Abstract
Ischemic conditioning involves the delivery of short cycles of reversible ischemic injury in order to induce protection against subsequent more prolonged ischemia. This randomized controlled trial was designed to determine the safety and efficacy of remote ischemic conditioning (RC) in live donor kidney transplantation.
This prospective randomized clinical trial, 80 patients undergoing live donor kidney transplantation were randomly assigned in a 1:1 ratio to either RC or to a control group. RC consisted of cycles of lower limb ischemia induced by an arterial tourniquet cuff placed around the patient's thigh. In the RC treatment group, the cuff was inflated to 200 mm Hg or systolic pressure +25 mm Hg for 4 cycles of 5 min ischemia followed by 5 min reperfusion. In the control group, the blood pressure cuff was inflated to 25 mm Hg. Patients and medical staff were blinded to treatment allocation. The primary end-point was renal function measured by estimated glomerular filtration rate (eGFR) at 1 and 3 months posttransplant.
Donor and recipient demographics were similar in both groups (P < 0.05). There were no significant differences in eGFR at 1 month (control 52 ± 14 vs RC 54 ± 17 mL/min; P = 0.686) or 3 months (control 50 ± 14 vs RC 49 ± 18 mL/min; P = 0.678) between the control and RC treatment groups. The RC technique did not cause any serious adverse effects.
RC, using the protocol described here, did not improve renal function after live donor kidney transplantation.
INTRODUCTION
Transplanted kidneys are subjected to a combination of warm and cold ischemic injury. This is followed by a sudden re-introduction of oxygen at the point of revascularization that may lead to further tissue injury. The overall process of ischemia–reperfusion (I/R) injury can lead to early graft dysfunction manifested clinically as delayed or slow initial graft function. Delayed graft function (DGF) is associated with higher rates of acute rejection, longer in-patient stay and therefore greater cost, and poorer long-term outcome.1 Interventions that reduce I/R injury have the potential to improve both the short-term and long-term outcomes of renal transplantation.
Ischemic conditioning involves the deliberate delivery of short cycles of nonlethal ischemia and reperfusion in order to induce a state of protection against a subsequent and more major I/R episode. This effect was first reported in the myocardium.2 In brief, the underlying mechanisms involve 2 phases of protection. In the early phase, starting in the first few minutes and lasting up to 4 h after the conditioning stimulus, humoral mediators such as adenosine and bradykinin are released.3 During the late phase, 24 h after the conditioning stimulus, there is upregulation of cytoprotective genes and the synthesis of protective proteins such as heat shock proteins.4
In renal transplantation, it would be possible to apply the conditioning stimulus directly to the transplant kidney by intermittent occlusion of the transplant renal artery. The disadvantage of this approach is its potential invasiveness. A more practical and less invasive approach is to use remote ischemic conditioning (RC), whereby brief ischemia in 1 region of the body protects distant organs or tissues from a subsequent more sustained ischemic injury.5
Conditioning stimuli can be delivered to a target organ before, during or after an ischemic event. Remote ischemic pre and postconditioning have been shown to be beneficial in reducing renal I/R injury in many different animal models.6,7
Ischemic conditioning during the renal transplant operation but before reperfusion of the allograft (peri-conditioning) would offer a pragmatic approach to renal protection, but there is a paucity of clinical trials in this area of study. We aimed to assess whether remote ischemic peri-conditioning (RC) induced by cycles of brief lower limb ischemia and reperfusion was effective in improving renal function in adults undergoing live donor kidney transplantation. Live donor kidneys were chosen for study as they are subjected to relatively consistent periods of warm and cold ischemic injury.
PATIENTS AND METHODS
The trial was approved by the local Ethics Committee and the Research and Development Department at the University Hospitals of Leicester (clinical trial registration ISRCTN66437627). Adult patients (≥18 years) undergoing a first or second kidney transplant from a live donor were recruited to the trial between October 2011 and August 2014. Recruited subjects gave signed written informed consent on the day before or on the day of their surgery. Patients with an ABO blood group incompatible donor, recipients of a first transplant <6 months ago, recipients of 3rd or subsequent transplants, patients with severe peripheral vascular disease and patients on ATP sensitive potassium channel medication were excluded from the trial.
Randomization and Masking
Patients were randomized to RC or to the control group in a 1:1 ratio. The randomization sequence was computer generated and converted into a closed envelope system by a trial administrator who was independent of all other aspects of the trial. The consecutively numbered opaque envelopes contained a slip identifying the study number and the treatment allocation. Patients were randomized after induction of anesthesia for their transplant operation. Randomization was performed by a research nurse who was independent from the transplant medical team. The medical staff and patients were blinded to the treatment groups.
RC Procedure
After induction of anesthesia, transplant recipients had an arterial tournquet cuff placed around the thigh contralateral to the side of the transplant operation. The cuff size was sufficient to take account of limbs in patients with high body mass indexes. In the treatment group, RC consisted of four 5-min cycles of the blood pressure cuff inflation to a pressure of 200 mm Hg or systolic blood pressure +25 mm Hg, whichever was the higher, interrupted by 5 min cycles of reperfusion by blood pressure cuff deflation. In the control group, the blood pressure cuff was inflated to a pressure of 25 mm Hg using the same cycle of 5 min inflation followed by 5 min deflation. Inflation sequences were timed to finish just before revascularization of the transplanted kidney.
Blinding
The research nurse who performed the randomization was also responsible for carrying out the cuff inflation and deflation cycles. The tourniquet cuff was covered in sterile drapes and attached to an automated inflation device. The surgical, anesthetic, and nursing teams were unaware of cuff inflation pressure being delivered and were therefore blinded to the treatment allocation.
Anesthesia and Transplant Surgery
All patients received a standardized general anesthetic given by 1 of 2 consultant anesthetists. Anesthesia was induced with 2.5 to 3 mg/kg propofol and 1 to 2 μg/kg fentanyl, and maintained with isofluorane and 50% oxygen in air. Muscle relaxation was achieved with 1 mg/kg atracurium. The Leicester Transplant Unit serves a population of 2.2 million people and performs 40 to 50 live donor kidney transplants per annum. All of the laparoscopic donor nephrectomy procedures and transplant operations were performed by, or under the direct supervision of the first author (MLN). All of the transplants were performed in the right iliac fossa with anastomosis of the renal artery to either the external or internal iliac artery and the renal vein to the external iliac vein. The ureter was anastomosed to the bladder as an extravesical onlay over a double J stent.
Immunosuppression
All the patients were immunosuppressed with a regimen of basiliximab (20 mg on days 0 and 4), tacrolimus 0.1 mg/kg/day to maintain trough blood levels of 6 to 10 ng/mL, mycophenolate mofetil 500 mg b.d. and prednisolone starting at a dose of 20 mg daily and reducing to 5 mg o.d. by 6 weeks posttransplantation.
Outcome Measures
The primary endpoint was estimated glomerular filtration rate (eGFR) using the modification of diet in renal disease formula at 1 and 3 months posttransplant. Secondary outcome measures were: renal function measured by serum creatinine at 1 and 3 months posttransplant; primary nonfunction defined as failure of the graft to ever function irrespective of cause; DGF defined as the requirement for dialysis in the first 7 days posttransplant; slow graft function defined as <10% fall in serum creatinine for 3 consecutive days in the first week posttransplant; creatinine reduction ratio day 2 posttransplant (CRR2 = creatinine day 1 – creatinine day 2/creatinine day 1); > 50% fall in serum creatinine in the first 24 h; biopsy-proven acute rejection and graft failure defined as the need for allograft nephrectomy or return to renal replacement therapy.
Biomarkers
Urine samples for the measurement of neutrophil gelatinase-associated lipocalin (NGAL) (Pathway Diagnostics Ltd, Dorking, UK) and liver type fatty acid binding protein (L-FABP) (Pathway Diagnostics Ltd) were taken pre, 24, 48, and 72 h posttransplant. NGAL was also measured in blood samples taken from the transplant renal vein 30 min postreperfusion.
Statistical Analysis
The trial size was calculated with respect to the primary end-point, which was eGFR at 1 and 3 months posttransplant. Based on 5 years of data from the Leicester Transplant Unit, the mean ± SD eGFR for live donor kidney transplants at 3 months was 52.3 ± 11.0 mL/min. The power calculation was based on the assumption that RC would increase the eGFR by 10%. Using a fixed sample size study, a total of 70 patients receiving a live donor kidney transplant would be required to detect this difference in eGFR with a power of 80% (β = 0.2, probability of a type II error = 20%) and a statistical significance of α = 0.05 (probability of a type I error = 5%). A total sample size of 80 was chosen to allow for a 12.5% dropout rate.
The trial coordinator collected and recorded all data prospectively in a specifically designed computerized database. Comparisons of outcome were made on an intention-to-treat basis. Data are presented as mean ± SD or median (range) for nonnormally distributed data. Normality testing was performed using the Kolmogorov–Smirnov test. Continuous variables were compared using the Student t test or the Mann–Whitney U test as appropriate. Categorical variables were compared by Fisher's exact test. Statistical analysis was performed using Prism® software for Macintosh (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Demographics
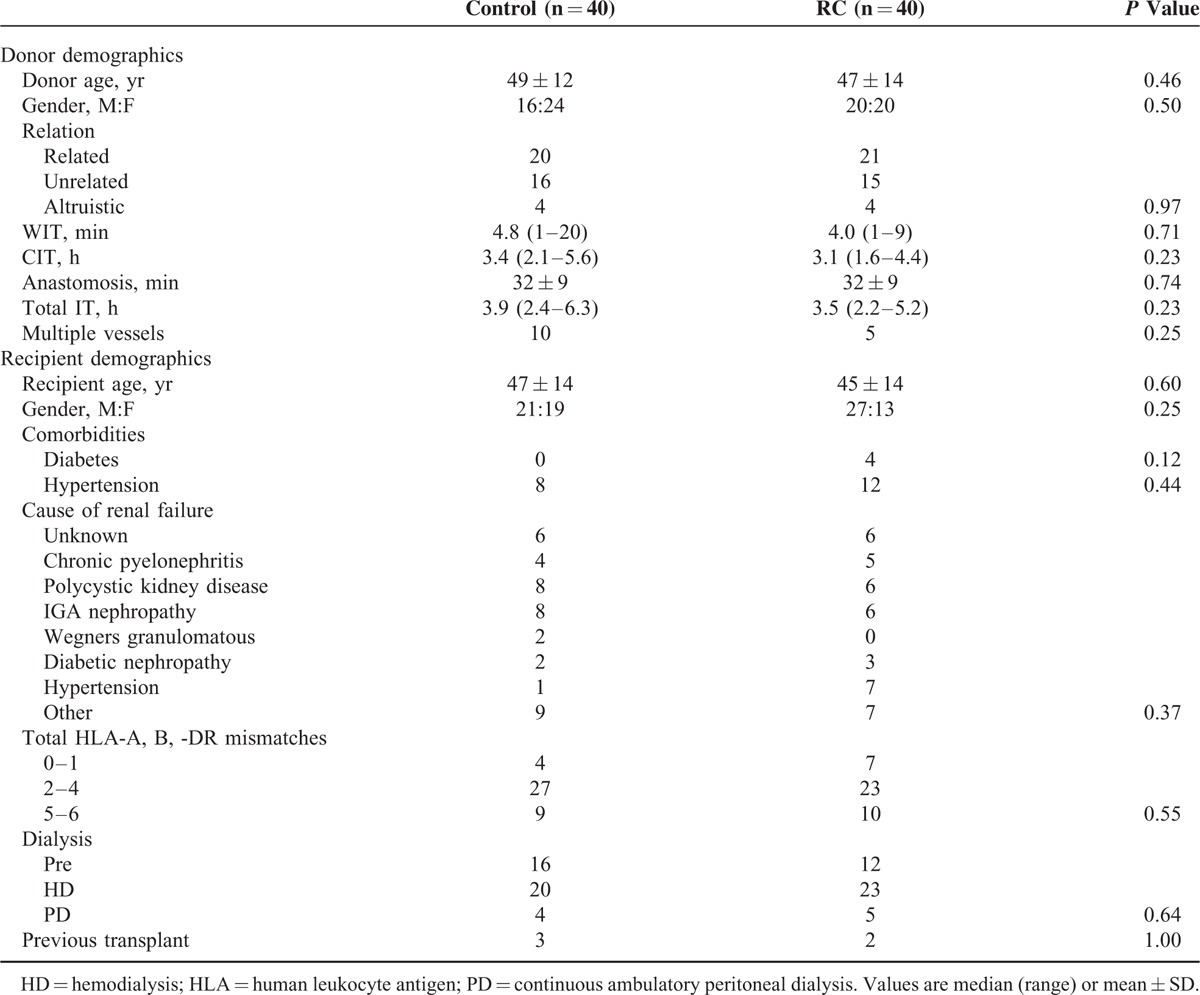
From October 2011 to August 2014, 80 out of 118 consecutive adult patients receiving a live donor kidney transplant were recruited into the study. Demographics and intraoperative variables are detailed in Table 1. The control and RC treatment groups were well matched with no significant differences between the groups.
TABLE 1.
Donor and Recipient Demographics
The CONSORT diagram is shown in Figure 1. Five patients were not eligible as they received a blood group incompatible transplant and 33 other recipients refused consent to the trial. The 80 recruited patients were randomized in a 1:1 ratio with 40 allocated to RC and 40 to the control group. Two patients in the control group did not receive the allocated intervention. One patient withdrew consent on the morning of the transplant operation and the other suffered bleeding requiring blood transfusion before cuff inflation. On an intention to treat basis they were included in the analysis and 40 patients were analyzed in both the control and treatment groups.
FIGURE 1.
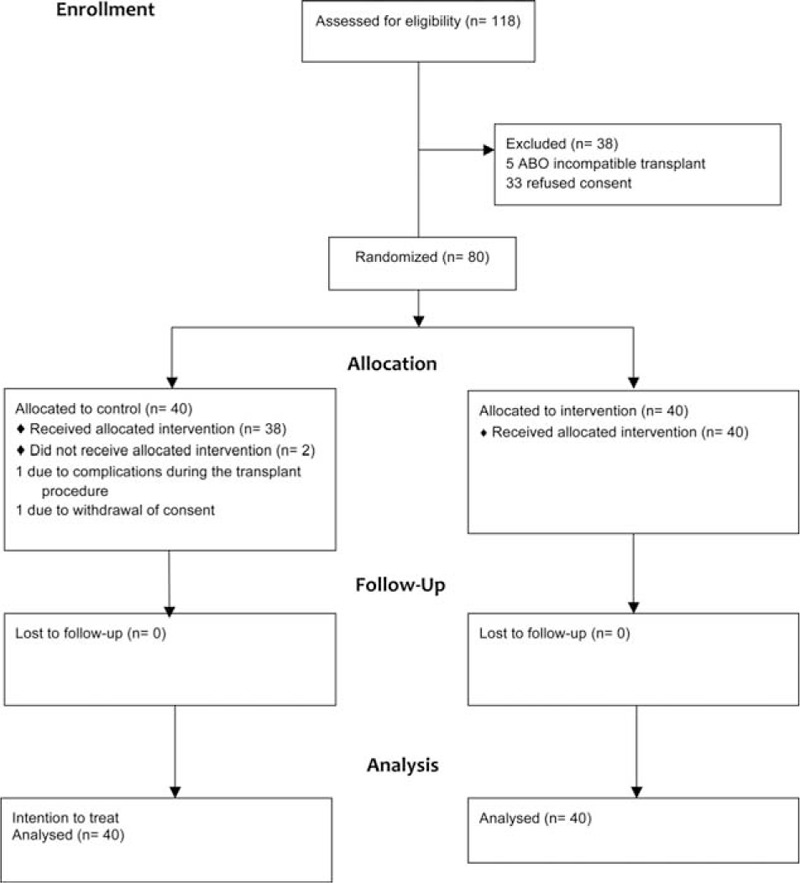
CONSORT flow diagram.
Outcome
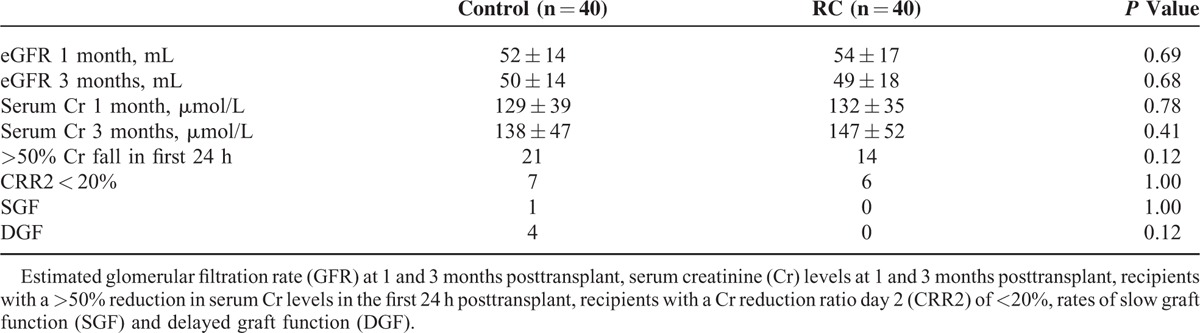
RC did not have a statistically significant effect on renal function at 1 and 3 months posttransplant, measured by either serum creatinine or eGFR. Numerically more recipients in the control group had a >50% reduction in serum creatinine levels within the first 24 h after transplantation compared with the RC group, but this did not reach statistical significance (P = 0.12). Four recipients in the control group had DGF compared with none in the RC group (P = 0.12). One patient in the control group had slow graft function compared with zero in the RC group (P = 1.00). There were no incidences of primary nonfunction (Table 2).
TABLE 2.
Outcome Data
In 3 out of the 4 episodes of DGF in the control group, there were identifiable factors that would explain the early graft dysfunction. One patient with a history of insulin dependent diabetes, hypertension, and ischemic heart disease suffered a peri-operative myocardial infarction. Although the serum creatinine fell on the first 2 postoperative days the patient required a blood transfusion and this was preceded by continuous veno-veno hemofiltration. A second patient had biopsy-proven acute cellular rejection on the second postoperative day and required hemofiltration for 24 h. The third patient's donor underwent a prolonged laparoscopic nephrectomy and there was also difficulty in extracting the kidney, which led to a warm ischemic time of 20 min. There was therefore only 1 unexplained episode of DGF in the control group. This patient required a single dialysis session on the second postoperative day.
The length of hospital stay was similar between the groups (control 7.7 ± 2.9 vs RC 7.5 ± 2.5 days; P = 0.56). Six recipients in the control group and 8 in the RC group were treated for acute rejection within the first 3 months (P = 0.77). There was 1 graft loss in the control group due to acute rejection 2 months posttransplant. Patient survival was 100% in both groups.
Effect of Hemodialysis
A post hoc analysis was performed to see if patients on hemodialysis (HD) responded differently to RC when compared with the predialysis patient group. There was no difference in eGFR at 3 months between HD and predialysis patients undergoing RC (P = 0.27) or control (P = 0.63).
Complications
Two patients in the control group had early postoperative lymphoceles but neither required operative intervention. There were 3 ureteric complications in the RC group and 1 in the control group (P = 0.62). One patient in the control group and 1 in the RC group had peri-operative non-ST elevation myocardial infarctions. In the RC group, 2 patients were re-explored for peri-transplant bleeding but neither involved the vascular anastomoses. One recipient in the RC group sustained minor bruising at the site of the blood pressure cuff. There were no other complications attributable to the intervention.
Biomarkers
There was no significant difference in the urinary levels of NGAL between the groups at any of the time points (Figure 2). Levels of L-FABP were significantly lower in the control group at 48 and 72 h posttransplant (P = 0.01 and 0.02, respectively) compared with the RC group (Figure 3). There was no statistical difference in the levels of NGAL in the renal vein samples 30 min after reperfusion (control 65.8 ± 16.3 vs RC 62.2 ± 12.5 pg/mL; P = 0.28).
FIGURE 2.
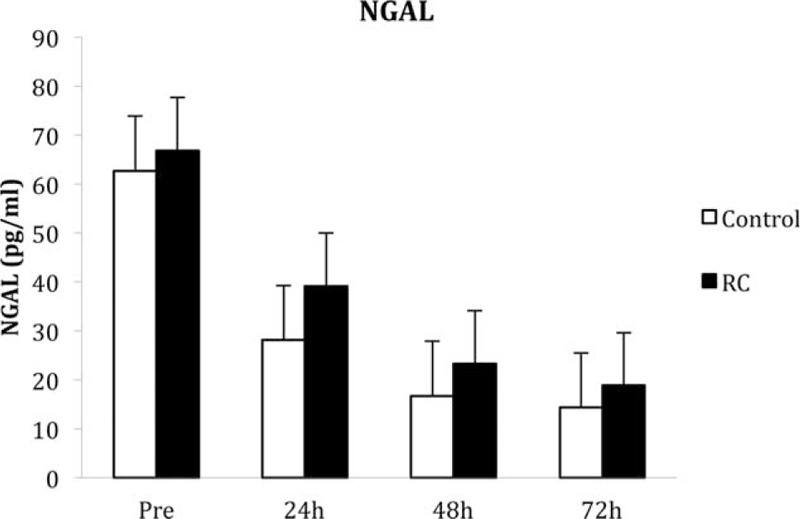
Levels of neutrophil gelatinase-associated lipocalin (NGAL) measured in the urine pretransplant and 24, 48, and 72 h posttransplant. Values are median and standard error.
FIGURE 3.
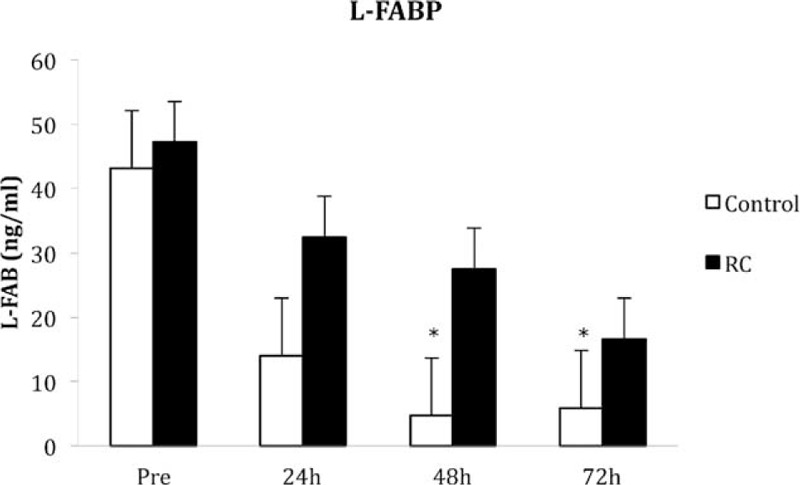
Levels of liver type fatty acid binding protein (L-FABP) measured in the urine pretransplant and 24, 48, and 72 h posttransplant (∗P < 0.05). Values are median and standard error.
DISCUSSION
In this randomized clinical trial, RC, induced by cycles of brief lower limb ischemia and reperfusion, was not effective in improving early renal function in adults undergoing live donor kidney transplantation. The ischemic conditioning stimulus in our protocol was delivered at the time of transplant surgery to coincide, as far as possible, with revascularization. This constitutes a pragmatic peri-ischemic conditioning approach. The technique used in this study proved to be straightforward and safe. The use of a blood pressure cuff to occlude leg circulation was noninvasive and there were no major complications.
Ischemic conditioning can be applied to the donor (preconditioning) or recipient during the transplant procedure (peri-conditioning) or after re-vascularization (postconditioning). However, the most effective conditioning strategy in renal transplantation has not been thoroughly investigated. Wu et al studied RC in a randomized controlled trial of paired kidneys from 24 deceased donors.8 Ischemia was induced by clamping the ipsilateral external iliac artery for three 5-min cycles during the renal transplant procedure. Renal function, measured by serum creatinine and eGFR, was improved in the RC group for the first 14 days posttransplant but there were no differences at 30 days. The validity of these findings is questionable as the sample size was limited and a power calculation was not reported. Moreover, the lack of an effect beyond 14 days suggests that RC was unlikely to influence long-term graft survival.
Chen et al9 performed a randomized trial of remote ischemic preconditioning in a series of live donor kidney transplants. The ischemic conditioning stimulus was similar to our method, involving inflation and deflation of a thigh blood pressure cuff to induce leg ischemic for three 5-min cycles interspersed with 5-min reperfusion periods. The conditioning stimulus was applied either to the donor (n = 20) or to the recipient (n = 20) and a control group did not receive RC (n = 20). There were no differences in the rates of DGF or renal function in the first 14 postoperative days. Plasma NGAL levels did not differ between the 3 groups.
In a nonrandomized pilot study, van den Akker et al10 examined ischemic postconditioning in 20 donation after circulatory death kidney transplants. Three 1-min cycles of ischemia and reperfusion were applied by simultaneous proximal and distal clamping of the external iliac artery after completion of the arterial and venous anastomoses. In comparison to historical controls, there were no significant differences in rates of DGF or renal function at 3 months.
Remote ischemic preconditioning induced by brief ischemia and reperfusion of the arm has been shown to reduce I/R injury to skeletal muscle11,12 and to provide myocardial protection during coronary artery bypass surgery.13,14 This raises the question of why RC appears to be less effective in kidney transplantation. The answer is likely to be multifactorial. In many of the cardiac studies, troponin was used as the main outcome measure. This is a highly sensitive marker of myocardial injury but does not give any information about functional outcome. More recently, Sloth et al15 demonstrated that RC, delivered by intermittent arm ischemia using a blood pressure cuff, did improve long-term outcomes in patients with ST-elevation myocardial infarction. In our study, the main endpoint was renal function measured by serum creatinine and eGFR. We also measured levels of NGAL and L-FABP as more sensitive markers of kidney injury. NGAL is regarded as one of the most sensitive indexes of proximal tubule injury.16 L-FABP has been studied less in renal transplantation but has a high predictive value of acute kidney injury.17 Urinary levels of NGAL and plasma levels taken from the renal vein showed no differences between control and RC treatment groups. Urinary levels of L-FABP decreased after transplantation in both groups but were significantly lower 48 and 72 h posttransplant in the control group suggesting a faster recovery. The significance of this is difficult to interpret but does imply more ischemic injury in the RC grafts.
HD produces repetitive episodes of ischemia–reperfusion injury, which could exert a conditioning effect.18 Conversely, it has been suggested that chronic repetitive ischemia may lead to a fatigue phenomenon that suppresses any conditioning effect.19 In view of these considerations, the possible confounding effects of pretransplant HD were addressed using a post hoc analysis and this did not show any difference in the effects of RC in predialysis and HD patients.
A combination of warm and cold ischemic injury is inevitable in all kidney transplants, albeit in variable degree depending on the donor type. In deceased donor transplantation the index ischemia may simply be too great for RC to be effective. On the other hand live donor kidney transplants are subjected to relatively minor ischemic insults and this may make it difficult to demonstrate an improvement with almost any therapy. In addition to the variation in ischemic insults, preexisting patient comorbidities such as diabetes, hypertension, obesity, age, and even gender may dampen or abolish the conditioning effect.20
Our trial had a number of strengths. The patients and medical team were blinded to the treatment intervention. The trial sample size was informed by an appropriate power calculation using historical information about renal function in live donor transplants from our unit and a realistic estimate of the level of improvement that RC might make. The dropout rate was very low at only 2.5%. This trial also has several limitations. The trial was designed with the aim of finishing the I/R cycles immediately before clamp release and revascularization of the kidney. The cycling process lasted for 40 min but as the anastomosis times varied between 16 and 62 min it proved difficult to match the timing of the last 5-min ischemic stimulus exactly to the point of organ revascularization. Nonetheless, the I/R cycling was completed before revascularization in all of the patients in the treatment group. The effectiveness of RC strategies is dependent on the exact algorithm used. A range of RC algorithms have been used in previous studies and there is no consensus view in defining the most favorable protocol. There is, however, agreement that the duration of the ischemic stimulus should be in proportion to the metabolic rate21–23 and this is why cycles of 5 min were chosen, rather than the shorter cycles used in small animals with high metabolic rates. Notwithstanding this it is possible that the chosen I/R cycling was inadequate and that a different algorithm might have proved to be more effective. A recent meta-analysis concluded that renal protection was better if the conditioning stimulus was delivered 24 h before the insult24 and this may explain why the protocol used in our study was ineffective. The volume of tissue rendered ischemic during conditioning is also an important factor and this will vary with patient weight. Although the leg has more muscle mass than the arm it may be that the mass of tissue undergoing I/R cycling in our study was insufficient to produce an effective conditioning stimulus. Further studies could be designed to deliver an ischemic insult to both legs. In addition, methods to assess the exact volume of the tissue undergoing the ischemic stimulus may prove helpful in refining RC protocols and a clearer understanding of the mechanisms may provide the basis for the development of pharmacological conditioning in the future.
In conclusion, RC, using the protocol described, did not improve renal function after live donor kidney transplantation. Further studies are required to assess the effects of different conditioning strategies.
Footnotes
Abbreviations: DGF = delayed graft function, eGFR = estimated glomerular filtration rate, HD = hemodialysis, IR = ischemia reperfusion, L-FABP = Liver type fatty acid binding protein, NGAL = neutrophil gelatinase-associated lipocalin, RC = remote ischemic conditioning.
This research was supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. This study was funded by the University of Leicester, Transplant Department.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ponticelli C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant 2014; 29:1134–1140. [DOI] [PubMed] [Google Scholar]
- 2.Murray CE, Jennings RB, Reimer KA. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation 1986; 74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 3.Sack S, Mohri M, Arras M, et al. Ischaemic preconditioning—timecourse of renewal in the pig. Cardiovasc Res 1993; 27:551–555. [DOI] [PubMed] [Google Scholar]
- 4.Marber MS, Latchman DS, Walker JM, et al. Cardiac stress protein elevation 24 hours after brief or heat stress is associated with resistance to myocardial infarction. Circulation 1993; 88:1264–1272. [DOI] [PubMed] [Google Scholar]
- 5.Przyklenk K, Bauer B, Ovize M, et al. Regional ischaemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993; 87:893–899. [DOI] [PubMed] [Google Scholar]
- 6.van den Akker EK, Maninveld OC, Hesselink DA, et al. Protection against renal ischaemia-reperfusion injury by ischaemic postconditioning. Transplantation 2013; 95:1299–1305. [DOI] [PubMed] [Google Scholar]
- 7.McCafferty K, Byrne C, Yaqoob MM. Ischaemic conditioning strategies for the nephrologist: a promise lost in translation? Nephrol Dial Transplant 2014; 29:1827–1840. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Feng X, Huang H, et al. Remote ischaemic conditioning enhanced the early recovery of renal function in recipients after kidney transplantation: a randomized controlled trial. J Surg Res 2014; 188:303–308. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Zheng H, Wang X, et al. Remote ischaemic preconditioning fails to improve early graft function of patients undergoing living-donor renal transplantation: a randomized controlled trial. Transplantation 2013; 95:e4–e6. [DOI] [PubMed] [Google Scholar]
- 10.van den Akker EK, Hesselink DA, Manintveld OC, et al. Ischaemic postconditioning in human DCD kidney transplantation is feasible and appears safe. Transpl Int 2013; 27:226–234. [DOI] [PubMed] [Google Scholar]
- 11.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb induces remote ischaemic preconditioning in vivo. Circulation 2002; 106:2881–2883. [DOI] [PubMed] [Google Scholar]
- 12.Loukogeorgakis SP1, Panagiotidou AT, Broadhead MW, et al. Remote ischaemic preconditioning provides early and late protection against endothelial-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 2005; 46:450–456. [DOI] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007; 370:575–579. [DOI] [PubMed] [Google Scholar]
- 14.Venugopal V, Hausenloy DJ, Ludman A, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 2009; 95:1567–1571. [DOI] [PubMed] [Google Scholar]
- 15.Sloth AD, Schmidt MR, Munk K, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 2014; 35:168–175. [DOI] [PubMed] [Google Scholar]
- 16.Kohei J, Ishida H, Tanabe K, et al. Neutrophil gelatinase-associated lipocalin is a sensitive biomarker for the early diagnosis of acute rejection after living-donor kidney transplantation. Int Urol Nephrol 2013; 45:1159–1167. [DOI] [PubMed] [Google Scholar]
- 17.Arthur JM, Hill EG, Alge JL, et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 2014; 85:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton JO, Jefferies HJ, Selby NM, et al. Haemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 2009; 4:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittaker P, Przyklenk K. From ischemic conditioning to “hyperconditioning”: clinical phenomenon and basic science opportunity. Dose Response 2014; 12:650–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCafferty K, Forbes S, Thiemermann C, et al. The challenge of translating ischaemic conditioning from animal models to humans: the role of comorbidities. Dis Model Mech 2014; 7:1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venugopal V, Ludman A, Yellon DM, et al. “Conditioning” the heart during surgery. Eur J Cardiothorac Surg 2009; 35:977–987. [DOI] [PubMed] [Google Scholar]
- 22.Weng X, Shen H, Kuang Y, et al. Ischaemic postconditioing inhibits the renal fibrosis induced by reperfusion injury in rats. Urology 2012; 80:484e1–484e7. [DOI] [PubMed] [Google Scholar]
- 23.Szwarc I, Soullier S, Gayrard N, et al. Ischaemic postconditioning prevents ischaemic acute renal failure. Transplant Proc 2007; 39:2554–2556. [DOI] [PubMed] [Google Scholar]
- 24.Wever KE, Menting TP, Rovers M, et al. Ischemic preconditioning in the animal kidney: a systematic review and meta-analysis. PLoS ONE 2012; 7:e32296.Published online February 28, 2012. doi: 10.1371/journal.pone.0032296. [DOI] [PMC free article] [PubMed] [Google Scholar]


