Abstract
The role of glutamine (GLN) supplementation in critically ill patients is controversial. Our aim was to analyze its potential effect in patients admitted to intensive care unit (ICU).
We performed a systematic literature review through Medline, Embase, Pubmed, Scopus, Ovid, ISI Web of Science, and the Cochrane-Controlled Trials Register searching for randomized clinical trials (RCTs) published from 1983 to 2014 and comparing GLN supplementation to no supplementation in patients admitted to ICU. A random-effect meta-analysis for each outcome (hospital and ICU mortality and rate of infections) of interest was carried out. The effect size was estimated by the risk ratio (RR).
Thirty RCTs were analyzed with a total of 3696 patients, 1825 (49.4%) receiving GLN and 1859 (50.6%) no GLN (control groups). Hospital mortality rate was 27.6% in the GLN patients and 28.6% in controls with an RR of 0.93 (95% CI = 0.81–1.07; P = 0.325, I2 = 10.7%). ICU mortality was 18.0 % in the patients receiving GLN and 17.6% in controls with an RR of 1.01 (95% CI = 0.86–1.19; P = 0.932, I2 = 0%). The incidence of infections was 39.7% in GLN group versus 41.7% in controls. The effect of GLN was not significant (RR = 0.88; 95% CI = 0.76–1.03; P = 0.108, I2 = 56.1%).
These results do not allow to recommend GLN supplementation in a generic population of critically ills. Further RCTs are needed to explore the effect of GLN in more specific cohort of patients.
INTRODUCTION
There is clear and sufficient evidence from preclinical and phase 2 clinical studies to suggest that glutamine (GLN) plays a central role of in several key metabolic pathways involved in the proper function of many organs.1 Moreover, GLN has been recognized as an essential substrate and the principal metabolic fuel for rapidly dividing cells, such as enterocytes, lymphocytes, and macrophages. For these reasons, although GLN is classified as a nonessential amino acid, it is commonly described as a conditionally essential amino acid in hypemetabolic states.2
During critical illness and the subsequent catabolism and inflammation, GLN plasma levels decrease and this relative deficiency has been associated with increased mortality in intensive care units (ICU).3,4 Therefore, the rational to supplement ICU patients with GLN has been emphasized repetitively.5 Indeed, GLN administration in critically ill patients has been associated with reduced mortality in several reports.6–8 However, large recent randomized clinical trials (RCT)9 were not able to confirm such an effect or even reported a trend to an harmful outcome.10
Since previous meta-analyses11–13 did not include the latest RCTs,10–14 the results of GLN supplementation in critically ill patients need to be updated by a more comprehensive meta-analysis to accept or reject the potential benefits of GLN.
METHODS
This research was conducted by following the guidelines and the PRISMA statement for reporting systematic reviews and meta-analyses of studies evaluating healthcare interventions. Ethical approval was not necessary according to local legislation because of the type of study (meta-analysis).
Literature Search
Two authors (MO, SC) independently performed a Medline, Embase, Pubmed, Scopus, Ovid, ISI Web of Science, and Cochrane Central Register of Controlled Trials and Cochrane Library database extended literature search of all studies published as original full-text article published between January 1983 and April 2014. The following medical subject heading terms and words was used for search, in all possible combination: “glutamine,” “dipeptide,” “L-glutamine,” “nutritional support,” “artificial nutrition,” “enteral nutrition,” “parenteral nutrition,” “immunonutrition,” “pharmaconutrition” AND “critically ill” or “critical care,” “intensive care,” “critical illness,” “intensive care unit,” “seriously ill,” “critical patients,” “surgical intensive care unit” “SICU” “ICU.”
The “related article” function was used to expand the search and the reference lists of articles selected for full-text review were searched for additional articles. In the event of overlap of authors, institutions, or patients, the most recent or highest quality article was chosen.
Study Selection
The term “glutamine supplementation” was defined as any treatment containing GLN or GLN dipeptide in combination or not with any form of artificial nutrition as reported in the articles reviewed.
We included trials with the following eligibility criteria:
Studies enrolling patients with age >18 years.
Patients admitted to ICUs.
Randomized trials with parallel group.
GLN supplementation.
Trials reporting at least 1 of the outcomes considered in the meta-analysis.
English language.
We considered all studies irrespectively if GLN was given with parenteral or enteral nutrition, or no artificial nutritional support. We also included studies with control groups who did not received isonitrogenous/isocaloric regimens.
We excluded trials with the following criteria:
GLN combined with other nutrients with potential immunometabolic activity (ie, arginine, nucleotides, and omega-3 fatty acids).
No full-text available articles, opinion pieces, and editorials.
Burn patients, because of their particular clinical features and because of a recent review focusing this specific group of patients.15
Data Extraction
An electronic database was created to collect all relevant trial data. The data were extracted independently by 2 investigators (MO and MS) and in case of disagreement 2 superpartes referents (LG and LN) cross-examined doubtful data and the decision was made after consensus meeting. Agreement between authors was calculated in order to investigate the risk of bias (Cohen κ = 0.88).
Information extracted from the trials involved: first author, country of origin, year of publication, number of patients randomized, type of nutritional support, GLN dosage, route of administration, and period of supplementation, regimen of the control groups, Acute Physiology and Chronic Health Evaluation (APACHE) score, Sequential Organ Failure Assessment (SOFA) score and number of patients in shock at study entry, intention-to-treat (ITT) reporting, double, single or no blindness, and the different outcome measures.
The primary purpose of this meta-analysis was to evaluate if GLN supplementation could affect mortality. As primary relevant outcomes we assessed the rate of in-hospital and ICU mortality. As secondary endpoint of the analysis we considered the rate of infectious morbidity, the length of in-hospital stay (IHS) and ICU stay (ICUS). All studies reporting on infection defined it as positive specimen culture.
Study quality was assessed by 2 independent reviewers (SC and LN) according to the Jadad score.16
Statistical Analysis
We performed a random-effects meta-analysis17 for each outcome of interest. For categorical outcomes (mortality, infectious morbidity) the effects size was estimated by the risk ratio (RR), while for continuous outcome (length of stay) the mean difference (MD) was used. In the calculation of the pooled RR, a correction factor of 0.5 was added to all cell frequencies of studies where no patient had the outcome in either GLN or control groups. We made sure of the absence of any possible bias due to sparse data by applying also the fixed method of Peto (results not shown) which confirmed the results of the present analysis.18 Mean and standard deviation of length of stay was calculated according to method of Hozo et al19 for the studies where only median and range (or interquartile range) were reported. The weights assigned to each study were computed according to the inverse of the variance. Heterogeneity was quantified using I2 and τ2 indexes and testing the null hypothesis that all studies share a common effect size. We used I2 = 30% as a threshold to establish the presence of moderate heterogeneity. Moreover, we investigated the presence of publication bias using funnel plots.20
Finally, some stratified analyses were performed according to the following indicators: GLN dosage (>0.3 g/kg/day or ≤0.3 g/kg/day), duration of GLN supplement (>5 days or ≤5 days), route of GLN administration (enteral or parenteral), ITT reporting (yes or not), blinding (single or double), Jadad score (≥3 or <3), and APACHE II score (>15 or ≤15). For all the analyses, we tested the presence of a different effect between subgroups.
P-values <0.05 were considered significant. All the analyses were performed using “meta” package within R, version 3.0.2.
RESULTS
Figure 1 depicts the flow diagram of the literature search and article selection. After duplicates removal, we identified 498 potentially relevant references through the electronic searches. A total of 414 studies were excluded screened after title and abstract evaluation, 54 articles were excluded for the following reasons: not critically ill patients (6 studies), pediatric population (12 studies), study design was not appropriate, and GLN administration in addition to other immunonutrients (14 studies). We also excluded 22 trials because they did not provide information on clinical outcomes.
FIGURE 1.

Flow diagram of the literature search according to the PRISMA statement.
Study Characteristics
Thirty RCTs were finally included in the meta-analysis with a total of 3696 patients, 1825 (49.4%) receiving GLN, and 1859 (50.6%) no GLN (control groups). The mean number of patients/study was 119.7 and 66.6% of the studies had less than 100 patients. Most RCTs were single center (24/30), 20 were double-blind and 10 were single blind, 20 trials (66.6%) reported ITT data, 4 studies (13%) were conducted in patients with acute pancreatitis, 7 studies (23.3%) in victims of trauma, 9 studies (30%) in mixed population, and 33.7% in unspecified critically ill ICU patients. Twenty-one trial used were intravenously GLN, 1 trial used both IV and enteral administration and in 8 trial GLN was supplemented enterally.
The median I.V. GLN dosage was 0.38 g/kg/day, the median enteral dosage was 25 g/day.
Table 1 reports the detailed information on all trials included in the meta-analysis.
TABLE 1.
Characteristics of the Included Trials

Primary Endpoints
Twenty-four trials including 2834 patients (n = 1423 treated and 1411 controls) provided data on hospital mortality. The rate was 27.6% in the patients receiving GLN and 28.6% in controls. The RR was 0.93 (95% CI = 0.81–1.07; P = 0.325). Heterogeneity among studies was low (I2 = 10.7%, P = 0.312) (Figure 2). No evidence of publication bias was detectable (Figure 3A).
FIGURE 2.

Forest plot of the effect of GLN supplementation on in-hospital mortality. Size of squares for RR reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % CI. CI = confidence intervals, GLN = glutamine, RR = relative risk.
FIGURE 3.

Funnel plots for (A) overall mortality, (B) intensive care unit (ICU) mortality, (C) infectious morbidity, (D) length of stay, and (E) ICU length of stay.
ICU mortality was reported in 14 studies including a total of 2230 subjects (n = 1112 treated and 1118 control). The rate was 18.0% in the patients receiving GLN and 17.6% in controls. The RR was 1.01 (95% CI = 0.86–1.19; P = 0.932). Heterogeneity among studies was absent (I2 = 0%, P = 0.989) (Figure 4). We detected no publication bias after the funnel plot analysis (Figure 3B).
FIGURE 4.

Forest plot of the effect of GLN supplementation on ICU mortality. Size of squares for RR reflects the weight of the trial in the pooled analyses. Horizontal bars 95% CI. CI = confidence intervals, GLN = glutamine, ICU = intensive care unit, RR = relative risk.
Secondary Endpoints
Fifteen RCTs (2795 patients; 1402 treated and 1393 control) described the rate of infections. The incidence was 39.7% in patients receiving GLN versus 41.7% in controls. The effect of GLN was not significant (RR = 0.88; 95% CI = 0.76–1.03; P = 0.1082). The tau-squared test for heterogeneity among studies was 56.1% with a significant P value (0.004) (Figure 5). Funnel plot suggested no evidence of publication bias (Figure 3C).
FIGURE 5.

Forest plot of the effect of GLN supplementation on infectious morbidity. Size of squares for RR reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % CI. CI = confidence intervals, GLN = glutamine, RR = relative risk.
The 19 studies reporting on IHS (1314 treated patients and 1321 controls) showed a nonsignificant reduction in the patients receiving GLN (MD = −1.73, 95% CI = −3.76–0.29; P = 0.094) with a significant heterogeneity among trials (I2 = 44%, P = 0.021) (Figure 6A). Funnel plot suggested no evidence of publication bias (Figure 3D).
FIGURE 6.

Forest plots of the effect of GLN on (A) hospital length of stay and (B) ICU length of stay comparing GLN and control. Size of squares for MD reflects the weight of the trial in the pooled analyses. Horizontal bars 95% CI. CI = confidence intervals, GLN = glutamine, ICU = intensive care unit, MD = mean difference, RR = relative risk
ICUS was described in 24 trials. The overall population analyzed was of 2816 patients (n = 1395 treated and 1421 controls). The mean ICUS was 15.9 days in the GLN supplemented group versus 16.6 days in the control group. The weight MD was a nonsignificant reduction in favor of the treated group (MD = −0.09; 95% CI = −0.76–+0.59) (Figure 6B). No heterogeneity was found (Figure 3E).
Subgroups Analyses
We performed different subgroup analyses to evaluate possible influences of GLN daily dosage (greater than 0.3 g/kg/day versus less or equal than 0.3 g/kg/day), period of supplementation (more than 5 days versus less or equal than 5 days), severity of illness (APACHE II > 15 versus less or equal of 15), ITT data reporting, blindness, and quality of trials (Jadad score > 3 points versus ≤3 points). The results are summarized in Table 2 .
TABLE 2.
Subgroup Analyses

TABLE 2 (Continued).
Subgroup Analyses
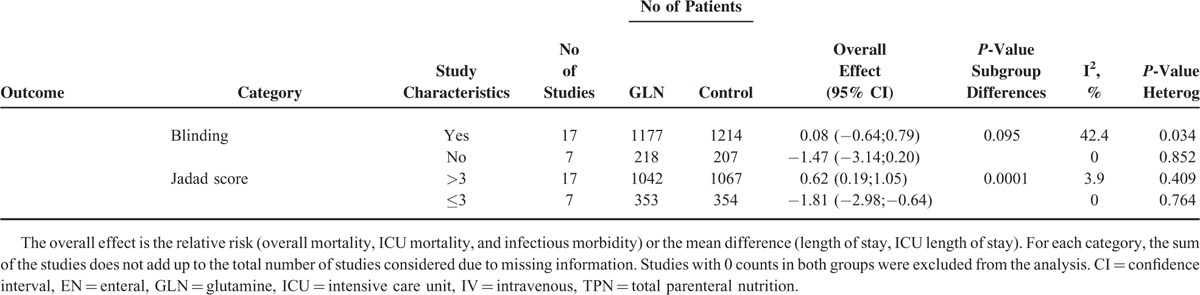
The daily dose of GLN did not affect any of the endpoints while a duration of supplementation longer than 5 days was associated with a significant reduction of infectious morbidity. A protective effect of GLN on hospital mortality and occurrence of infections was more evident when given parenterally and in less critically ill patients (APACHE II score lower or equal then 15) even though the number of studies and subjects analyzed in this last cohort was extremely limited. In the lower quality studies (Jadad ≤ 3) and lack of blindness we identified a reduction of IHS and ICUS in the treated group.
DISCUSSION
The results of the present meta-analysis suggest that GLN supplementation given to a mixed population of critically ill patients does not significantly affect primary outcome measures such as hospital and ICU mortality. The reasons for this lack of benefit are unidentified. The rational reason for giving supernormal doses of GLN in severely ills was the correlation between mortality rate and low levels of this amino acid in the plasma and tissues.3,4 Nevertheless, it is still unclear if the decline of circulating GLN contributes to death or is a simple marker of disease severity. Moreover, recent findings did not confirm GLN deficiency in ICU patients with shock and multiple organ dysfunction.10
Our stratified analysis implies that hospital mortality, but not ICU mortality is decreased only in patients receiving IV GLN and in patients entering in the trials with APACHE II equal or less than 15, keeping in mind that the mortality rate of patients with such score is usually negligible.
This observation is difficult explain. It may be speculated that GLN does not have a protective role on mortality in the most severe patients because in these subjects death is mainly determined by MODS and GLN supplementation is not sufficient to affect the recovery of organ dysfunction. When disease severity is less than GLN supplementation may be effective in modulated function and protect organs.
Overall, we could not even demonstrate a protective effect of GLN on the occurrence infections. This observation is in line with what we recently showed in patients undergoing major abdominal surgery.48,49 These data suggest that GLN supplementation may be not so effective in preventing the injury-induced immune deficiency as previously reported by others.50,51 Yet, in subgroup analyses, infectious morbidity was significantly reduced in patients receiving parenteral GLN, for more than 5 days and with less severe disease, although these findings need to be confirmed by a future large RCT designed with these specific inclusion criteria and type of treatment.
The dissimilarity of our results with previous meta-analyses11–13 can be explained mainly by the criteria assumed to select studies and by the recent publication of other large RCTs. The present meta-analysis included 30 RCTs that enrolled patients needing intensive care treatments for several different conditions. We excluded burn injury because of the peculiarity of the patients and care elements which are not comparable with any other types of critical illnesses. In fact, these subjects are treated in specific burn units and not in generic ICUs. Moreover, the GLN effect in this specific cohort was recently reviewed and analyzed by Lin et al.15
Bollhalder et al,11 included 11 RCTs and they concluded with an advantage of GLN on infectious complications and hospital stay. Subgroup analyses suggested that GLN given at a dose greater that 0.2 g/kg/day and for at least 9 days was associated with a decreased mortality rate. The main differences with the present analysis are the exclusion of trials using enteral GLN and the inclusion of burn patients and the additional 9 studies22,23,25,28,30,32,39,41,43 that we evaluated. Moreover, at the time of their publication, the results of 2 relevant RCTs10,14 were not yet available.
In 2014, Chen et al12 published a meta-analysis including the REDOX trial but the authors did not split the 4 different study groups, including therefore also antioxidant supplementation. This did not allowed an independent evaluation on the effect of GLN.10 However, the results were much more similar to ours showing no benefit of GLN on mortality and LOS, although they reported a significant reduction of infections in the treated group. Conversely, they included burn injury,52 a quasi randomized trial,53 and a study where GLN was given in combination with probiotics in adults and children.54 Once more, we found 16 additional trials to be analyzed.14,21,24,26,28,30–33,35,38,39,41,43,44
The systematic review by Wischmeyer et al13 concluded that GLN was effective in reducing hospital mortality and LOS. There was also a trend toward an improved infectious morbidity rate and ICU stay. The profound divergence with our results may be attributed to the inclusion of 3 trials written in Chinese,55–57 2 trials on burns,52,58 and 1 published in abstract form59 and the exclusion of 11 studies10,14,22–25,28,30,32,36,45 that instead we found relevant for a comprehensive review.
The most recent review by Tao et al60 showed a moderate evidence that GLN supplementation can reduce infections and a low quality evidence that GLN supplementation reduces length of hospital stay for critically ill patients. They reported no effect on the risk of mortality and length of ICU stay in the overall results and in subgroup analyses. Again, the main difference with the present analysis is the different study selection criteria. Tao et al included quasi random studies, RCTs on burn patients,52,61–67 trials written in Chinese,68–71 1 trial written in Hungarian,72 1 trial with multiple doses of GLN,73 and 1 trial without clinical outcomes74 and did not include 10 studies that we found relevant.22,27,28,30,33–35,38,39,45
The present meta-analysis has several limitations. We realize that our results may have been partially skewed by including the data of the REDOX study10 for its considerable weight on the summary of the analysis. On the contrary, it seems unreasonable to exclude such trial for its strength and scientific robustness including a large and adequate number of patient, blindness, rigorous determination and adjudication of infection, and ITT analysis, all of which augment the validity of the trial. By excluding this RCT, the results of a meta-analysis may artificially appear in favor of the treatment.
To help clinicians in the difficult decision process of accepting or rejecting a treatment, it is necessary to summarize the findings of all published RCTs evaluating the controversial consequences of GLN supplementation. It is also true that the harmful effect of GLN in the REDOX study10 was mostly driven by a subgroup of patients who died with renal failure as suggested by a post-hoc analysis of the same authors.75
Our findings may have also been influenced by pooling trials where GLN was provided only enteral or through both the enteral and parenteral routes. This may appear as a confounding element in the analysis because of the different metabolic pathways and utilization of GLN. In fact, when given enterally GLN should be mainly active on the gut mucosal layer being the preferential substrate for the enterocytes and intestinal immune cells. Subsequently, the intestinal barrier function, which plays a critical role in critically ills, may control permeability and protect against the occurrence of systemic infections by decreasing bacterial translocation. When given intravenously, GLN should protect tissues against oxidative stress, toxic agents, or pathologic insults by increasing gluthation production and enhancing heat shock protein expression. On the other hand, many of the potential protective mechanisms of GLN given enterally or parenterally are overlapping and quite similar.76 For these reasons, we decided that there was a strong rational in pooling both routes.
An additional shortcoming of the present study is the lack of separate analyses by more specific ICU patient cohorts. Unfortunately in most of the trials, it was difficult or impossible to distinguish further the type of subjects admitted or the attempt to categorization would have compelled an excessive subgrouping with loss of reliability of results. Moreover, the secondary outcome is variable between the present review and the previous ones, but this may be because of subgroup analysis which may throw unexpected results based on differing inclusion criteria and study selection.
In conclusion, at present, our results do not allow to recommend GLN supplementation in a generic population of critically ill patients. Further RCTs are needed to confirm or deny the potential protective or harmful effect of GLN in more specific cohort of patients treated in ICUs. In particular, as our data suggest that GLN given parenterally, for more than 5 days, in patients with APACHE II < 15 might have a protective role. To confirm this trend a adequately powered RCT with this inclusion criteria is deserved.
Footnotes
Abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation, GLN = glutamine, ICU = intensive care unit, ICUS = intensive care unit stay, IHS = in-hospital stay, ITT = intention to treat, IV = intravenous, MD = mean difference, RCT = randomized clinical trial, RR = risk ratio.
The results were presented in part at the 27th annual meeting of the Surgical Infection Society-Europe, June 5 to 7 2014, Vienna.
The study was supported by a research grant of the Milano-Bicocca University.
MO, MS, SC, and LN had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: LG, MO, MS. Acquisition of data: MO, SC, MS, LN. Analysis and interpretation of data: LG, MO, MS, LN, SC, DPB. Drafting of the manuscript: LG, MO. Critical revision of the manuscript for important intellectual content and final approval: MO, MS, LN, DPB, SC. Statistical analysis: DPB. Study supervision: LG.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Preiser JC, Wernerman J. Glutamine, a life-saving nutrient, but why? Crit Care Med 2003; 31:2555–2556. [DOI] [PubMed] [Google Scholar]
- 2.Coeffier M, Dechelotte P. The role of glutamine in intensive care unit patients: mechanisms of action and clinical outcome. Nutr Rev 2005; 63:65–69. [DOI] [PubMed] [Google Scholar]
- 3.Oudermans-van Straaten HM, Bosman RJ, Treskes M, et al. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 2001; 27:84–90. [DOI] [PubMed] [Google Scholar]
- 4.Rodas PC, Rooyackers O, Herbert C, et al. Glutamine and glutathione at ICU admission in relation to outcome. Clin Sci 2012; 122:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wischmeyer PE. Glutamine: role in critical illness and ongoing clinical trials. Curr Opin Gastroenterol 2008; 24:190–197. [DOI] [PubMed] [Google Scholar]
- 6.Goeters C, Wenn A, Mertes N, et al. Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Crit Care Med 2002; 30:2032–2037. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths RD, Allen KD, Andrews FJ, et al. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine-supplemented parenteral nutrition on acquired infection. Nutrition 2002; 18:546–555. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths RD, Jones C, Palmer TE. Six month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 1997; 13:295–302. [PubMed] [Google Scholar]
- 9.Andrews PJ, Avenell A, Noble DW, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011; 342:d1542. [DOI] [PubMed] [Google Scholar]
- 10.Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013; 368:1489–1497. [DOI] [PubMed] [Google Scholar]
- 11.Bollhalder L, Pfeil AM, Tomonaga Y, et al. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr 2013; 32:213–223. [DOI] [PubMed] [Google Scholar]
- 12.Chen QH, Yang Y, He HL, et al. The effect of glutamine therapy on outcomes in critically ill patients: a meta-analysis of randomized controlled trials. Crit Care 2014; 18:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wischmeyer PE, Dhaliwal R, McCall M, et al. Parenteral glutamine supplementation in critical illness: a systematic review. Crit Care 2014; 18:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Bárcena J, Marsé P, Zabalegui-Pérez A, et al. A randomized trial of intravenous glutamine supplementation in trauma ICU patients. Intensive Care Med 2014; 40:539–547. [DOI] [PubMed] [Google Scholar]
- 15.Lin JJ, Chung XJ, Yang CY, et al. A meta-analysis of trials using the intention to treat principle for glutamine supplementation in critically ill patients with burn. Burns 2013; 39:565–570. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 18.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23:1351–1375. [DOI] [PubMed] [Google Scholar]
- 19.Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol 2001; 54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 21.Powell-Tuck J, Jamieson CP, Bettany GE, et al. A double blind, randomised, controlled trial of glutamine supplementation in parenteral nutrition. Gut 1999; 45:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones C, Palmer TE, Griffiths RD. Randomized clinical outcome study of critically ill patients given glutamine-supplemented enteral nutrition. Nutrition 1999; 15:108–115. [DOI] [PubMed] [Google Scholar]
- 23.Conejero R, Bonet A, Grau T, et al. Effect of a glutamine-enriched enteral diet on intestinal permeability and infectious morbidity at 28 days in critically ill patients with systemic inflammatory response syndrome: a randomized, single-blind, prospective, multicenter study. Nutrition 2002; 18:716–721. [DOI] [PubMed] [Google Scholar]
- 24.Ockenga J, Borchert K, Rifai K, et al. Effect of glutamine enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr 2002; 21:409e16. [DOI] [PubMed] [Google Scholar]
- 25.Hall JC, Dobb G, Hall J, et al. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med 2003; 29:1710–1716. [DOI] [PubMed] [Google Scholar]
- 26.Xian-li H, Qing-jiu M, Jian-guo L, et al. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP). Clin Nutr 2004; 1:43. [Google Scholar]
- 27.Fuentes-Orozco C, Anaya-Prado R, Gonzalez-Ojeda A, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin Nutr 2004; 23:13–21. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler TR, Ogden LG, Singleton KD, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Med 2005; 31:1079–1086. [DOI] [PubMed] [Google Scholar]
- 29.Dechelotte P, Hasselmann M, Cynober L, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med 2006; 34:598–604. [DOI] [PubMed] [Google Scholar]
- 30.Spindler-Vesel A, Bengmark S, Vovk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr 2007; 31:119–126. [DOI] [PubMed] [Google Scholar]
- 31.Sahin H, Mercanligil SM, Inanc N, et al. Effects of glutamine-enriched total parenteral nutrition on acute pancreatitis. Eur J Clin Nutr 2007; 61:1429–1434. [DOI] [PubMed] [Google Scholar]
- 32.McQuiggan M, Kozar R, Sailors RM, et al. Enteral glutamine during active shock resuscitation is safe and enhances tolerance of enteral feeding. JPEN J Parenter Enteral Nutr 2008; 32:28–35. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Kumar R, Sharma SB, et al. Effect of oral glutamine administration on oxidative stress, morbidity and mortality in critically ill surgical patients. Indian J Gastroenterol 2007; 26:70–73. [PubMed] [Google Scholar]
- 34.Estivariz CF, Griffith DP, Luo M, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. JPEN J Parenter Enteral Nutr 2008; 32:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duska F, Fric M, Waldauf P, et al. Frequent intravenous pulses of growth hormone together with glutamine supplementation in prolonged critical illness after multiple trauma: effects on nitrogen balance, insulin resistance, and substrate oxidation. Crit Care Med 2008; 36:1707–1713. [DOI] [PubMed] [Google Scholar]
- 36.Fuentes-Orozco C, Cervantes-Guevara G, Mucino-Hernandez I, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr 2008; 32:403–411. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Barcena J, Regueiro V, Marse P, et al. Glutamine as a modulator of theimmune systemof critical care patients: effect on Toll-like receptor expression. A preliminary study. Nutrition 2008; 24:522–527. [DOI] [PubMed] [Google Scholar]
- 38.Cai G, Yan J, Zhang Z, et al. Immunomodulatory effects of glutamine-enriched nutritional support in elderly patients with severe sepsis: a prospective, randomized, controlled study. J Organ Dysfunct 2008; 4:31. [Google Scholar]
- 39.Ozgultekin A, Turan G, Durmus Y, et al. Comparison of the efficacy of parenteral and branched-chain amino acid solutions given as extra supplements in parallel to the enteral nutrition in head trauma. E-SPEN, Euro e-J Clin Nutr Metab 2008; 3:211–216. [Google Scholar]
- 40.Luo M, Bazargan N, Griffith DP, et al. Metabolic effects of enteral versus parenteral alanyl-glutamine dipeptide administration in critically ill patients receiving enteral feeding: a pilot study. Clin Nutr 2008; 27:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eroglu A. The effect of intravenous alanyl-glutamine supplementation on plasma glutathione levels in intensive care unit trauma patients receiving enteral nutrition: the results of a randomized controlled trial. Anesth Analg 2009; 109:502–505. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Barcena J, Crespi C, Regueiro V, et al. Lack of effect of glutamine administration to boost the innate immune system response in trauma patients in the intensive care unit. Crit Care 2010; 14:R233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grau T, Bonet A, Minambres E, et al. The effect of L-alanyl- L-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit Care Med 2011; 39:1263–1268. [DOI] [PubMed] [Google Scholar]
- 44.Wernerman J, Kirketeig T, Andersson B, et al. Scandinavian glutamine trial: a pragmatic multi-centre randomised clinical trial of intensive care unit patients. Acta Anaesthesiol Scand 2011; 55:812–818. [DOI] [PubMed] [Google Scholar]
- 45.Schneider A, Markowski A, Momma M, et al. Tolerability and efficacy of a low-volume enteral supplement containing key nutrients in the critically ill. Clin Nutr 2011; 30:599–603. [DOI] [PubMed] [Google Scholar]
- 46.Çekmen N, Aydin A, Erdemli O. The impact of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on clinical outcome in critically patients. e-SPEN, Eur e-J Clin Nutr Metab 2011; 6:e64. [Google Scholar]
- 47.Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol 2013; 19:2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gianotti L, Braga M, Biffi R, et al. Perioperative intravenous glutamine supplemetation in major abdominal surgery for cancer: a randomized multicenter trial. Ann Surg 2009; 250:684–690. [DOI] [PubMed] [Google Scholar]
- 49.Sandini M, Nespoli L, Oldani M, et al. Effect of glutamine dipeptide supplementation on primary outcomes for elective major surgery: systematic review and meta-analysis. Nutrients 2015; 7:481–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu CY, Shih YL, Sun LC, et al. The inflammatory modulation effect of glutamine-enriched total parenteral nutrition in postoperative gastrointestinal cancer patients. Am Surg 2011; 77:59–64. [PubMed] [Google Scholar]
- 51.Engel JM, Pitz S, Mühling J, et al. Role of glutamine administration on T-cell derived inflammatory response after cardiopulmonary bypass. Clin Nutr 2009; 28:15–20. [DOI] [PubMed] [Google Scholar]
- 52.Wischmeyer PE, Lynch J, Liedel J, et al. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med 2001; 29:2075–2080. [DOI] [PubMed] [Google Scholar]
- 53.Schulman AS, Willcutts KF, Claridge JA, et al. Does the addition of glutamine to enteral feeds affect patient mortality? Crit Care Med 2005; 33:2501–2506. [DOI] [PubMed] [Google Scholar]
- 54.Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond) 2004; 106:287–292. [DOI] [PubMed] [Google Scholar]
- 55.Tian H, Wang KF, Wu TJ. Effect of total parenteral nutrition with supplementation of glutamine on the plasma diamine oxidase activity and D-lactate content in patients with multiple organ dysfunction syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006; 18:616–618. [PubMed] [Google Scholar]
- 56.Zhang Z, Qin HD, Ni HB, et al. Effect of early enriched parenteral alanyl-glutamine on heat shock protein 70 (HSP70) expression in critical patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007; 19:481–484. [PubMed] [Google Scholar]
- 57.Yang SQ, Xu JG. Effect of glutamine on serum interleukin-8 and tumor necrosis factor-alpha levels in patients with severe pancreatitis. Nan Fang Yi Ke Da Xue Xue Bao 2008; 28:129–131. [PubMed] [Google Scholar]
- 58.Ye-Ping Zhou Z-MJ, Yong-Hua S, Gui-Zhen H, et al. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: a randomized, double-blind, controlled clinical trial. Clin Nutr Suppl 2004; 1:55–60. [Google Scholar]
- 59.Ziegler T, May A, Hebbar G, et al. Glutamine dipeptide-supplemented parenteral nutrition in surgical ICU patients: Results of an American randomized, double-blind, multicenter trial. Clin Nutr Suppl 2012; 7:265. [Google Scholar]
- 60.Tao KM, Li XQ, Yang LQ, et al. Glutamine supplementation for critically ill adults. Cochrane Database Syst Rev 2014; Issue 9. Art. No.: CD010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cucereanu-Badica I, Luca I, Negres S, et al. The effects of intravenous glutamine supplementation in severely burned, multiple traumatized patients. Farmacia 2013; 61:212–219. [Google Scholar]
- 62.Garrel D, Patenaude J, Nedelec B, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Crit Care Med 2003; 31:2444–2449. [DOI] [PubMed] [Google Scholar]
- 63.Pattanshetti VM, Powar RS, Godhi AS, et al. Enteral glutamine supplementation reducing infectious morbidity in burns patients: a randomised controlled trial. Indian J Surg 2009; 71:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng X, Yan H, You Z, et al. Clinical and protein metabolic efficacy of glutamine granules-supplemented enteral nutrition in severely burned patients. Burns 2005; 31:342–346. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Y, Sun Y, Jiang Z, et al. The effects of glutamine dipeptide on the improvement of endotoxemia in severely burned patients. Zhonghua Shao Shang Za Zhi 2002; 18:343–345. [PubMed] [Google Scholar]
- 66.Zhou YP, Jiang ZM, Sun YH, et al. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN J Parenter Enteral Nutr 2003; 27:241–245. [DOI] [PubMed] [Google Scholar]
- 67.Zhou YP, Jiang ZM, Sun YH, et al. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: a randomized, double-blind, controlled clinical trial. Clin Nutr Suppl 2004; 1:55–60. [Google Scholar]
- 68.Cai GL, Yan J, Yu YH, et al. Influence of glutamine and growth hormone intensified nutrition support on immunomodulation in critically ill elderly patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006; 18:595–598. [PubMed] [Google Scholar]
- 69.Tian H, Wang KF, Wu TJ. Effect of total parenteral nutrition with supplementation of glutamine on the plasma diamine oxidase activity and D-lactate content in patients with multiple organ dysfunction syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006; 18:616–618. [PubMed] [Google Scholar]
- 70.Yang SQ, Xu JG. Effect of glutamine on serum interleukin-8 and tumor necrosis factor-alpha levels in patients with severe pancreatitis. Nan Fang Yi Ke Da Xue Xue Bao 2008; 28:129–131. [PubMed] [Google Scholar]
- 71.Zhang Z, Qin HD, Ni HB, et al. Effect of early enriched parenteral alanyl-glutamine on heat shock protein 70 (HSP70) expression in critical patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007; 19:481–484. [PubMed] [Google Scholar]
- 72.Hajdú N, Belágyi T, Issekutz A, et al. Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis – a prospective randomized clinical study. Magy Seb 2012; 65:44–51. [DOI] [PubMed] [Google Scholar]
- 73.Tremel H, Kienle B, Weilemann LS, et al. Glutamine dipeptide-supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology 1994; 107:1595–1601. [DOI] [PubMed] [Google Scholar]
- 74.Tjader I, Rooyackers O, Forsberg AM, et al. Effects on skeletal muscle of intravenous glutamine supplementation to ICU patients. Intensive Care Med 2004; 30:266–275. [DOI] [PubMed] [Google Scholar]
- 75.Heyland DK, Elke G, Cook D, et al. Glutamine and antioxidants in the critically ill patient: A post hoc analysis of a large-scale randomized trial. JPEN J Parenter Enteral Nutr 2014; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gianotti L, Oldani M, Coppola S, et al. Glutamine supplementation in major surgery and intensive care. In “Glutamine in Clinical Nutrition”. Rajendram R, Preedy VR. Patel VB (Eds.). New York: Springer Science+Business Media; 2015:152–168. ISBN 978-1-4939-1931-4, DOI 10.1007/978-1-4939-1932-1_12 [Google Scholar]


