Abstract
Isoflurane is a volatile halogenated anesthetic used especially for anesthesia maintenance whereas propofol is a venous anesthetic utilized for anesthesia induction and maintenance, and reportedly an antioxidant. However, there are still controversies related to isoflurane-induced oxidative stress and it remains unanswered whether the antioxidant effects occur in patients under propofol anesthesia.
Taking into account the importance of better understanding the role of anesthetics on oxidative stress in anesthetized patients, the present study was designed to evaluate general anesthesia maintained with isoflurane or propofol on antioxidant status in patients who underwent minimally invasive surgeries.
We conducted a prospective randomized trial in 30 adult patients without comorbidities who underwent elective minor surgery (septoplasty) lasting at least 2 h admitted to a Brazilian tertiary hospital.
The patients were randomly allocated into 2 groups, according to anesthesia maintenance (isoflurane, n = 15 or propofol, n = 15). Peripheral blood samples were drawn before anesthesia (baseline) and 2-h after anesthesia induction.
The primary outcomes were to investigate the effect of either isoflurane or propofol anesthesia on aqueous plasma oxidizability and total antioxidant performance (TAP) by fluorometry as well as several individual antioxidants by high-performance liquid chromatography. As secondary outcome, oxidized genetic damage (7,8-dihydro-8-oxoguanine, known as 8-oxo-Gua) was investigated by the comet assay.
Both anesthesia techniques (isoflurane or propofol) for a 2-h period resulted in a significant decrease of plasma α-tocopherol, but not other antioxidants including uric acid, carotenoids, and retinol (P > 0.05). Propofol, in contrast to isoflurane anesthesia, significantly increased (P < 0.001) anti-inflammatory/antioxidant plasma γ-tocopherol concentration in patients. Both anesthesia types significantly enhanced hydrophilic antioxidant capacity and TAP, with no significant difference between them, and 8-oxo-Gua remained unchanged during anesthesia in both groups. In addition, both anesthetics showed antioxidant capacity in vitro.
This study shows that anesthesia maintained with either propofol or isoflurane increase both hydrophilic and total antioxidant capacity in plasma, but only propofol anesthesia increases plasma γ-tocopherol concentration. Additionally, both types of anesthetics do not lead to oxidative DNA damage in patients without comorbidities undergoing minimally invasive surgery.
INTRODUCTION
Among the inhaled anesthetics, isoflurane is a halogenated ether widely used because of its low metabolism rate and low blood–gas partition coefficient. Preconditioning effects of halogenated anesthetics have been suggested to provide cardiac protection in patients undergoing coronary artery bypass graft surgery, which triggers ischemia–reperfusion injury.1,2 However, there are still controversies related to isoflurane-induced oxidative stress.
Propofol is a short-acting intravenous (IV) anesthetic widely employed for induction and maintenance of general anesthesia. Apart from its several anesthetic advantages, it has been reported that this agent exerts a number of nonanesthetic effects.3 The antioxidant capacity of this alkylphenol has been related to its chemical structure, which is similar to that of phenol-based scavengers, such as tocopherol.4 Various in vitro and animal studies have reported that propofol presents a scavenging activity against a broad range of free radicals and reactive oxygen species (ROS).5–7 However, it remains unanswered whether these effects occur in anesthetized patients.
In general, surgical procedures can increase ROS and decrease the biological defense system, leading to oxidative changes. During surgery, activated neutrophils convert oxygen into ROS, such as superoxide radicals, which are dismutated to toxic nonradical hydrogen peroxide, capable of destroying microorganisms. ROS produced by neutrophils can be inhibited by some anesthetics, such as isoflurane,8 and may provide a benefit against ischemia–reperfusion injury.9
The involvement of ROS in surgical stress has been reported most commonly in major surgeries,10 but not during minor surgeries without ischemia–reperfusion injury. Moreover, anesthetics used in surgery have demonstrated an important role in the oxidative status of patients. The overproduction of ROS can damage many macromolecules, including deoxyribonucleic acid (DNA). Among such DNA damage, 7,8-dihydro-8-oxoguanine (8-oxo-Gua) is a well-known modification resulting in transversion mutation (GC → TA).11 The intricate network of cellular defense mechanisms includes enzymatic and nonenzymatic antioxidant compounds. Among them, vitamins A and E have shown potent antioxidant activities that ameliorate ROS damage, protecting tissues against oxidative stress.12
Taking into account the importance of better understanding the role of anesthetics on oxidative stress in anesthetized patients, the present study was designed to evaluate general anesthesia maintained with isoflurane or propofol on antioxidant status in patients without comorbidities who underwent minimally invasive surgeries. The primary outcomes were to investigate both hydrophilic and total antioxidant capacity as well as several individual antioxidants during different anesthetic techniques. As secondary outcome, 8-oxo-Gua, which is recognized by the 8-hydroxyguanine DNA-glycosylase 1 (OGG1) was investigated.
PATIENTS AND METHODS
Study Design
This study was performed at Sao Paulo State University Hospital (UNESP, Botucatu, Brazil) from 2009 to 2011, was approved by the Institutional Review Board (Human Research Ethics Committee of the Botucatu Medical School-UNESP, #3449–2010), and was registered on the Clinical Trials (NCT00854178).
Fasted adults of both sexes participated in this prospective randomized study. The inclusion criteria consisted of nonobese, nonsmoking, and nonalcoholic adults (18–45 years old) patients classified by the American Society of Anesthesiologists (ASA) as physical status I (healthy patient with no disease other than a surgical abnormality), scheduled for elective minor surgery (septoplasty) lasting at least 2 h. These patients could not be under recent (within 3 months) or current medication or antioxidant supplementation, radiation or exposure to any environmental pollutant.
An anesthesiologist who was not involved in the perioperative management of the patients printed 30 group identification tags (15 for each group) and placed them in 30 envelopes (1 identification tag per envelope). Then the same anesthesiologist sealed, mixed, and listed the envelopes sequentially, that is, numbered in ascending order. Just before each anesthesia induction, 1 envelope was opened following the sequential order. Thus, the qualified patients were randomly allocated into the following groups: anesthesia maintained with the inhalation isoflurane (isoflurane group) or the IV propofol (propofol group). All participants provided written informed consent before the study. Random allocation, participant inclusion, and intervention distribution were performed by different investigators.
Anesthesia Protocol
In the operating room (OR), a 20-gauge peripheral IV line was inserted in the cephalic vein of the arm. Fluid deficits were replaced with lactated Ringer's solution at 4 mL/kg/h. A Primus anesthesia workstation (Dräger Medical, Lübeck, Germany) was used in all cases. The following standard clinical monitoring was performed: electrocardiogram, saturation of peripheral oxygen (SpO2), noninvasive arterial pressure (systolic and diastolic), and monitoring of neuromuscular blockade by train-of-four count at the adductor pollicis (TOF-Guard, Organon Teknika/Biometer, Odense, Denmark). The inspiratory and expiratory isoflurane concentrations (isoflurane group), oxygen concentrations, end-tidal carbon dioxide (PETCO2), and ventilation parameters were monitored with the Primus built-in monitor. All the patients received active forced-air warming with a specific blanket on the lower limbs using a warming device (Bair Hugger®, model 750, Arizant Healthcare, Minneapolis, MN).
All patients were premedicated in the OR with IV benzodiazepine midazolam (Dormonid, Roche, Rio de Janeiro, Brazil) (3 mg). In the isoflurane group, anesthesia was induced using the opioid fentanyl (GlaxoSmithKline, Parma, Italy) (5 μg/kg IV) and the hypnotic propofol 2 mg/kg (Diprivan®, AstraZeneca, Milan, Italy), and was maintained with an end-tidal isoflurane (Abbott, São Paulo, Brazil) at approximately 1.0 minimum alveolar concentration (MAC) equivalent to 1.2%. In the IV group, after the fentanyl (5 μg/kg IV), a target controlled infusion of 1% propofol (Diprivan, AstraZeneca) was administered using a computer-controlled infusion pump (Diprifusor, Fresenius Vial, Brezins, France) programmed with the pharmacokinetic model for propofol.13 The initial target propofol plasma concentration was set at 6.0 μg/mL. The target propofol concentrations were maintained between 3.0 and 5.0 μg/mL until the end of surgery. All groups received the neuromuscular blocker rocuronium bromide (Esmeron, Organon, Oss, The Netherlands) (0.6 mg/kg IV). After tracheal intubation, patients’ lungs were mechanically ventilated using the volume-controlled mode of the Primus anesthesia workstation with a tidal volume of 8 mL/kg of 40% oxygen (0.8 L/min) in air (1.2 L/min) and a respiratory rate of 10 to 12 breaths/min to maintain a PETCO2 of approximately 35 mm Hg. The intraoperative distal esophageal (core) temperatures were measured after tracheal intubation using a thermocouple sensor (Mon-a-therm 90044®, Mallinckrodt Medical, Veracruz, Mexico) attached to an electronic thermometer (4070, Mallinckrodt Medical, St Louis, MO). The effectiveness of anesthesia was monitored by assessing the hemodynamic responses (systemic arterial blood pressure and cardiac rate values within ±20% of baseline). Additional doses of fentanyl (2 μg/kg) and rocuronium (0.2 mg/kg) were administered if the patient was considered to be inadequately anesthetized.
Tracheal extubation was accomplished after the complete reversal of the neuromuscular blockade. If necessary, neuromuscular blockade was reversed with neostigmine 30 μg/kg IV (Normastig, União Química, São Paulo, Brazil) and atropine 10 μg/kg IV (Atropion, Ariston, São Paulo, Brazil) at the end of surgery. Patients received dipyrone 1 g IV (Novalgina, Hoechst, Rio de Janeiro, Brazil) and tramadol 100 mg IV (Tramal, Pfizer, São Paulo, Brazil) at the end of surgery. Patients were taken to the Post-Anesthetic Care Unit (PACU) following surgery.
Blood Sampling
Venous blood samples were collected from all patients before (baseline) and 2-h after anesthesia induction, then placed on ice and protected from light. Isolated lymphocytes and plasma were obtained by centrifugation. Aliquots were appropriately frozen at −80°C until analysis. All samples were coded and analyzed blindly. Techniques were carried out under indirect light.
Detection of Lipophilic Antioxidants
Using a reverse phase high-performance liquid chromatography (HPLC) system, the fat-soluble antioxidants—namely carotenoids, retinol (vitamin A), and tocopherols—were measured. Plasma samples were extracted with 2 mL of chloroform:methanol (2:1) followed by 3 mL of hexane. Samples were dried under nitrogen, resuspended into 100 μL ethanol, of which 20 μL was injected into the HPLC. The HPLC system was a Waters Alliance 2695 (Waters, Wilmington, MA) consisting of a pump with chromatography bound to a 2996 programmable photodiode array detector and a 474 fluorescence detector, a semibore C30 column (3.0 × 150 mm), and the software Empower2 Pro. The Waters 2996 programmable photodiode array detector was set at 450 nm for carotenoids, 340 nm for retinoids, and 292 nm for tocopherols. Using this method, lutein, zeaxanthin, cryptoxanthin, carotene, lycopene, retinol, and tocopherol were adequately separated. Carotenoids and retinoids were quantified by determining peak areas in the HPLC chromatograms calibrated against known amounts of standards. The amounts were corrected for extraction and handling losses by monitoring the recovery of the internal standards (echinenone or retinyl acetate). The coefficients of variation (CV) were 4% for inter-assay and 3% for intra-assay. Recovery of internal standard averaged 98%. All sampling was carried out under red light.
Determination of Uric Acid
Hydrophilic antioxidant uric acid was determined by a colorimetric method using a clinical chemistry analyzer (AutoAnalyzer, VITROS 5.1/FS model, Johnson & Johnson, UK), according to the manufacturers’ instructions. Data were expressed as μmol/L.
Measurement of Plasma Aqueous Compartment Oxidation
This assay was fluorometrically determined as previously described,14 with slight modifications. Aqueous plasma oxidizability was initiated by 2,2′-azobis (4-amidinopropane) dihydrochloride (AAPH), used as a peroxyl radical initiator whereas 2′,7′-dichlorodihydrofluorescein (DCFH) was employed as the marker of oxidative reaction. Briefly, 100 μL of plasma, 100 μL of phosphate saline, and 100 μL of delipidized human serum (DHS) were incorporated and 100 μL of DCFH was added and vortexed, and samples were incubated at 37°C for 10 min. AAPH (500 μL) was added in each tube with 200 μL of phosphate saline. DHS and also samples (200 μL) were evaluated in triplicate in a 96-well plate. Oxidation was monitored by the fluorescent oxidation product of DCFH using a microplate reader (Wallac Victor 2, Perkin Elmer Life Sciences, Boston, MA) at 37°C. Samples for each subject and per time point, from both groups, were analyzed within the same run for every assay performed. The experiments were run under red light.
Determination of Total Antioxidant Performance (TAP)
Phosphatidylcholine (PC) liposomes were prepared as previously reported,15 and TAP was determined following the protocol already described16 with slight modifications, by using a lipophilic radical initiator 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (MeO-AMVN) and 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY581/591) as a lipophilic fluorescence probe to monitor the lipid compartment plasma oxidation. Plasma (100 μL) was added to PC (100 μL), which was used as control/standard, and to 300 μL of phosphate buffer saline. Samples were incubated at 37°C for 10 min. Then, phosphate buffer (485 μL, pH 7.4) was added and gently vortexed. The radical initiator, MeO-AMVN (15 μL, final concentration 2 mM), was added slowly to the samples with a syringe, and gently mixed with a magnetic stirring bar. Oxidation was monitored by the green fluorescent oxidation product of BODIPY (final concentration λex = 500, λem = 520 nm, slit 10 nm) using a microplate reader (Wallac Victor 2, Perkin Elmer Life Sciences), at 37°C for 3 h (36 cycles × 5 min). Wallac workout 2 was used and the following formula was applied: TAP = [(AUC control – AUC plasma)/AUC control] × 100, where AUC = area under the curve; control = PC and plasma sample. The experiments were run under red light.
In order to verify whether the anesthetics could have antioxidant capacity per se, PC liposomes were used for in vitro studies to determine TAP of propofol or isoflurane. Based on patients’ plasma concentrations (3–5 μg/mL), propofol (Diprivan®) was added into the solution before the incubation at final concentrations of 1, 3, 5, 7, and 10 μg/mL (mM). For the volatile anesthetic, based on theoretical blood concentrations (MAC and partition coefficient blood:gas; 0.2–0.8 mM),17 isoflurane (Abbott) was added into the incubation solution by reverse pipetting on ice at final concentrations of 0.1, 0.3, 0.5, 0.7, and 1.0 mM. The antioxidant α-tocopherol was evaluated as a control (from 5 to 20 mM). All samples were performed and analyzed in triplicate.
Oxidized Purine (8-oxo-Gua) Detected by the Comet Assay
The protocol used followed the procedures previously described,18 with slight modifications. Every step was carried out under dim light. Volumes of 10 μL of lymphocytes were added to 120 μL of 0.5% low melting point agarose at 37°C. The mixtures were layered onto slides precoated with 1.5% normal agarose, covered with a coverslip and left for 5 min at 4°C to solidify the agarose. Afterwards, coverslips were carefully removed and slides immersed, overnight, into a cold lysis solution (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid [EDTA], 10 mM Tris at pH 10, with 1% Triton X-100 and 10% dimethylsulfoxide added fresh). Slides were washed in phosphate buffer saline for 5 min, washed again (3 × 5 min each) in a buffer 1× (40 mM 2-[4-(2-hydroxyethyl) piperazin-1-yl] ethanesulfonic acid [HEPES], 100 mM potassium chloride [KCl], 0.2 mg/mL bovine serum albumin, and 0.5 mM EDTA at pH 8), and incubated at 37°C for 30 min in a moist chamber with 50 μL of OGG1 (1:1000) or 50 μL of enzyme buffer only (control). Then, slides were left for 15 min at 4°C and coverslips were removed. Afterwards, the slides were exposed to a freshly prepared alkaline buffer (1 mM EDTA, 300 mM NaOH at pH 13) in a horizontal electrophoresis tank. After a 40-min DNA unwinding period, electrophoresis was conducted at 25 V and 300 mA for 30 min. Following 15 min of neutralization with 0.4 M Tris (pH 7.5), slides were fixed in absolute ethanol and stored at 4°C. Slides were stained with 75 μL Sybr Gold and analyzed in a fluorescent microscope at 400× magnification. Images from 100 nucleoids from each treatment/time point/patient were scored using the Comet Assay IV Image System (Perceptive Instruments, Haverhill, Suffolk, UK). Tail intensity was used to estimate the extent of DNA damage.
Statistical Analysis
Sample size was calculated from a pilot study and also from previous studies19,20 based on antioxidant level. Fifteen patients per group allowed a 95% statistical power (type II error – β of 0.05) to detect a between-group difference of 3.4 μM in γ-tocopherol concentrations (SD of ±2.6 μM) with an α error of 0.05. The characteristics of the 2 groups were compared by Student t test. The repeated measures ANOVA followed by Tukey's test were used to compare time points and groups for all measurements. For all the analyses, a P value <0.05 was considered statistically significant.
RESULTS
The flow diagram of the study procedure is shown in Figure 1. Characteristics of the studied population are shown in Table 1. None of the patients presented any surgical or clinical complications, and all of them were discharged from the hospital according to the established guidelines. Patients from both groups were hemodynamically stable (data not shown).
FIGURE 1.
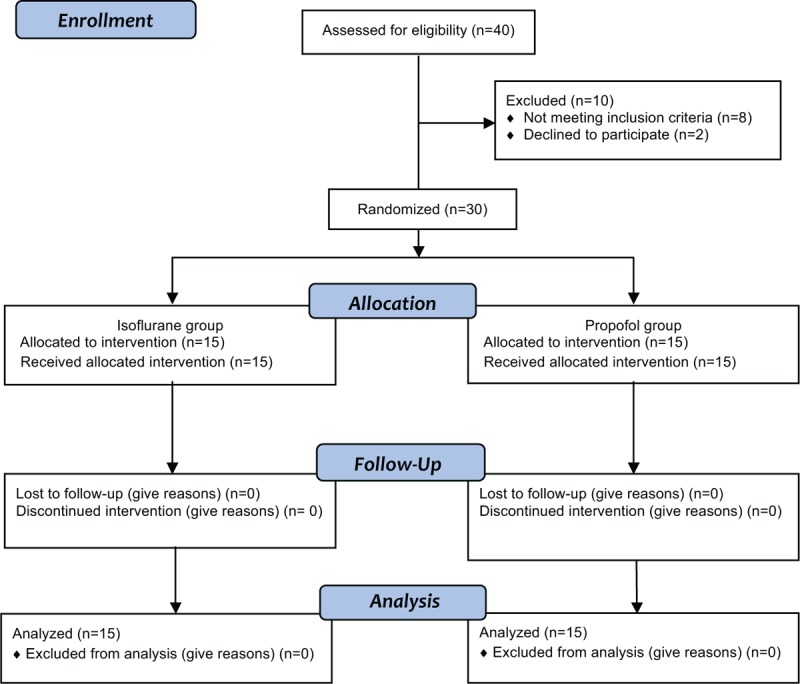
Flow diagram of the study.
TABLE 1.
Characteristics of the Studied Populations
Individual Antioxidants
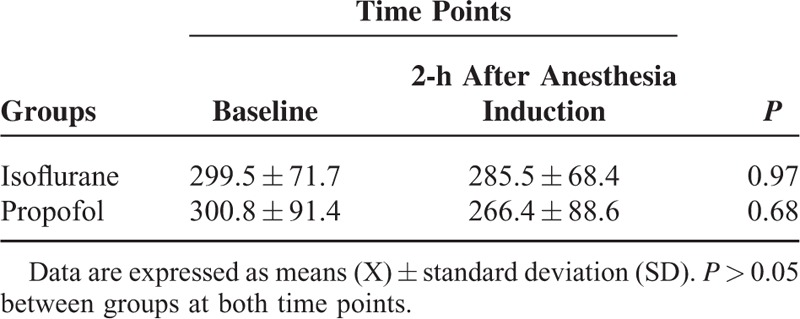
All the carotenoids and retinol evaluated slightly decreased during 2 h of anesthesia, but without statistical significance, and with no difference between the groups (Table 2). Both types of anesthesia significantly decreased α-tocopherol, but there was no statistically difference between groups. Isoflurane or propofol anesthesia did not alter the uric acid concentrations in plasma (Table 3).
TABLE 2.
Micronutrients (μM) Detected in Patients Undergoing Surgery Maintained With Isoflurane (n = 15) or Propofol (n = 15) Anesthesia
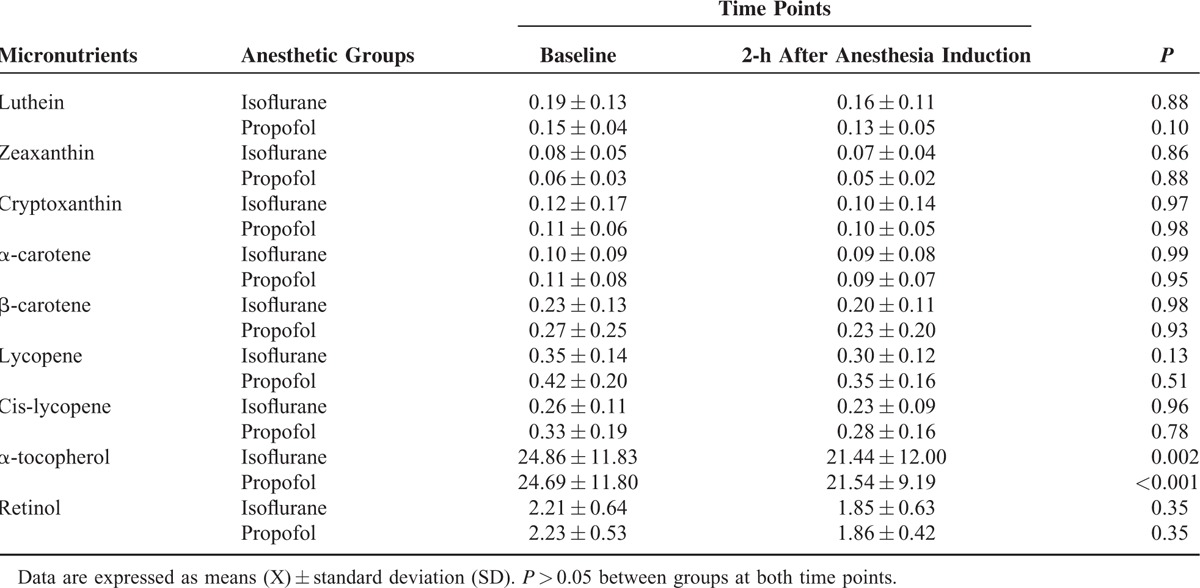
TABLE 3.
Uric Acid Levels (μM) in Patients Undergoing Surgery Maintained With Isoflurane (n = 15) or Propofol (n = 15) Anesthesia
Patients from the 2 groups presented similar levels of γ-tocopherol before anesthesia (baseline), but they differed at 2-h after anesthesia induction (P = 0.02). Plasma γ-tocopherol concentration significantly increased with propofol (1.6× fold) (P < 0.001), but remained unchanged during isoflurane anesthesia (Figure 2).
FIGURE 2.
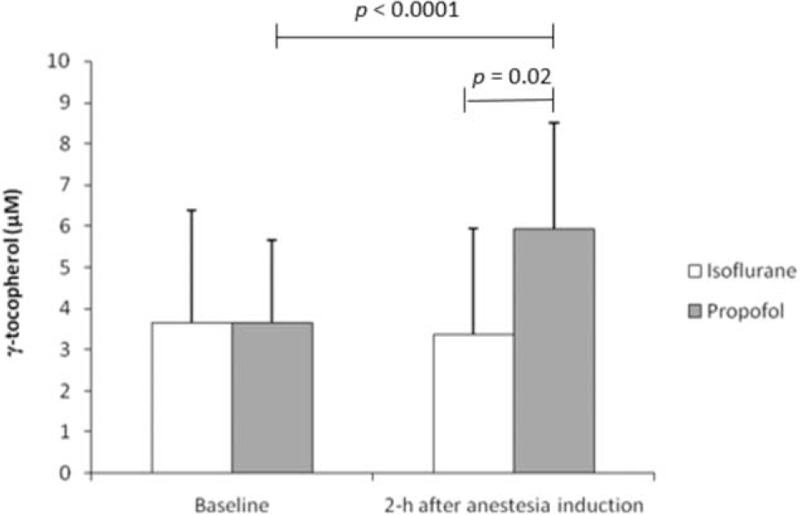
Changes in γ-tocopherol detected in patients undergoing surgery maintained with isoflurane (n = 15) or propofol (n = 15) anesthesia. P > 0.05 between groups at baseline.
Antioxidant Capacity
Both isoflurane and propofol anesthesia significantly increased the antioxidant capacity in the aqueous compartment of plasma (P < 0.05) as well as TAP (P < 0.05) evaluated in patients during surgery (Figures 3 and 4, respectively). An enhancement of antioxidant status at 2-h of anesthesia was observed in patients anesthetized with isoflurane or propofol, without any significant differences between the groups.
FIGURE 3.
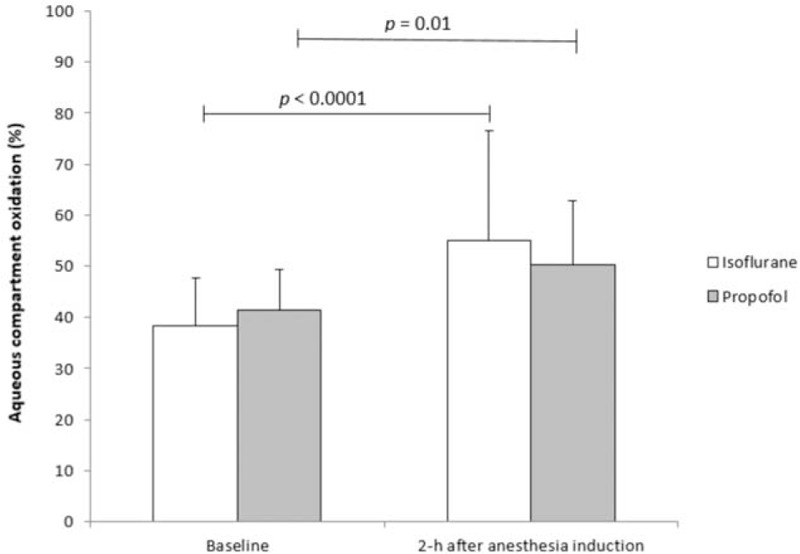
Hydrophilic antioxidant capacity evaluated in patients undergoing surgery maintained with isoflurane (n = 15) or propofol (n = 15) anesthesia. P > 0.05 between groups at both time points.
FIGURE 4.
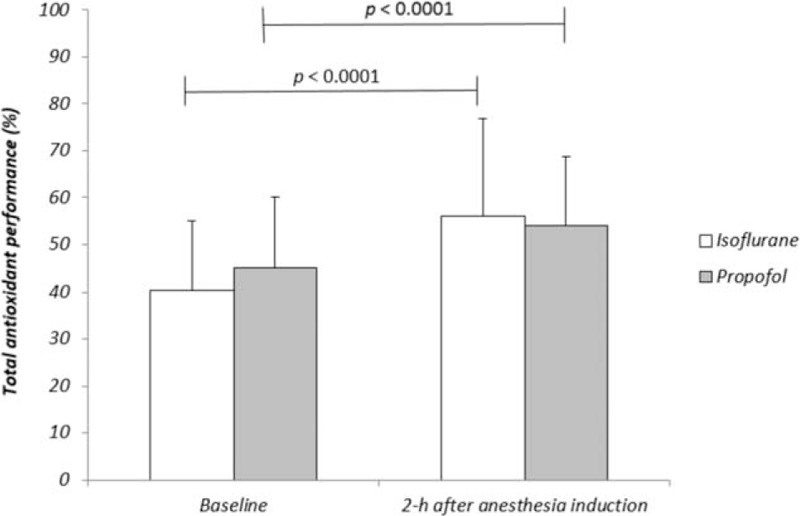
Total antioxidant performance evaluated in patients undergoing surgery maintained with isoflurane (n = 15) or propofol (n = 15) anesthesia. P > 0.05 between groups at both time points.
Figure 5 shows the oxidation kinetics of PC liposomes in the presence of either isoflurane or propofol, as determined by TAP. Both anesthetics presented similar responses and showed dose-dependent antioxidant potential in the equivalent blood concentration during anesthesia. Figure 6 shows the antioxidant protection of both anesthetics at different concentrations, including those utilized for anesthesia maintenance. Both anesthetics showed antioxidant capacity (around 11% and 14% for isoflurane and propofol, respectively).
FIGURE 5.
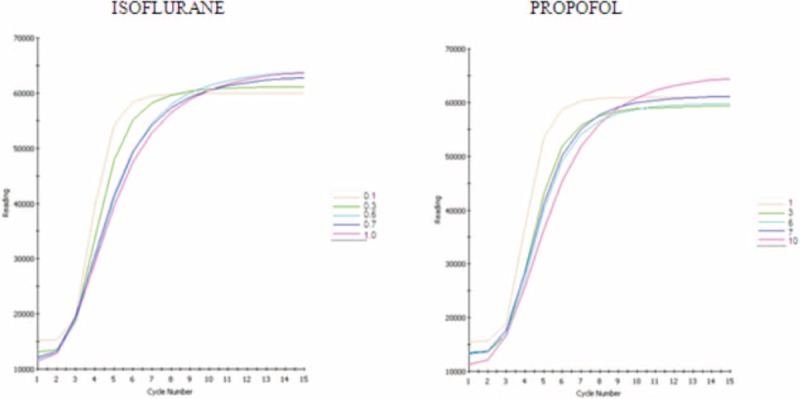
Oxidation kinetics of total antioxidant performance assay (fluorescence) with different concentrations of isoflurane or propofol. Each cycle corresponds to 5 min.
FIGURE 6.
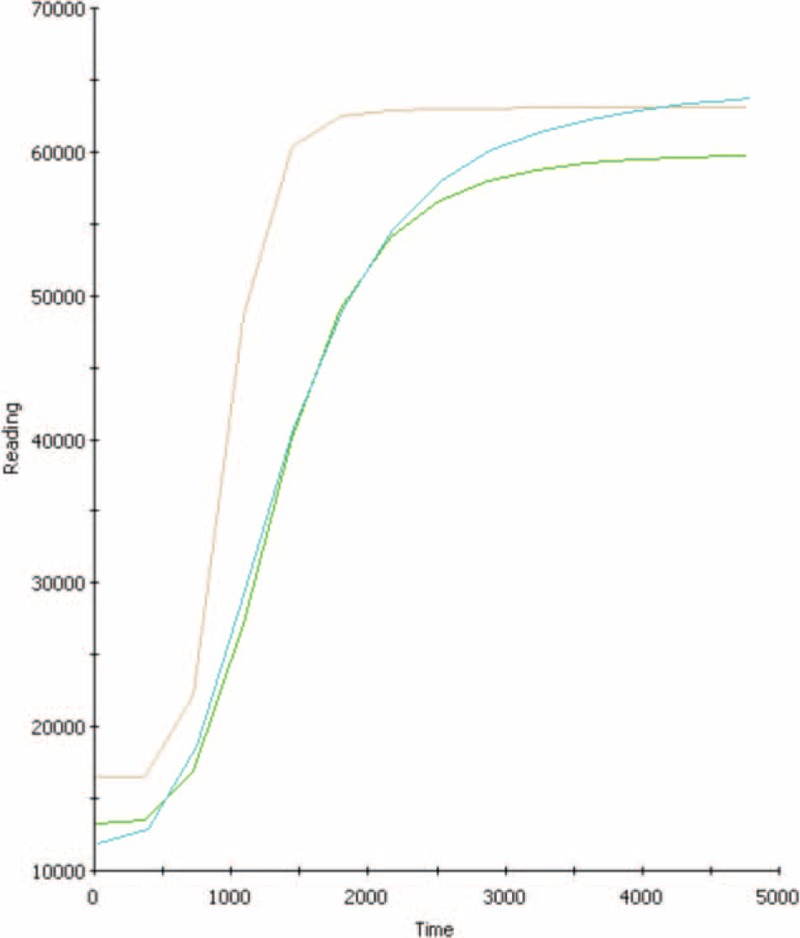
Total antioxidant performance of control (phosphatidylcholine—brown line), isoflurane (blue line), or propofol (green line), at blood concentrations.
Oxidative DNA Damage
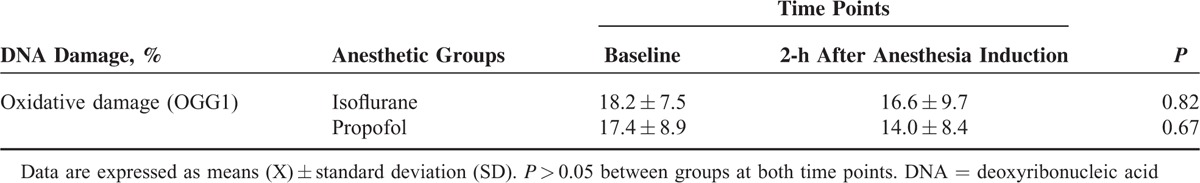
General anesthesia maintained with isoflurane or propofol did not induce 8-oxo-Gua damage in lymphocytes of patients 2 h after anesthesia induction as measured by the comet assay, when specific glycosylase OGG1 was used (Table 4). Neither the time points nor groups differed significantly (P > 0.05).
TABLE 4.
Oxidative DNA Damage (Using Lesion-Specific-Endonuclease) Evaluated in Patients Undergoing Surgery Maintained With Isoflurane (n = 15) or Propofol (n = 15) Anesthesia
DISCUSSION
In this randomized and prospective clinical trial study, we showed the antioxidative role of propofol and isoflurane anesthesia in patients without comorbidities undergoing minimally invasive surgery without ischemia–reperfusion injury. Antioxidant activity of isoflurane itself may have resulted in increases of plasma antioxidant capacity in adult patients whereas propofol may have contributed to the increase of γ-tocopherol and also antioxidative status in these patients. Both anesthetics seem to prevent systemic oxidative DNA damage.
All the nonenzymatic antioxidants determined in the present study are able to quench the peroxyl radical, which is involved in lipid peroxidation. Despite the slight decline of some antioxidant concentrations (but still at normal ranges), neither isoflurane nor propofol anesthesia had any effect on malondialdehyde (MDA) values, as demonstrated in our previous study.21 Isoflurane plus nitrous oxide (N2O) anesthesia had no effect on the plasma antioxidant enzymatic system, but decreased some erythrocyte enzyme activities and trace elements in patients undergoing abdominal surgery.22 In this same type of surgery, patients under total IV propofol anesthesia showed enhancement of antioxidant glutathione-peroxidase measured in platelets.23
We believe one of the novelties observed in the present study is the increase of γ-tocopherol in patients anesthetized with propofol, but not under isoflurane, in minor elective surgery, since humans cannot synthesize tocopherol, and patients were fasted and not under supplementation. The γ-tocopherol is the major form of vitamin E in many plant seeds and vegetable oils.24 Since propofol is usually eluted in an intralipid solution (Diprivan®) containing soybean oil with a mixture of saturated and unsaturated fatty acids, which are rich in γ-tocopherol, this could explain our findings. The increase of γ-tocopherol concentration only in the propofol group could also be attributed to the interaction of this anesthetic with Cytochrome P450 3A4 (CYP3A4), since the anesthetic and tocopherols share this metabolic pathway.19
Drawing a parallel, a 6-week γ-tocopherol supplementation results in high concentrations of this antioxidant (similarly to our observations in the propofol group) thus reducing biomarkers of oxidative stress and inflammation in metabolic syndrome patients.25 A study reported that propofol anesthesia (plasma concentrations of 2.5–4.0 μg/mL) enhances γ-tocopherol during cardiac surgery with cardiopulmonary bypass, returning to basal values 24 h after surgery.19 These findings indicate an anti-inflammatory effect of propofol, which may be relevant for controlling inflammatory response related to tissue injury after reperfusion.
Plasma γ-tocopherol was increased during propofol anesthesia whereas α-tocopherol was decreased during continuous infusion with propofol or even with isoflurane. Previous studies reported that antioxidant supplementation leads to a negative correlation between α- and γ-tocopherol.26,27 Another report supports our data in isoflurane group since the authors showed a decrease of vitamin E 1 h after the introduction of this volatile anesthetic in patients who underwent cholecystectomy.28 In contrast, this same antioxidant (α-tocopherol) remained unchanged during and after propofol or isoflurane anesthesia in patients scheduled for lumbar spinal surgery.29 Possible discrepancies in these data could be explained by different anesthetic regimens, including the use of isoflurane (0.5% to 0.75%) together with N2O (60%), and different plasma concentrations of propofol.
Compared with α-tocopherol, γ-tocopherol is a slightly less potent antioxidant as an electron donor despite being much superior in detoxifying electrophiles, including peroxynitrite, and presents an anti-inflammatory effect. Interestingly, an inflammatory response occurred earlier in patients who received isoflurane compared with propofol anesthesia.30 Moreover, like propofol, γ-tocopherol reduces inflammation through inhibition of cyclooxygenase type 2 activity.31
Most of previous studies using propofol and derivatives were performed under aqueous (nonmembranous) conditions in vitro.32,33 Thus, our findings indicate that propofol administered by a computer-controlled infusion pump is able to increase antioxidant capacity when evaluated in the hydrophilic compartment of the patient's plasma.
Propofol has a great affinity for lipid bilayers, being preferentially distributed in liposomal and cellular membranes, accounting for its much higher concentration in membranes than in blood. This anesthetic reacts with peroxynitrite, leading to formation of a propofol-derived phenoxyl radical, and acts similarly to tocopherol.4,34 The majority of in vitro and in vivo studies have attributed the protective effect of bioactive polyphenols to their chemical reactivity toward free radicals and their capacity to prevent oxidation of important intracellular components. This anesthetic displays antioxidant capacity and inhibits lipid peroxidation at low μM levels, which correspond to the human plasma concentration during anesthesia.7 Moreover, propofol showed great potential as a free radical scavenger in patients undergoing surgery with extracorporeal circulation.35
In order to assess the magnitude of the active interactions between the 2 compartments in plasma, we used the fluorometric method denominated TAP to determine the total antioxidant effects of patients undergoing anesthesia. TAP measures both aqueous and lipid compartment oxidizability.14 Thus, our data showed that both propofol and isoflurane are able to augment both the aqueous compartment as well as the hydrophilic and lipophilic antioxidant capacity.
It has been suggested that polyphenols induce the heme oxygenase (HO)-1 pathway.36 In the same manner, it has already been reported that anesthetics also modulate HO, which exerts anti-inflammatory, anti-proliferative, antioxidative, and vasodilatory effects.37 Therefore, both propofol and isoflurane share similar mechanisms of HO-1 induction via the nuclear factor kappa B.38 Our findings reinforce the notion that patients anesthetized with propofol or isoflurane have increased antioxidant capacity, even when administered in minor surgeries without ischemia–reperfusion injury.
To elucidate whether the increase of hydrophilic antioxidant capacity and TAP in patients undergoing an anesthetic procedure could be a consequence of antioxidant capacities from anesthetics, the oxidation kinetics of either propofol or isoflurane were determined in vitro. Anesthetics by themselves showed antioxidant capacities, reinforcing our findings related to the increase of antioxidant status in anesthetized patients. Thus, isoflurane may have contributed to enhanced antioxidant status in patients even during a relatively short surgical-procedure duration. This volatile anesthetic is being utilized especially in major surgeries, including coronary ones, related to ischemia–reperfusion injury. Moreover, this anesthetic can attenuate oxidative stress and has neuroprotective effects in vitro, and its pretreatment protects cardiac cells from damage by oxidative stress and reduces myocardial infarction size by modulating mitochondrial ROS at clinical concentrations.39–41
Additionally, there was no oxidative genetic-induced lesion, evaluated by the use of OGG1 in the modified comet assay, during isoflurane anesthesia. There are controversial findings related to the cellular toxicity of volatile anesthetics. Isoflurane might induce cytotoxicity in vitro,42 but anesthesia maintained with isoflurane did not lead to genotoxicity or apoptotic T lymphocytes in patients.43 It must be highlighted that only 0.2% of isoflurane undergoes metabolism, and does not alter the extent of DNA damage induced by in vitro treatment with hydrogen peroxide.43 Hence, these data support the hypothesis that 1.2% isoflurane does not induce oxidative lesions in patients without comorbidities undergoing minor elective surgeries.
In relation to genetic damage, propofol prevented DNA cleavage in peroxynitrite injury in primary astroglial cell cultures.6 Although we have already observed a reduction of genetic lesions evaluated by formamidopyrimidine DNA glycosylase (FPG) sites during propofol anesthesia,44 we did not detect a decrease in oxidized DNA using OGG1 when total IV anesthesia with propofol was administered. This could be explained by different types of oxidative damage recognized by OGG1 or FPG repair enzyme. The OGG1 is more specific for detecting methyl-fapy-guanine and 8-oxo-Gua, which is the most abundant metabolite of guanine and seems to play a major role in mutagenesis and in carcinogenesis.18,45 In a previous study, we reported a decrease of hydrogen peroxide-induced genotoxicity in lymphocytes as well as a reduction of apoptotic T helper cells collected from patients during surgical procedures with propofol anesthesia.44
One limitation of our study is that we have used propofol for anesthesia induction in isoflurane group, but since this IV anesthetic was only administered in a single dose, and considering its high clearance,46 it seems to have had only a minimum influence on oxidative status during surgery.
This is the first study to show that both types of anesthesia increase antioxidant status and do not lead to oxidized (8-oxo-Gua) genetic damage in adult patients during surgical procedure. Additionally, propofol anesthesia contributes to enhance the anti-inflammatory/antioxidant plasma γ-tocopherol concentration in these patients. Consequently, the clinical application of this study is very important. We recommend the anesthesiologists and surgeons to apply this knowledge into routine clinical practice. These findings can provide support for the translational concept by further elucidating the antioxidative role of 2 anesthetics widely used in daily practice during surgeries. Further studies are also required to better understand the possible action mechanisms of such anesthetics related to antioxidant capacity in patients undergoing major surgeries or with comorbidities.
In conclusion, the present study shows that anesthesia maintained with either propofol or isoflurane increase both hydrophilic and total antioxidant capacity in plasma, but only propofol anesthesia increases plasma γ-tocopherol concentration. In addition, both types of anesthetics do not lead to oxidative DNA damage in patients without comorbidities undergoing minimally invasive surgery.
ACKNOWLEDGMENTS
We thank the biostatisticians Prof José Eduardo Corrente and Prof Lídia Raquel de Carvalho (UNESP, Botucatu, Brazil) for statistical analyses, and James Welsh (Native English speaker) to correct our manuscript.
Footnotes
Abbreviations: 8-oxo-Gua = 7,8-dihydro-8-oxoguanine, AAPH = 2,2′-azobis (4-amidinopropane) dihydrochloride, ASA = American Society of Anesthesiologists, AUC = area under the curve, BODIPY = 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid, CABG = coronary artery bypass graft, CV = coefficient of variation, CYP3A4 = Cytochrome P450 3A4, DCFH = 2′,7′-dichlorodihydrofluorescein, DHS = delipidized human serum, DNA = deoxyribonucleic acid, EDTA = ethylenediaminetetraacetic acid, FPG = formamidopyrimidine DNA glycosylase, HEPES = 2-[4-(2-hydroxyethyl) piperazin-1-yl] ethanesulfonic acid, HO = heme oxygenase, HPLC = high-performance liquid chromatography, IV = intravenous, KCl = potassium chloride, MAC = minimum alveolar concentration, MDA = malondialdehyde, MeO-AMVN = 2,2′-azobis (4-methoxy-2,4-dimethylvaleronitrile), N2O = nitrous oxide, NaCl = sodium chloride, NaOH = sodium hydroxide, NFκB = nuclear factor kappa B, OGG1 = 8-hydroxyguanine DNA-glycosylase 1, OR = operating room, PACU = Post-Anesthetic Care Unit, PC = phosphatidylcholine, PETCO2 = partial pressure of end-tidal carbon dioxide, ROS = reactive oxygen species, SpO2 , saturation of peripheral oxygen = TAP, total antioxidant performance.
This work was supported by the grant #2010/05611-0, São Paulo Research Foundation (FAPESP), and the U.S. Department of Agriculture (USDA/USA) under number 58-1950-7-07. MGB was supported by a Postdoctoral Fellowship program, grant #2009/17344-9, from the São Paulo Research Foundation (FAPESP) and São Paulo State University (UNESP).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Belhomme D, Peynet J, Louzy M, et al. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation 1999; 100:II340–II344. [DOI] [PubMed] [Google Scholar]
- 2.Kottenberg E, Thielmann M, Bergmann L, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 2012; 56:30–38. [DOI] [PubMed] [Google Scholar]
- 3.Vasileiou I, Xanthos T, Koudouna E, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol 2009; 605:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Murphy PG, Myers DS, Davies MJ, et al. The antioxidant potential of propofol (2,6-diisopropyl-phenol). Br J Anaesthesiol 1992; 68:613–618. [DOI] [PubMed] [Google Scholar]
- 5.Kokita N, Hara A, Abiko Y, et al. Propofol improves functional and metabolic recovery in ischemic reperfused isolated rat hearts. Anesth Analg 1998; 86:252–258. [DOI] [PubMed] [Google Scholar]
- 6.Acquaviva R, Campisi A, Murabito P, et al. Propofol attenuates peroxynitrite-mediated DNA damage and apoptosis in cultured astrocytes: an alternative protective mechanism. Anesthesiology 2004; 101:1363–1371. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya H, Ueno T, Tanaka T, et al. Comparative study on determination of antioxidant and membrane activities of propofol and its related compounds. Eur J Pharm Sci 2010; 39:97–102. [DOI] [PubMed] [Google Scholar]
- 8.Fröhlich D, Rothe G, Schwall B, et al. Effects of volatile anaesthetics on human neutrophil oxidative response to the bacterial peptide FMLP. Br J Anaesthesiol 1997; 78:718–723. [DOI] [PubMed] [Google Scholar]
- 9.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth 2008; 22:263–277. [DOI] [PubMed] [Google Scholar]
- 10.Aivatidi C, Vourliotakis G, Georgopoulos S, et al. Oxidative stress during abdominal aortic aneurysm repair-biomarkers and antioxidant's protective effect: a review. Eur Rev Med Pharmacol Sci 2011; 15:245–252. [PubMed] [Google Scholar]
- 11.Cheng KC, Cahill DS, Kasai H, et al. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G–T and A–C substitutions. J Biol Chem 1992; 267:166–172. [PubMed] [Google Scholar]
- 12.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 1993; 300:535–543. [DOI] [PubMed] [Google Scholar]
- 13.Marsh B, White M, Morton N, et al. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesthesiol 1991; 67:41–48. [DOI] [PubMed] [Google Scholar]
- 14.Aldini G, Yeum KJ, Russell RM, et al. A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free Radic Biol Med 2001; 31:1043–1050. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira AL, Yeum KJ, Matsubara LS, et al. Doxorubicin as an antioxidant: maintenance of myocardial levels of lycopene under doxorubicin treatment. Free Radic Biol Med 2007; 43:740–751. [DOI] [PubMed] [Google Scholar]
- 16.Beretta G, Aldini G, Facino RM, et al. Total antioxidant performance: a validated fluorescence assay for the measurement of plasma oxidizability. Anal Biochem 2006; 354:290–298. [DOI] [PubMed] [Google Scholar]
- 17.Jaloszynski P, Kujawski M, Wasowicz M, et al. Genotoxicity of inhalation anesthetics halothane and isoflurane in human lymphocytes studied in vitro using the comet assay. Mutat Res 1999; 439:199–206. [DOI] [PubMed] [Google Scholar]
- 18.Smith CC, O’Donovan MR, Martin EA. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis 2006; 21:185–190. [DOI] [PubMed] [Google Scholar]
- 19.Cavalca V, Colli S, Veglia F, et al. Anesthetic propofol enhances plasma (-tocopherol levels in patients undergoing cardiac surgery. Anesthesiology 2008; 108:988–997. [DOI] [PubMed] [Google Scholar]
- 20.Jambazian PR, Haddad E, Rajaram S, et al. Almonds in the diet simultaneously improve plasma alpha-tocopherol concentrations and reduce plasma lipids. J Am Diet Assoc 2005; 105:449–454. [DOI] [PubMed] [Google Scholar]
- 21.Braz MG, Braz LG, Braz JR, et al. Comparison of oxidative stress in ASA physical status I patients scheduled for minimally invasive surgery under balanced or intravenous anesthesia. Minerva Anestesiol 2013; 79:1030–1038. [PubMed] [Google Scholar]
- 22.Türkan H, Bukan N, Sayal A, et al. Effects of halothane, enflurane, and isoflurane on plasma and erythrocyte antioxidant enzymes and trace elements. Biol Trace Elem Res 2004; 102:105–112. [DOI] [PubMed] [Google Scholar]
- 23.De La Cruz JP, Zanca A, Carmona JA, et al. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg 1999; 89:1050–1055. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin PJ, Weihrauch JL. Vitamin E content of foods. J Am Diet Assoc 1979; 75:647–665. [PubMed] [Google Scholar]
- 25.Devaraj S, Leonard S, Traber MG, et al. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med 2008; 44:1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handelman GJ, Machlin LJ, Fitch K, et al. Oral α-tocopherol supplements decrease plasma γ-tocopherol levels in humans. J Nutr 1985; 115:807–813. [DOI] [PubMed] [Google Scholar]
- 27.Meydani M, Cohn JS, Macauley JB, et al. Postprandial changes in the plasma concentration of alpha- and gamma-tocopherol in human subjects fed a fat-rich meal supplemented with fat-soluble vitamins. J Nutr 1989; 119:1252–1258. [DOI] [PubMed] [Google Scholar]
- 28.Zunić J, Stavljenić-Rukavina A, Granić P, et al. Changes in vitamin E concentration after surgery and anesthesia. Coll Antropol 1997; 21:327–334. [PubMed] [Google Scholar]
- 29.Hans P, Canivet JL, Pincemail J, et al. Plasma vitamin E, total lipids and myeloperoxidase levels during spinal surgery. A comparison between two anesthetic agents: propofol and isoflurane. Acta Anaesthesiol Scand 1991; 35:302–305. [DOI] [PubMed] [Google Scholar]
- 30.Mazoti MA, Braz MG, de Assis Golim M, et al. Comparison of inflammatory cytokine profiles in plasma of patients undergoing otorhinological surgery with propofol or isoflurane anesthesia. Inflamm Res 2013; 62:879–885. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Q, Elson-Schwab I, Courtemanche C, et al. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA 2000; 97:11494–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gülçin I, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005; 53:281–285. [DOI] [PubMed] [Google Scholar]
- 33.Rigobello MP, Stevanato R, Momo F, et al. Evaluation of the antioxidant properties of propofol and its nitrosoderivative. Comparison with homologue substituted phenols. Free Radic Res 2004; 38:315–321. [DOI] [PubMed] [Google Scholar]
- 34.Mathy-Hartert M, Mouithys-Mickalad A, Kohnen S, et al. Effects of propofol on endothelial cells subjected to a peroxynitrite donor (SIN-1). Anaesthesia 2000; 55:1066–1071. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SH, Wang SY, Yao SL. Antioxidative effect of propofol during cardiopulmonary bypass in adults. Acta Pharmacol Sin 2004; 25:334–340. [PubMed] [Google Scholar]
- 36.Scapagnini G, Vasto S, Abraham NG, et al. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 2011; 44:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoetzel A, Schmidt R. Regulatory role of anesthetics on heme oxygenase-1. Curr Drug Targets 2010; 11:1495–1503. [DOI] [PubMed] [Google Scholar]
- 38.Li Volti G, Basile F, Murabito P, et al. Antioxidant properties of anesthetics: the biochemist, the surgeon and the anesthetist. Clin Ter 2008; 159:463–469. [PubMed] [Google Scholar]
- 39.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology 2006; 105:98–104. [DOI] [PubMed] [Google Scholar]
- 40.Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth Analg 2009; 109:1506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirata N, Shim YH, Pravdic D, et al. Isoflurane differentially modulates mitochondrial reactive oxygen species production via forward versus reverse electron transport flow: implications for preconditioning. Anesthesiology 2011; 115:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuoka H, Kurosawa S, Horinouchi T, et al. Inhalation anaesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 2001; 95:467–472. [DOI] [PubMed] [Google Scholar]
- 43.Braz MG, Mazoti MA, Giacobino J, et al. Genotoxicity, cytotoxicity and gene expression in patients undergoing elective surgery under isoflurane anaesthesia. Mutagenesis 2011; 26:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braz MG, Braz LG, Mazoti MA, et al. Lower levels of oxidative DNA damage and apoptosis in lymphocytes from patients undergoing surgery with propofol anesthesia. Environ Mol Mutagen 2012; 53:70–77. [DOI] [PubMed] [Google Scholar]
- 45.Fortini P, Pascucci B, Parlanti E, et al. 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat Res 2003; 531:127–139. [DOI] [PubMed] [Google Scholar]
- 46.Reves JG, Glass P, Lubarsky DA. Miller RD. Intravenous anesthetics. Miller's Anesthesia 7th ed.Philadelphia: Elsevier; 2010. 719–768. [Google Scholar]


