorbidity score, low risk was more prevalent in lower GGT tertiles, and intermediate risk was more prevalent in higher GGT tertiles. The prevalence of high risk was not significantly different among the GGT categories. Modified Charlson comorbidity score was higher in the highest GGT tertile. There was also no significant difference in use of medication such as aspirin, ACE inhibitor, ARB, β-blocker, and vitamin D among the GGT categories. Patients with high GGT levels had higher hemoglobin levels, serum levels of AST, ALT, calcium, and ferritin, and had lower serum levels of phosphorus and intact parathyroid hormone. Patients with higher GGT levels had a higher prevalence of HBS Ag positivity. There was no significant difference in BMI, duration of dialysis therapy, systolic blood pressure, diastolic blood pressure, serum levels of albumin, total cholesterol, triglyceride, uric acid, high-sensitivity C-reactive protein, and residual urine volume among the groups.
TABLE 1.
Baseline Characteristics of the Study Population by Tertiles of Gamma-Glutamyltransferase
Determinants of Serum GGT Levels
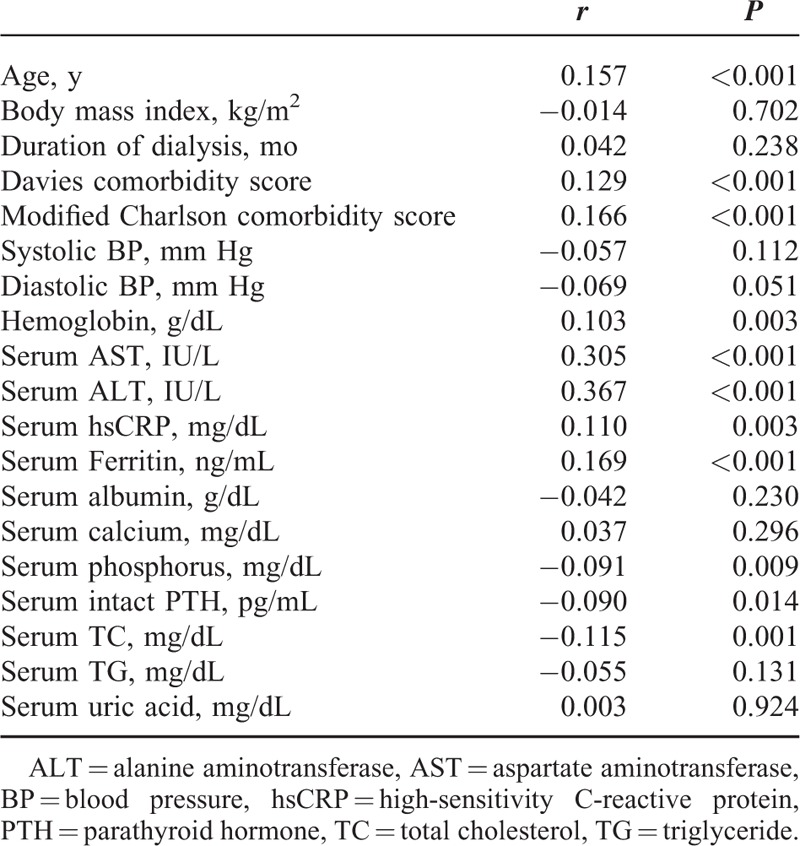
Serum GGT levels were positively correlated with age, Davies comorbidity score, modified Charlson comorbidity score, hemoglobin levels, serum levels of AST, ALT, high-sensitivity C-reactive protein levels, and ferritin, and negatively correlated with serum levels of phosphorus, intact parathyroid hormone, and total cholesterol (Table 2). In stepwise multiple regression models, serum GGT levels were positively associated with male sex (β = 0.12, P = 0.004), modified Charlson comorbidity score (β = 0.12, P = 0.003), serum AST levels (β = 0.23, P < 0.001), and serum ferritin levels (β = 0.87, P = 0.033), and they were negatively associated with BMI (β = −0.91, P = 0.027) and serum phosphorus levels (β = −0.92, P = 0.028). The above factors explained 13% of the interindividual variability in serum GGT levels.
TABLE 2.
Spearman Correlation of Serum Gamma-Glutamyltransferase Levels and Other Factors
Association Between Serum GGT Levels and All-Cause Mortality
The median follow-up period was 34 months (interquartile range, 18–49 months). During the follow-up period, 276 patients left the study. The reasons for censoring included kidney transplantation (n = 64), transfer to a nonparticipating hospital (n = 43), refusal of further participation (n = 20), or others (n = 33). There were 116 deaths during the follow-up period.
Cardiovascular diseases were the leading cause of death (33.6% of all deaths), followed by infectious diseases (32.8% of all deaths). Table 3 shows the causes of death of the study population by tertiles of serum GGT levels. There was no significant difference in the causes of death among the 3 groups (P = 0.785). The absolute mortality rate was 5.2 deaths per 100 person-years. Figure 1 shows the Kaplan–Meier plot of patient survival according to tertiles of serum GGT levels. The log-rank test showed that all-cause mortality rate was significantly increased in patients with the highest tertile of serum GGT levels (P = 0.001).
TABLE 3.
Causes of Deaths of the Study Population by Tertiles of Gamma-Glutamyltransferase
FIGURE 1.
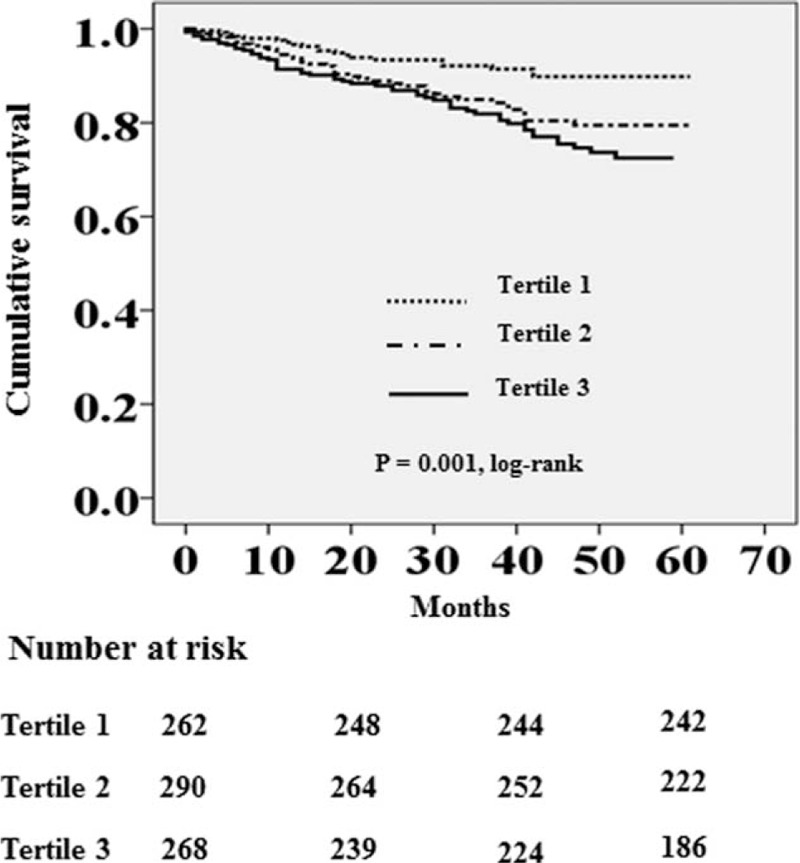
Kaplan–Meier survival curve for all-cause mortality according to tertiles of serum GGT levels (tertile 1, GGT < 16 U/L; tertile 2, GGT = 16–27 U/L; and tertile 3, GGT > 27 U/L).
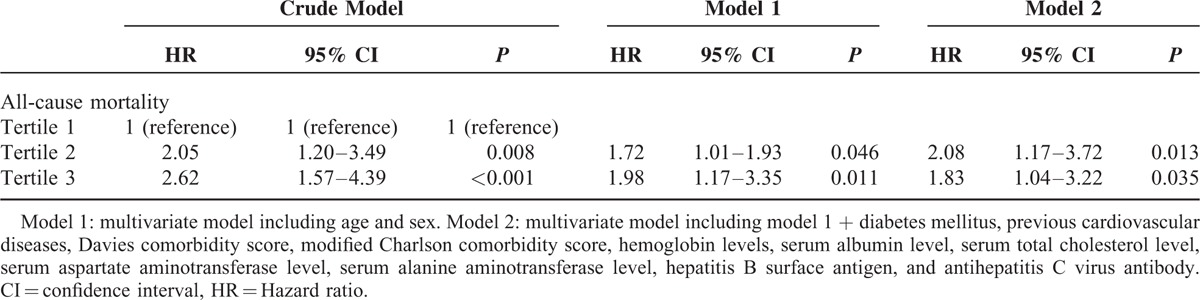
Univariate and multivariate Cox regression analyses for all-cause mortality are shown in Table 4. In the crude model, the HRs for all-cause mortality of patients in tertile 2 and tertile 3 of serum GGT levels were 2.05 (95% CI, 1.20–3.49, P = 0.008) and 2.62 (95% CI, 1.57–4.39, P < 0.001), respectively, using tertile 1 as the reference category. In multivariate Cox regression analysis, higher tertiles had a significantly higher risk for all-cause mortality in model 1 (tertile 2: HR 1.72, 95% CI, 1.01–1.93, P = 0.046; tertile 3: HR 1.98, 95% CI, 1.17–3.35, P = 0.011) and model 2 (tertile 2: HR 2.08, 95% CI, 1.17–3.72, P = 0.013; tertile 3: HR 1.83, 95% CI, 1.04–3.22, P = 0.035), which indicate that predictive power of GGT for all-cause mortality was independent of liver disease as well as other potential confounder, including age, sex, diabetes mellitus, previous cardiovascular diseases, comorbidity score, hemoglobin levels, and serum albumin level.
TABLE 4.
The Univariate and Multivariate Cox Regression Analysis for All-Cause Mortality
DISCUSSION
In this prospective observational study, we demonstrated that a higher serum GGT level was significantly associated with increased all-cause mortality in PD patients independent of traditional risk factors or liver diseases. These findings are compatible with previous studies reporting serum GGT levels as a predictor for mortality in the general population,4 as well as in patients with coronary artery disease and diabetes mellitus.15,16 There are few data on the association between serum GGT level and morality in ESRD patients undergoing dialysis. One previous study reported that a high serum GGT level was associated with all-cause and cardiovascular mortality in HD patients.10 In our knowledge, this is the first study investigating the impact of serum GGT level on all-cause mortality in PD patients.
The mechanism underlying the association between GGT and mortality in PD patients is unclear. However, some explanations can be proposed.
First, oxidative stress might be the link between high serum GGT levels and increased all-cause mortality.9 GGT is the key enzyme involved in the extracellular catabolism of glutathione. This reaction produces cysteinylglycine, which reduces Fe3+ to Fe2+ and leads to the formation of superoxide anion radical and hydrogen peroxide.3,17 In PD patients, oxidative stress is increased by chronic inflammation, loss of small molecules including the antioxidants into the peritoneal cavity and continuous exposure of the peritoneum to PD fluids that are hyperosmolar and have a low pH and glucose degradation products.18–21 Increased oxidative stress is associated with all-cause mortality in PD patients.11 Therefore, it may be speculated that increased serum GGT levels reflect the increased oxidative stress and associated with increased all-cause mortality in PD patients.
Second, GGT is a marker of liver function, and liver diseases may be the link between high serum GGT levels and increased all-cause mortality in PD patients.22 In this study, patients with HBS Ag positivity were more prevalent in tertile 3 of serum GGT level, and serum AST or ALT levels were higher in higher tertile 3. However, high serum GGT levels had prognostic value only for all-cause mortality after adjustment for liver function (serum AST and ALT levels), HBS Ag positivity, and antihepatitis C virus antibody positivity. These findings suggest that the association between serum GGT levels and all-cause mortality in PD patients may be independent of liver disease.
Third, oral or intravenous iron preparations are commonly used for the correction of anemia in PD patients. Administration of ferrous salts may lead to high transferrin saturation levels and subsequently promote the formation of nontransferrin-bound iron, which can induce oxidative stress. Numerous studies have demonstrated that iron overload is associated with increased overall and cardiovascular morbidity and mortality in ESRD patients.23,24 Considering that the effect of GGT generating reactive oxygen species seems to occur when GGT is expressed in the presence of iron or other transition metals, iron overload may contribute to increased serum GGT levels. In this study, serum GGT levels were strongly correlated with serum ferritin levels, a conventional marker of stored body iron (r = 0.169, P < 0.001). Therefore, it may be speculated that iron overload is related to increased serum GGT levels which reflect the increased oxidative stress and associated with increased all-cause mortality in PD patients.
One of the interesting findings of this study is that high serum GGT levels predicted all-cause mortality in PD patients, which is similar with the findings for HD patients from previous studies. Intensity of oxidative stress is different according to dialysis modality, and HD patients have higher oxidative stress levels than PD patients.11 Therefore, considering the strong link between serum GGT levels and oxidative stress,1–3 it may be postulated that the predictive effect of serum GGT level for mortality may be different between HD and PD patients. However, serum GGT level had predictive power for all-cause mortality in PD patients. These findings suggest that serum GGT level is a clinically important predictor even in patients whose oxidative stress is relatively low, not only in PD patients, but also in HD patients.
Another interesting finding of this study is that physiological high levels of serum GGT level had a predictive value for all-cause mortality in PD patients. In this study, tertile 2 of serum GGT levels (16–27 IU/L) included substantial portion of patients with normal high levels of serum GGT,4 and was associated with all-cause mortality using tertile 1 of serum GGT levels (<16 IU/L) as reference category. These findings suggest that close monitoring might be needed even at physiological high levels of serum GGT level in PD patients.
Our study has several limitations. First, the design of our study was not a randomized, controlled study, but a prospective observational study. Second, in spite of the multicenter nature of the study the cohort consisted of Korean patients and all were Asian. Thus, it is uncertain whether our results can be generalized to other ethnic groups with ESRD. Third, our study did not specifically exclude current or ex-alcoholics, which may be confounders for the association between serum GGT levels and mortality because serum GGT level is affected by alcohol intake. Forth, the use of statin may have effect on the relationship of GGT with cardiovascular diseases.25 Unfortunately, our study did not include data for the use of medication such as stain. Fifth, serum GGT levels can be changed over time by individual changes of BMI, dietary habit, alcohol consumption, physical activity, or use of medication.26 However, repeated GGT measurements were not performed in this study. Therefore, we could not determine the prognostic value of longitudinal change of serum GGT levels on mortality.
In conclusion, our study demonstrated that high serum GGT levels were an independent risk factor for all-cause mortality in PD patients. Our findings suggest that serum GGT levels might be a useful biomarker to predict all-cause mortality in PD patients.
Acknowledgments
The authors thank the study coordinators Hye Young Lim, Nam Hee Kim, Mi Joung Moon, Hwa Young Lee, Mi Joung Kwon, Su Yeon An, Su Joung Oh, and Hye Young Kwak for their contributions to this study.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidence interval, CRC = Clinical Research Center, ESRD = end-stage renal disease, GGT = gamma-glutamyltransferase, HBS Ag = hepatitis B surface antigen, HD = hemodialysis, HR = hazard ratio, PD = peritoneal dialysis.
This work was supported by a grant of the Korea Healthcare Technology R & D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Dominici S, Paolicchi A, Corti A, et al. Prooxidant reactions promoted by soluble and cell-bound gamma-glutamyltransferase activity. Methods Enzymol 2005; 401:484–501. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Blomhoff R, Jacobs DR., Jr Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res 2004; 38:535–539. [DOI] [PubMed] [Google Scholar]
- 3.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation 2005; 112:2078–2080. [DOI] [PubMed] [Google Scholar]
- 4.Ruttmann E, Brant LJ, Concin H, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005; 112:2130–2137. [DOI] [PubMed] [Google Scholar]
- 5.Jiang S, Jiang D, Tao Y. Role of gamma-glutamyltransferase in cardiovascular diseases. Exp Clin Cardiol 2013; 18:53–56. [PMC free article] [PubMed] [Google Scholar]
- 6.Sung CC, Hsu YC, Chen CC, et al. Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Oxid Med Cell Longev 2013; 2013:301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handelman GJ. Evaluation of oxidant stress in dialysis patients. Blood Purif 2000; 18:343–349. [DOI] [PubMed] [Google Scholar]
- 8.Del Vecchio L, Locatelli F, Carini M. What we know about oxidative stress in patients with chronic kidney disease on dialysis—clinical effects, potential treatment, and prevention. Semin Dial 2011; 24:56–64. [DOI] [PubMed] [Google Scholar]
- 9.Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant 2003; 18:1272–1280. [DOI] [PubMed] [Google Scholar]
- 10.Postorino M, Marino C, Tripepi G, et al. Gammaglutamyltransferase in ESRD as a predictor of all-cause and cardiovascular mortality: another facet of oxidative stress burden. Kidney Int Suppl 2008; 111:S64–66. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Watanabe M, Qureshi AR, et al. Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients [published online ahead of print March 1, 2014]. Perit Dial Int. doi:10.3747/pdi.2013.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies SJ, Russell L, Bryan J, et al. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis 1995; 26:353–361. [DOI] [PubMed] [Google Scholar]
- 13.Davies SJ, Phillips L, Naish PF, et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002; 17:1085–1092. [DOI] [PubMed] [Google Scholar]
- 14.Hemmelgarn BR, Manns BJ, Quan H, et al. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 2003; 42:125–132. [DOI] [PubMed] [Google Scholar]
- 15.Stojakovic T, Scharnagl H, Trauner M, et al. Serum gamma-glutamyl transferase and mortality in persons undergoing coronary angiography-The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2010; 208:564–571. [DOI] [PubMed] [Google Scholar]
- 16.Sluik D, Beulens JW, Weikert C, et al. Gamma-glutamyltransferase, cardiovascular disease and mortality in individuals with diabetes mellitus. Diabetes Metab Res Rev 2012; 28:284–288. [DOI] [PubMed] [Google Scholar]
- 17.Marques de Mattos A, Marino LV, Ovidio PP, et al. Protein oxidative stress and dyslipidemia in dialysis patients. Ther Apher Dial 2012; 16:68–74. [DOI] [PubMed] [Google Scholar]
- 18.Witowski J, Jörres A, Korybalska K, et al. Glucose degradation products in peritoneal dialysis fluids: do they harm? Kidney Int Suppl 2003; 84:S148–151. [DOI] [PubMed] [Google Scholar]
- 19.Churchill DN, Thorpe KE, Nolph KD, et al. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 1998; 9:1285–1292. [DOI] [PubMed] [Google Scholar]
- 20.Wang T, Heimbürger O, Waniewski J, et al. Increased peritoneal permeability is associated with decreased fluid and small-solute removal and higher mortality in CAPD patients. Nephrol Dial Transplant 1998; 13:1242–1249. [DOI] [PubMed] [Google Scholar]
- 21.Brimble KS, Walker M, Margetts PJ, et al. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 2006; 17:2591–2598. [DOI] [PubMed] [Google Scholar]
- 22.Wang SM, Liu JH, Chou CY, et al. Mortality in hepatitis C-positive patients treated with peritoneal dialysis. Perit Dial Int 2008; 28:183–187. [PubMed] [Google Scholar]
- 23.Kletzmayr J, Hörl WH. Iron overload and cardiovascular complications in dialysis patients. Nephrol Dial Transplant 2002; 17 suppl 2:25–29. [DOI] [PubMed] [Google Scholar]
- 24.Fishbane S. Upper limit of serum ferritin: misinterpretation of the 2006 KDOQI anemia guidelines. Semin Dial 2008; 21:217–220. [DOI] [PubMed] [Google Scholar]
- 25.Ritzel U, Leonhardt U, Näther M, et al. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol 2002; 36:454–458. [DOI] [PubMed] [Google Scholar]
- 26.Nilssen O, Førde OH. Seven-year longitudinal population study of change in gamma-glutamyltransferase: the Tromsø Study. Am J Epidemiol 1994; 139:787–792. [DOI] [PubMed] [Google Scholar]


