Abstract
The impact of thiopurines (TP) on the long-term outcome of early Crohn disease (CD) is still controversial. The present study designed as a comparison of conventional step-care to alternative treatment paradigms for disease progression.
This longitudinal cohort study examined the established CD patients from a university-based inflammatory bowel disease referral center. Outcomes of mucosal healing (MH), CD-related surgery or hospitalization, and clinical remission were compared based on timing of initiation of TP therapy. The cumulative incidence of events was estimated by Kaplan–Meier method.
One-hundred ninety patients with early CD were included. After a median follow-up of 57 months (interquartile range, 31.3–76.2), 29 patients undergone abdominal surgeries, 48 patients hospitalized, and 68 patients experienced clinical flares. A higher cumulative proportion of patients in the top-down (TD) group achieving MH than both the accelerated step-up (AC) group and conventional management (CM) group at month 36 (78.8% vs 39.9% and 42.2%, respectively; P = 0.001). There was a trend, albeit not significant, for an increased proportion of patients free of CD-related intestinal surgery in the TD group at month 60 (P = 0.16). However, among secondary outcomes, an early TP-based AC or TD strategy was not associated with improvement in clinical remission rates compared with a CM strategy at month 60 (P = 0.79). No significant difference was observed between early TP and CM for rates of MH, CD-related intestinal surgery or hospitalization, and clinical remission.
Both AC and CM strategy were minimally effective for disease modification. TD strategy has the potential of achieving higher rates MH. Our results support the TD strategy in patients with early CD at risk for a disabling course.
INTRODUCTION
Crohn disease (CD) is a chronic, progressive, disabling, and destructive inflammatory disorder.1 Current guidelines advocate a step-up strategy to treatment, with the addition of more powerful therapies as the severity of disease or refractoriness to therapy increases. In this conventional step-care approach, anti-tumor necrosis factors (TNFs) are reserved for refractory or intolerance to conventional therapies. This approach has not been proven to slow or prevent bowel damage. In contrast to conventional step-up approach, the proactive top-down (TD) regimen or an accelerated step-up (AC) algorithm advocates biological and immunomodulator therapy at an early stage, shortly after diagnosis of CD. Whether such treatment paradigms outperformed the conventional step-care for the endpoints of disease progression and bowel damage remains a matter of active debate.
Accumulating evidence has pointed to the lack of efficacy of early use of thiopurines (TP) for achieving CFREM. Cosnes et al2 and Panes et al3 published their trials on early use of azathioprine (AZA) in adult CD for achievement of corticosteroid-free remission (CFREM). Both trials concluded that an AZA-based AD strategy was not associated with improvement in CD remission rates compared to a conventional step-up treatment strategy. Instead, trials with targets addressing bowel structure damage, evident as delay of disease progression or avoidance of bowel surgery, can be achieved by TP started early in CD course. Preliminary data from the Randomized Evaluation of an Algorithm for Crohn's Treatment (REACT) trial,4 which assigned patients with CD to either a conventional step-care or an AC algorithm with early use of combined antimetabolite/ADA therapy, also demonstrated marginally higher proportion of patients with AC in clinical remission at 12 months. However, after 24 months, a significant reduction in rates for complication, surgeries, and hospitalizations was observed using the AC approach.4 It is hence worth considering a treat-to-target strategy of TP treatment in CD.
Thus the present study designed as a comparison of conventional step-care to alternative treatment paradigms for the endpoints of bowel damage in a relatively large cohort of patients with CD.
METHODS
Patients and Design
This is an observational study where a prospectively designed standardized follow-up schedule was used for all patients.
All consecutive patients with a diagnosis of CD who received AZA/6-mercaptopurine (6-MP) treatment at the gastroenterology outpatient clinic of the first affiliated hospital of Sun Yat-Sen University between 2003 and 2013 were included in this study. Diagnoses of CD were established according to the criteria of Lennard-Jones,5 disease phenotype was determined according to the Montreal classification.6
Criteria for inclusion in the study were patients ages between 18 and 80 years old; early CD according to Paris definition7; at high risk for disabling disease8; continuous treatment with AZA/6-MP for at least 17 weeks.9
Exclusion criteria of this study: patients with an immediate need for surgery, or with contraindication to TP.
The study protocol was approved by the clinical research ethics committee of the first affiliated hospital of Sun Yat-Sen University.
Definitions
Early CD is defined by disease duration ≤18 months and no previous use of disease-modifying agents.7 Patients were categorized into 1 of 3 groups based on the 3 main strategies which have been widely proposed to treat CD: conventional step-up therapy, early TD strategy, and the AC strategy. The AC strategy was defined as AZA/6-MP prescribed concomitantly with the first course of corticosteroids. Early TD strategy was referred to the combination of AZA/6-MP with anti-TNFs. The conventional management (CM) was classified as TP in condition of corticosteroid dependency or refractoriness, chronic active disease with frequent flares, or development of severe perianal disease.2 Patients applied the former 2 strategies were consisted the early TP group.
Treatment Schedules: Dosing and Duration
According to the major available guidelines,10–12 AZA dose was targeted at 2.0 to 2.5 mg/kg body weight and 6-MP at 1.0 to 1.5 mg/kg body weight to achieve the therapeutic window of 250 to 400 pmol/8 × 108 erythrocyte by regular monitoring the 6-thioguanine nucleotides(6-TGN) concentrations.13
Endoscopic Documentation
All endoscopic procedures were performed by skilled endoscopists with standard protocol. The endoscopy reports were recorded in the patients’ pro forma questionnaire sheet and also saved as a digital version in the endoscopy registry. Score was assessed retrospectively by these 2 experts (B-LC and YH) according to the saved endoscopy images. Of note, the subsequent endoscopic assessments were usually a priori planned within 6 months by the treating physician to assess response to therapy.
Clinical Follow-Up
All clinical follow-up information documented in the medical files of the patients was reassessed by 2 experienced gastroenterologists (B-LC, YH). A predetermined structured datasheet was used to collect data from the medical files, the inflammatory bowel disease (IBD) register, and the endoscopic register, which including age, gender, CD classification, smoking history, symptoms at presentation, presence of extra-intestinal manifestations, perianal lesions, endoscopic appearance, disease duration, TP initiation dates and dosage, and co-medication and start dates (eg, steroids, immunosuppressants, anti-TNFs use), need for bowel surgery or hospitalization. The laboratory values of hematology, immunology, chemistry, and microbiology were collected. The number of patients with mucosal healing (MH) was also recorded at the time of each endoscopic procedure.
Outcomes
The primary efficacy outcome was the proportion of the achievement of MH and CD-related hospitalization, intestinal surgery at the successive visits throughout patient follow-up. MH was defined as in the study by Schnitzler et al14 in which complete MH was described as “the absence of ulcerations at follow-up endoscopy in patients who had ulcerations present at baseline ileocolonoscopy.” Surgery was defined as any intra-abdominal surgical procedure for active CD. CD-related hospitalizations were defined as those resulting from adverse events (AE) or CD-related treatment or complications.15
Prespecified secondary outcomes were the proportion of patients remaining in CFREM at the successive visits. CFREM was defined as the absence of flare, with no corticosteroid or anti-TNF use. Flare was defined by the Crohn's Disease Activity Index (CDAI) score >150 or an increase in CDAI of ≥70 points. Any new symptom or sign, any significant laboratory abnormality, or worsening of a preexisting condition or abnormality that occurred after initiation of TP was considered an AE.
When patients were lost to follow-up, the last date was taken to calculate the duration of TP therapy following guidelines of intention-to-treat.
Statistical Analysis
Demographic and clinical parameters were described using medians with interquartile range (IQR) for continuous data and percentages for discrete data. Fisher's exact test and χ2 tests were used to compare the nonparametric categorical data between groups, and analysis of variance for continuous parameters. Cumulative probabilities of remaining free of events (perianal surgery, intestinal resection, and hospitalization) were calculated using the Kaplan–Meier method and compared with the log-rank test. Time-to-event analysis was performed with the Kaplan–Meier curve.
The SPSS 16.0 software (SPSS, Chicago, IL) were used to conduct all statistical analyses. For all tests, the P value for statistical significance is defined as P < 0.05.
RESULTS
Demographic Characteristics
The baseline characteristics of the 190 patients with early CD (129 male; median age, 26.9 years [IQR, 19.6–33.2 years]; median duration, 3 months [IQR, 1.3–6.8 months]) that we evaluated were shown in Table 1.
TABLE 1.
Comparison of Baseline Characteristics
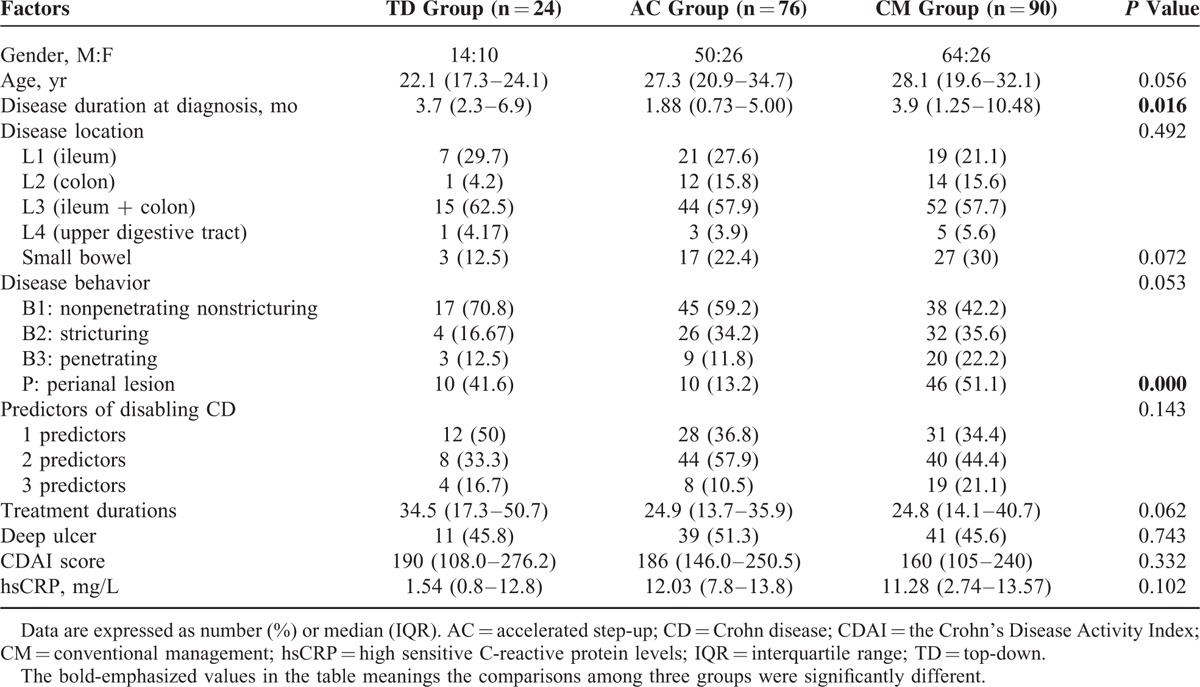
These 190 patients were then divided into 3 homogeneous arms by 3 main strategies which have been widely proposed to treat CD: the TD group (referred to the combination of AZA/6-MP with anti-TNFs, n = 24), the AC group (referred to AZA/6-MP prescribed concomitantly with the first course of corticosteroids, n = 76), and the CM group (referred to TP only in cases of corticosteroid dependency or refractory or development of severe perianal disease, n = 90) (Figure 1). There was no significant difference regarding age, gender, prior disease outcomes before referral, IFX use, ESR, new steroid use, and paralleled medication except for disease duration (P = 0.016) and perianal lesion (P < 0.001, Table 1). These patients were followed-up for a median of 57 months (IQR, 31–76 months).
FIGURE 1.
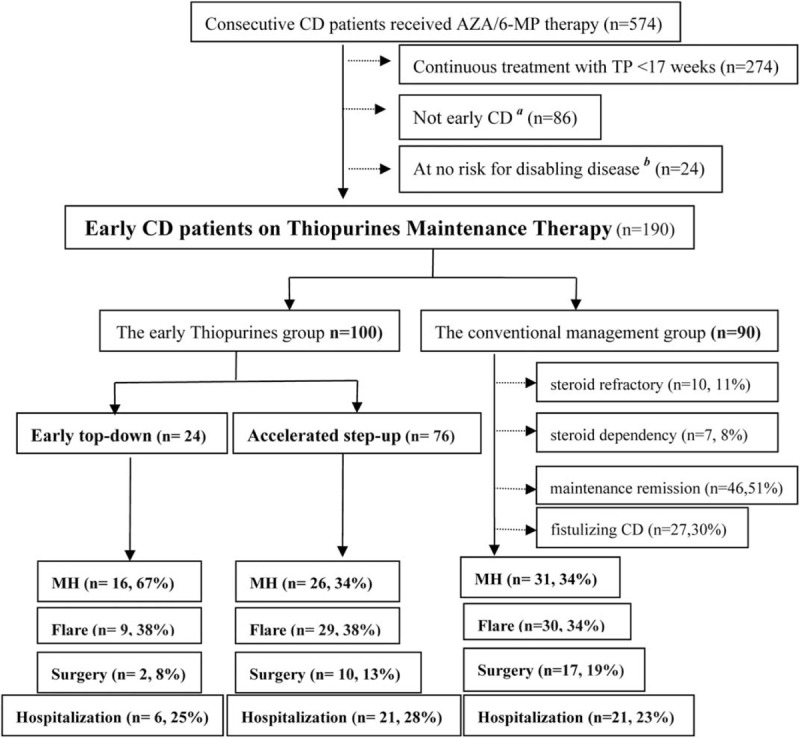
Study population. aEarly Crohn disease is defined by disease duration ≤18 months, no previous use of disease-modifying agents.7bDefined as thiopurines only in cases of corticosteroid dependency, chronic active disease with frequent flares, poor response to corticosteroids, or development of severe perianal disease.2
Primary Efficacy End Point
Endoscopic Procedures and Outcomes
MH was observed in 73 patients: 16 (16/24, 66.7%) were in the TD group, 26 (26/76, 34.2%) in the AC group, and 31 (31/90, 34.4%) in the CM group. A significant higher proportion of patients achieved MH in the TD group at month 36: 78.8 ± 9.4% in the TD group versus 39.9 ± 9.2% in the AC group, 42.2 ± 7.5% in the CM group (P < 0.01, Figure 2A).
FIGURE 2.

Kaplan–Meier plot showing probability of main events: (A) mucosal healing; (B) CD-related bowel surgery; (C) CD-related hospitalization; (D) flare; and (E) adverse events among patients assigned with different strategies. CD = Crohn disease.
Surgical Intervention and Hospitalizations
Due to persisting disease activity and strictures, 29 patients underwent intestinal resection: 2 (8.3%) were in the TD group, 10 (13.2%) in the AC group, and 17 (18.9%) in the CM group. There was a trend, albeit not significant, for an increased proportion of patients remaining free of intestinal surgery in the TD group at month 60: 83.2 ± 11% in the TD group versus 82.5 ± 6% in the AC group, 60.3 ± 9% in the CM group (P = 0.16, Figure 2B).
Hospitalization was observed in 48 patients: 6 were in the TD group, 21 in the AC group, and 21 in the CM group. There was no difference regarding the cumulative proportion of patients remaining free of hospitalization among the 3 groups at month 60: 63.9 ± 12.6% in the TD group versus 67.8 ± 6.4% in the AC group, 57.4 ± 12.6% in the CM group (P = 0.58, Figure 2C).
Secondary Efficacy End Points
There was a total 68 disease-flare attack within a median time 49.3 months (IQR, 28.6–70.4 months). Of the 68 patients experienced flare, 9 (37.5%) were in the TD group, 29 (38.2%) in the AC group, and 30 (33.3%) in the CM group. There was no significant difference regarding the cumulative proportion of patients in CFREM between the 3 groups at month 60: 55.4 ± 11.4% in the TD group, 24.9 ± 18.1% in the AC group, and 42.7 ± 11.5% in the CM group (P = 0.79, Figure 2D).
Toxicity
AEs were observed in 80 patients: 10 (41.6%) were in the TD group, 31 (40.8%) in the AC group, and 39 (43.3%) in the CM group. No difference regarding the cumulative proportion of patients free of AEs between the 3 groups at month 60: 51.1 ± 11.0% in the TD group versus 40.3 ± 13.0% in the AC group, 48.2 ± 7.0% in the CM group (P = 0.73, Figure 2E).
Most common AEs included myelotoxicity (13.2%), infections (9.5%), arthralgia (8.9%), flu-like symptoms (7.9%), and GI reactions (7.4%) were observed in the present study. The documented incidence of infections (P = 0.67) and arthralgia (P = 0.51) were comparable between the 3 groups. However, the incidence of leucopoenia was significantly lower in the CM group (P = 0.01).
Overall 21% of patients with CD discontinued therapy in our study. A discontinuation rate due to side effects was up to 11.6%, followed by refractoriness (4.7%) or patient's request (3.7%). And the incidence of withdraw were comparable among 3 groups except for that the myelosuppression caused withdraw was more frequently occurred in the TD group.
Early TP Compared With CM
The former 2 strategies (TD and AC strategies) consisted the early TP group (n = 100). Considering both the primary and secondary endpoints, no significant difference was found between early TP group and conventional TP group (Figure 3).
FIGURE 3.
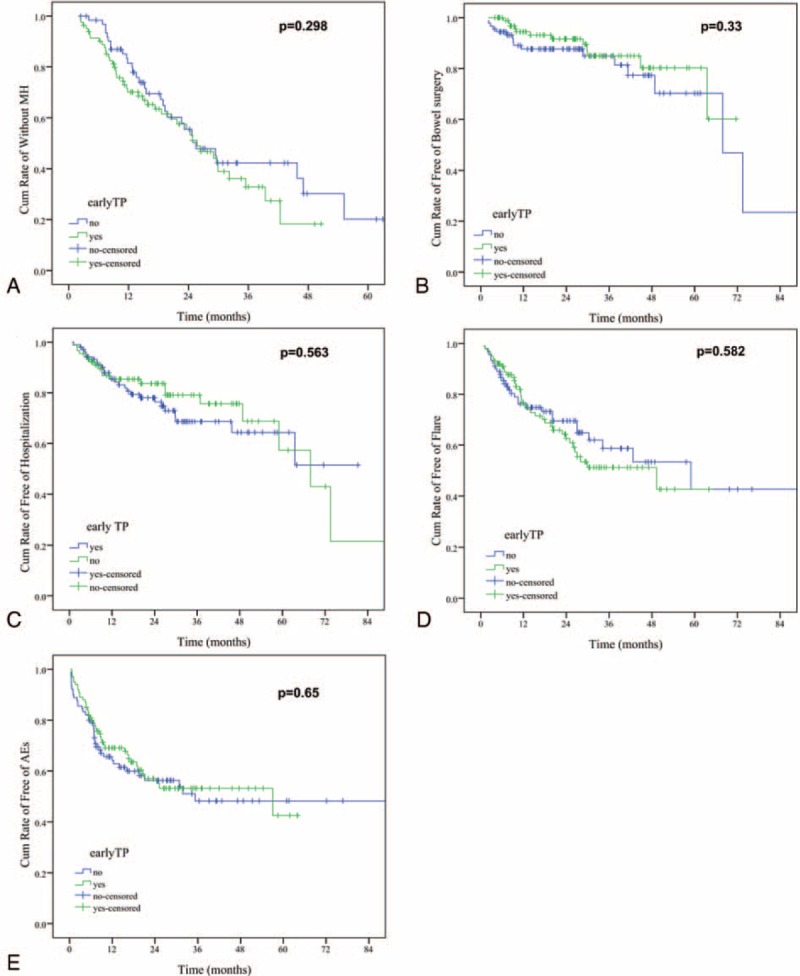
Kaplan–Meier plot showing probability of main events: (A) mucosal healing; (B) CD-related bowel surgery; (C) CD-related hospitalization; (D) flare; and (E) adverse events between early TP and conventional TP group. CD = Crohn disease; TP = thiopurines.
DISCUSSION
The present study focused on the long-term follow-up evaluation of 3 main strategies of TP treatment in early CD at risk for a disabling course. The efficacy of TP for maintenance of remission in patients with CD, albeit modest, is well established when used at the appropriate dosage and duration (a minimum of 17 weeks).16 In order to prevent progression to a complicated phenotype, it is of great importance to intervene in an early stage of the disease when irreversible damage has not yet occurred,17,18 the so-called “window of opportunity.”19
Recently, both the AZTEC study3 and the GETAID study2 failed to reproduce the efficacy of AZA in newly diagnosed adult CD, with the conclusion that AZA-based AC approach was no more effective for achieving clinical remission compared to a CM approach. However, both patient selection and the definition of the primary end point20 should be taken into account when interpreting the data.21 Importantly, nearly 30% of patients with complicated disease phenotype at diagnosis were excluded by definition from both AZTEC3 and RAPID trials.2
In the present study, only early CD defined as a disease duration of <18 months from diagnosis and also naïve to disease-modifying agents according to the Paris definition7 were included. TP and/or anti-TNFα were induced within a median time of 3 months (95% confidence interval 1.3–6.8) from diagnosis. One-seventh (14.7%) patients had undergone bowel complication at referral. In this selective cohort, we again failed to demonstrated early AZA/6-MP therapy (either TD or AC strategy) was more effective than CM for maintaining CFREM (P = 0.79).
Evidence is accumulating about the limitations of CDAI. Recently, a post hoc analysis of the SONIC trial further confirmed the discrepancy between clinical symptoms and objective findings of endoscopic inflammation,22 with an overall accuracy of clinical symptoms, relative to endoscopic inflammation of only 56%. Reliance merely on symptoms to guide treatment may be not an optimal management strategy.
MH has been received increasing attention based on observations that treatment aiming at clinical symptoms resolution alone does not reduce or prevent long-term bowel damage in patients with CD.14,23–25 MH as the treatment goal is being increasingly considered in patients with CD. Early introduction of TNF antagonists in the course of the disease, particularly in combination with immunosuppressives, is 1 strategy for improving MH, as shown in the SONIC and step-up/TD trials.26,27 Combination therapy with TP and infliximab could reduce anti-infliximab antibodies and approximately doubles the serum level of infliximab,26,28 which may lead to optimized clinical outcomes to either agent alone in both ulcerative colitis and CD.26,29 Whereas AZA monotherapy is minimally effective as a disease-modification agent in CD.30 In our cohort, there was a significant greater proportion of patients achieving MH in the early TP group (P = 0.001). The well-established evidence support that maintenance of MH is predictive of other desired disease-modification benefits such as prevention of bowel damage. The present study demonstrated a trend, albeit not significant, for an increased proportion of patients remaining free of intestinal surgery in the early TD groups at 5 years: 83.2% in the TD group versus 60.3% in the CM group (P = 0.16). As surgery is a relatively uncommon event in the first postdiagnostic 5 years in CD, it is possible a larger cohort with a longer follow-up would reveal differences between different strategies over time. As demonstrated in the REACT trial,4 a significant reduction in rates for complication, surgeries, and hospitalizations was observed using the AC approach after 24 months instead of 12 months. Thus, although due to the relative small group sample size, and low incidence rates of hospitalization and intestinal surgery, we did not demonstrate such benefits in the present cohort, it is promising such benefits exist due to its nature of MH on disease modification.
Safety issues should also be taken into account when initiating TD strategy. TP, when used in combination with anti-TNF agents, may be associated with an increased risk of opportunistic infections in patients with IBD.31 In the present study, safety was identical throughout the 3 groups (P = 0.89) during the 5 years’ follow-up. The rates of myelotoxicity (13.2%), infections (9.5%), arthralgia (8.9%), flu-like symptoms (7.9%), and GI reactions (7.4%) observed in the present study did not significantly differ from literature data.32–35 Overall 21% of patients with CD discontinued TP therapy mainly due to side effects which made up to 11.6% of our study population. In previous studies, TP discontinuation rate due to side effects varies from 5–6%35 to 30%.36 And the incidence of withdraw were comparable among 3 groups except for that withdraw due to myelosuppression was more frequently occurred in the TD group, possibly due to the synergetic effect of AZA and anti-TNFs.
This study had several limitations. First, the retrospective design could induce a bias of patient selection and information gathering. However, most of the data were collected prospectively and made these biases minimal. Second, tertiary-center referral bias was also likely to have influenced the outcomes considering the data source from a referral teaching hospital. Third, compliance to TP treatment was not assessed. But the regular check of 6-TGN concentration37 within the target therapeutic window suggested good compliance of our cohort. Fourth, the lack of a validated endoscopic scoring system was an important limitation. Finally, the lack of a uniformed tool to evaluate bowel damage is an important limitation. The development of novel tools, such as the Lemann score,7 will further our understanding of the potency of different strategies of TP in changing the natural course of CD.
In our single-center experience, both the AC and CM strategies are minimally effective for disease modification. TD strategy with potential of achieving higher rates MH, thus our results support the TD strategy in adult patients with early CD at risk for a disabling course.
Footnotes
Abbreviations: 6-MP = 6-mercaptopurine, AC = accelerated step-up, AE = adverse events, AZA = azathioprine, CD = Crohn's disease, 6-TGNs = 6-thioguanine nucleotides, TNF = tumor necrosis factor, CDAI = the Crohn's Disease Activity Index, CFREM = corticosteroid-free remission, CM = conventional management, IBD = inflammatory bowel disease, IQR = interquartile range, MH = mucosal healing, REACT = Randomized Evaluation of an Algorithm for Crohn Treatment, TD = top-down, TP = thiopurines.
YQ and B-LC have contributed equally to this work.
Author contributions: YQ and B-LC: study design, data collection, statistical analysis, interpretation, and manuscript drafting/revision; M-HC: conceive and design, critical revision of the manuscript for important intellectual content; and study supervision; RM, S-HZ, YH, and Z-RZ: study concept and design; and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
This study was financially supported in part by the National Natural Science Foundation of China (NSFC grant no. 81470821 and 81270473).
The authors alone are responsible for the content and writing of the paper.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002; 8:244–250. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn's disease: a randomized controlled trial. Gastroenterology 2013; 145:758–765. [DOI] [PubMed] [Google Scholar]
- 3.Panes J, Lopez-Sanroman A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn's disease. Gastroenterology 2013; 145:766–774. [DOI] [PubMed] [Google Scholar]
- 4.Khanna R, Levesque BG, Bressler B, et al. 1053 Early combined immunosuppression for the management of Crohn's disease: a community-based cluster randomized trial. Gastroenterology 2014; 146:S-187. [Google Scholar]
- 5.Lennard-Jones J. Classification of inflammatory bowel disease. Scand J Gastroenterol 1989; 24:2–6. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19:5–36. [DOI] [PubMed] [Google Scholar]
- 7.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011; 17:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn's disease. Gastroenterology 2006; 130:650–656. [DOI] [PubMed] [Google Scholar]
- 9.Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2013; 4:CD000545.doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 10.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis 2010; 4:28–62. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol 2009; 104:465–483. [DOI] [PubMed] [Google Scholar]
- 12.Hu P-J, Qian J-M, Wu K-C, et al. The consensus statement on diagnoses and treatment of inflammatory bowel disease (2012 Guangzhou). Chin J Intern Med 2012; 10:818–831. [Google Scholar]
- 13.Derijks LJ, Gilissen LP, Engels LG, et al. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: implications for therapy. Ther Drug Monit 2004; 26:311–318. [DOI] [PubMed] [Google Scholar]
- 14.Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis 2009; 15:1295–1301. [DOI] [PubMed] [Google Scholar]
- 15.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol 2014; 12:414–422. [DOI] [PubMed] [Google Scholar]
- 16.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev (Online) 2009; 4:CD000545.doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 17.Ramadas AV, Gunesh S, Thomas GA, et al. Natural history of Crohn's disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut 2010; 59:1200–1206. [DOI] [PubMed] [Google Scholar]
- 18.Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn's disease & quest; surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977–2009. Am J Gastroenterol 2012; 107:579–588. [DOI] [PubMed] [Google Scholar]
- 19.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut 2010; 59:141–147. [DOI] [PubMed] [Google Scholar]
- 20.Allen PB, Peyrin-Biroulet L. Moving towards disease modification in inflammatory bowel disease therapy. Curr Opin Gastroenterol 2013; 29:397–404. [DOI] [PubMed] [Google Scholar]
- 21.Lakatos PL, Peyrin-Biroulet L. Azathioprine in early Crohn's disease: time to revisit patient selection and end points for clinical trials and/or azathioprine efficacy? Gastroenterology 2014; 146:867–868. [DOI] [PubMed] [Google Scholar]
- 22.Peyrin-Biroulet L, Reinisch W, Colombel J-F, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 2014; 63:88–95. [DOI] [PubMed] [Google Scholar]
- 23.Frøslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007; 133:412–422. [DOI] [PubMed] [Google Scholar]
- 24.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology 2010; 138:463–468. [DOI] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Korzenik JR, Hur C. Can mucosal healing be a cost-effective endpoint for biologic therapy in Crohn's disease? A decision analysis. Inflamm Bowel Dis 2013; 19:37–44. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010; 362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 27.D’Haens G, Baert F, Van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008; 371:660–667. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009; 30:210–226. [DOI] [PubMed] [Google Scholar]
- 29.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014; 146:392–400. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ. The future of inflammatory bowel disease therapy: where do we go from here? Dig Dis 2012; 30 Suppl 3:140–144. [DOI] [PubMed] [Google Scholar]
- 31.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008; 134:929–936. [DOI] [PubMed] [Google Scholar]
- 32.Chebli JM, Gaburri PD, De Souza AF, et al. Long-term results with azathioprine therapy in patients with corticosteroid-dependent Crohn's disease: open-label prospective study. J Gastroenterol Hepatol 2007; 22:268–274. [DOI] [PubMed] [Google Scholar]
- 33.Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993; 34:1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 1989; 111:641–649. [DOI] [PubMed] [Google Scholar]
- 35.Warman JI, Korelitz BI, Fleisher MR, et al. Cumulative experience with short- and long-term toxicity to 6-mercaptopurine in the treatment of Crohn's disease and ulcerative colitis. J Clin Gastroenterol 2003; 37:220–225. [DOI] [PubMed] [Google Scholar]
- 36.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002; 50:485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha C, Dassopoulos T. Thiopurine therapy in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2010; 4:575–588. [DOI] [PubMed] [Google Scholar]


