Abstract
The contributions of micro RNAs (miRNAs) to rheumatoid arthritis (RA) are beginning to be uncovered during the last decade. Many studies in efforts to use miRNAs as biomarkers in disease diagnosis, prognosis, and treatment are ongoing.
We conducted a systematic literature review to reveal the role of miRNAs in the pathogenesis of RA in order to inform future research.
We analyzed all the literature which is searched by keywords “microRNA” and “arthritis” in PubMed from December 2007 to June 2015, and the references cited by the articles searched were also considered.
Relevant literature focusing on the field of miRNAs and RA was identified. The searching process was conducted by 5 independent investigators. The experts in the field of miRNAs and Rheumatology were involved in the process of analyzing.
Relevant literature was analyzed according to the objective of this review and the availability of full text.
The crucial role of miRNAs in maintaining immune and inflammatory responses is revealed. In addition, it is now clear that miRNAs are implicated in the development of RA synovial phenotype including synovial hyperplasia and joint destruction. Intriguingly, the biomedical application of several miRNAs may result in the effects of “double-edged sword.” Moreover, there appears to have a feedback loop for expression of some miRNAs related to disease activity in inflammatory milieu of rheumatoid joint.
This review underscores the potential importance of miRNAs to diagnosis, prognosis, and treatment of RA. Further investigations are required to identify the unique miRNAs signatures in RA and characterize the mechanisms mediated by miRNAs in the pathology of RA.
INTRODUCTION
There have been only few events in the history of molecular biology that could be compared with the discovery of micro RNAs (miRNAs) and their roles in cell physiology and the pathogenesis of human diseases.1 MiRNAs are a group of approximately 20 to 22 nucleotides noncoding RNAs and have been proven to regulate gene expression at the posttranscriptional level.2,3 In addition, miRNAs exhibit tissue-specific or developmental stage-specific expression patterns and are associated with diverse biological events such as cell growth, apoptosis, cell differentiation, cancer, and autoimmune arthritis.4,5
Rheumatoid arthritis (RA) is a most common autoimmune disease that affects 0.5% to 2.0% of the human population worldwide.6,7 Progression of RA will finally lead to joint destruction, functional disability, and sometimes death.8,9 However, the etiology and pathogenesis of RA remain largely unknown, and thus there are no satisfactory therapeutic strategies to cure this disease so far.10,11 It is well known that the pathology of RA is characterized by synovial lining cell hyperplasia and irreversible joint destruction because of chronic synovial inflammation.9,12 RA synoviocytes is considered as the effector cells of cartilage and bone destruction.13 Resident cells in rheumatoid joints, such as RA synovial fibroblasts and fibroblast-like synoviocytes (FLS), play a crucial role in RA.14 These cells show an “intrinsically” activated and aggressive phenotype, which results in the increased production of matrix metalloproteinases (MMPs) and adhesion molecules that play a key role in joint bone destruction.13,14
In the past few years, the importance of miRNAs in the pathogenesis of RA has been elucidated.15 MiRNAs have been demonstrated to play an important role in inflammatory responses, cell proliferation of synoviocytes, and production of MMPs in rheumatoid joints.12 For instance, recently a series of miRNAs were identified to be dysregulated in cell subsets within the articular compartment of patients with RA,16 and more recently several studies suggest that miRNAs regulate leukocyte activation and cytokine production that in turn contribute to the immunologic component of effector synovial pathology.16 Importantly, therapeutic trials aimed at targeting miRNA in arthritis have been conducted in in vivo models.5,12 Thus, targeting miRNA will enable a new advanced strategy toward arthritis treatment.12 In this review, we analyzed the roles of miRNAs in immune and inflammatory responses, as well as RA synovial phenotype including synovial hyperplasia and joint bone destruction.
ETHICAL REVIEW
The study presented here is a literature review and it does not need any ethical approval. This study does not involve interaction with any human subjects and does not collect any identifiable private information.
METHODS
Relevant literature focusing on the field of miRNAs and RA was identified through searching in PubMed (MEDLINE) database by keywords “microRNA” and “arthritis” from December 2007 to June 2015. There were no limitations imposed on language and study types. The references cited by the articles searched were also analyzed.
Five independent investigators (contributing authors), R-YH, X-MC, S-LY, Y-HY, and YH, conducted the searching process. The experts in the field of miRNAs, LH (contributing author) and C-JL (acknowledged in the section of acknowledgment), as well as the experts in Rheumatology (contributing authors), Q-CH, Y-LC, and R-YH, were involved in the process of analyzing the role of miRNAs in RA pathogenesis. Relevant literature was chosen according to the objective of this review and the availability of full text.
Role of miRNAs in Immune and Inflammatory Responses
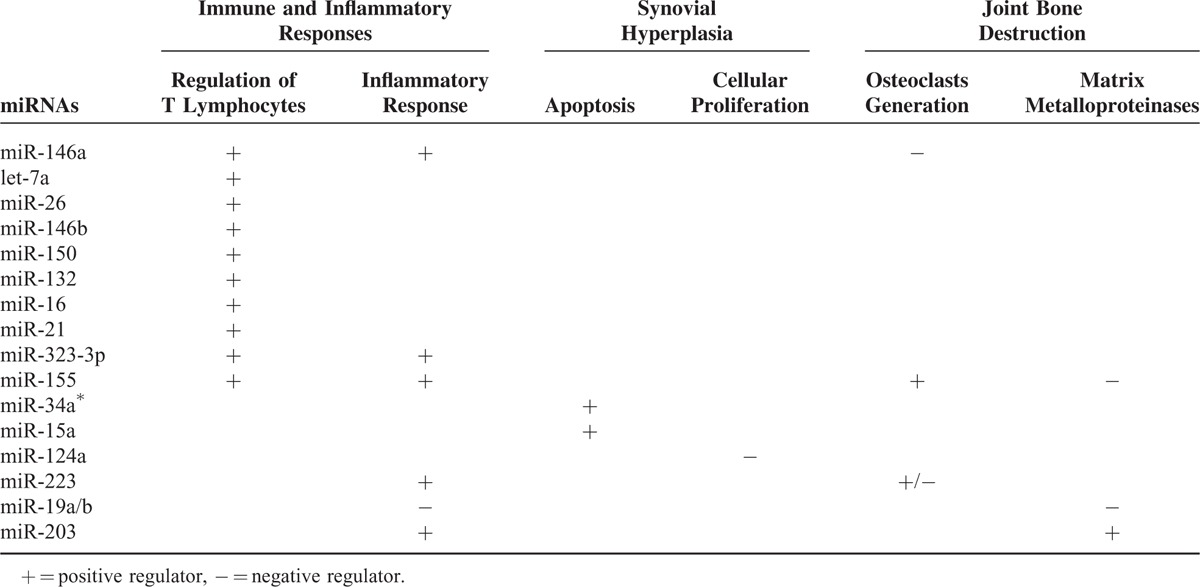
Through regulating the expression of many genes, miRNAs play a crucial role in maintaining immune system development and function, and may participate in the development of numerous autoimmune diseases.17,18 It is now clear that inflammatory cytokines, interleukin-17 (IL-17) for instance, promote inflammatory autoimmune diseases,19 and it has been found that 6 miRNAs, let-7a, miR-26, miR-146a/b, miR-150, and miR-155, are significantly up-regulated in the IL-17 producing T cells.20 In addition, miR-146a, miR-155, miR-132, and miR-16 are increased in peripheral blood mononuclear cells (PBMCs) from RA patients, as compared with healthy control patients.21 The tissues or cells that are main sources of miRNAs in RA are shown in Table 1. Among those miRNAs, miR-146a has been well elucidated to be a regulator of immune and inflammatory responses, and it has been demonstrated to be strongly expressed in synovial tissue, synoviocytes, PBMCs, and other IL-17 expressing cells from patients with RA.20–23 By using quantitative reverse transcription-polymerase chain reaction (qRT-PCR), a research team determined the expression of miR-146 in synovial tissues obtained from 5 patients with RA, 5 patients with osteoarthritis (OA), and 1 normal patient.24 The results showed that mature miR-146a and primary miR-146a/b, company with tumor necrosis factor (TNF)-α, are over-expressed in RA synovial tissues, as compared with OA and normal synovial tissues.24 This study further demonstrated that primary miR-146a is mainly expressed in cells of the superficial and sublining layers in synovial tissues from RA patients.24 In this study, cells positive for miR-146a are primarily CD68+ macrophages. In addition, miR-146a is also expressed in several CD3+ T cell subsets and CD79a+ B cells.24 Moreover, the expression profile of miRNAs in CD4+ T cells from synovial fluid and peripheral blood of 33 patients with RA was determined in another study by microarray assay and validated by qRT-PCR analysis.25 The results showed that the expression of miR-146a is significantly up-regulated in CD4+ T cells of RA patients, and its level is positively correlated with the level of TNF-α.25 Importantly, TNF-α is able to increase miR-146a expression in T lymphocyte cells.25 Moreover, it is reported that over-expression of miR-146a can suppress apoptosis of Jurkat T cells, an immortalized line of human T lymphocyte cells.26 Transcriptome analysis of miR-146a over-expression in T cells has identified FAS associated factor 1 as an miR-146a-regulated gene, which is critically involved in modulating T cell apoptosis.25 All together, these data strongly indicate that miR-146a is associated with inflammation and regulates cell proliferation of immune-regulated cells. Thus, a positive role of miR-146a in immune and inflammatory responses of RA is revealed, and it may hold the potential to be novel target for RA therapy.
TABLE 1.
Tissues or Cells That are Main Sources of miRNAs in RA

In addition to the therapeutic target, miR-146a could also be used as a biomarker for prognosis of RA, as the increased miR-146a is found to be positively correlated with TNF-α and 2 disease activity indicators, erythrocyte sedimentation rate and disease activity score in 28 joints, in patients with RA.27
Of note, several other miRNAs have also been addressed to regulate immune or inflammatory responses in patients with RA (Table 2). For example, it is reported that miR-19a/b can act as the negative regulators of inflammation in humans.14 In addition, miR-21 was demonstrated to have a role in maintaining the balance between immune activation and tolerance.28 Recently, the gene encoding miR-323-3p, a biomarker in immune and inflammatory responses, was found to be increased in RA synovial fibroblast.29 In addition to these miRNAs, miR-155 has powerful regulatory potential in a wide variety of immune cells.30 For example, it is reported that compared with PBMCs from RA, synovial fluid monocytes obtained from RA patients displayed higher levels of miR-155.31 In addition, microarray analysis of miRNAs expressed in synovial fibroblast treated with TNF-α revealed a significant up-regulation of miR-155,31 whereas repression of miR-155 in RA synovial CD14+ cells reduced expression of TNF-α.32 These observations strongly suggest a positive correlation between miR-155 and inflammation in rheumatoid joints. Moreover, miR-155-deficient mice showed the resistance to collagen-induced arthritis, with profound suppression of antigen-specific Th17 cells and autoantibody responses,32,33 supporting a view of that miR-155 is essentially involved in the immune reactions leading to autoimmune arthritis.33 Therefore, miR-155 may provide another novel target for the treatment of patients with RA. The further understanding the role of these miRNAs could shed light on the cause and progression of RA and eventually lay the groundwork for therapeutic options.34
TABLE 2.
Role of Several Well Characterized miRNAs in Pathogenesis of RA
Role of miRNAs in Synovial Hyperplasia
Synovial hyperplasia is a phenotypical manifestation of RA.35 Resident cells in rheumatoid joints, synovial fibroblasts, and FLS, in principle, are characterized by a resistance to apoptosis, and this contributes to tumor-like cell proliferation and the related synovial hyperplasia.9,36,37 Proliferation of RA resident cells plays a crucial role in the progression of RA, as these cells produce inflammatory cytokines, chemokines, and angiogenic factors, which are critically involved in inflammatory responses and joint destruction in patients with RA.9,12
Several lines of evidence have suggested the role of miRNAs in proliferation of resident cells in rheumatoid joints. For instance, it is found that basal expression level of miR-34a∗ is reduced in RA synovial fibroblasts from RA patients compared with OA patients, and importantly the decreased expression of miR-34a∗ results in up-regulation of its direct target X-linked inhibitor of apoptosis protein, thereby contributing to the resistance of RA synovial fibroblasts to apoptosis.38 On the contrary, miR-15a is reported to induce cell apoptosis by negatively regulating the expression of B-cell lymphoma 2 (Bcl-2), which suppresses the apoptotic processes.5 In this study, Nagata et al established a model of autoantibody-mediated arthritis in male DBA/1J mice. The double-stranded miR-15a was injected into the knee joint, and they found that Bcl-2 protein was down-regulated and the expression of caspase-3 was increased as compared with that in the control group.5 Furthermore, in another study, by using quantitative stem-loop RT-PCR, synoviocytes derived from surgical specimens obtained from RA patients were compared with those obtained from OA patients for the expression of a panel of 156 miRNAs.39 The results showed the dramatical decrease of miR-124a in RA synoviocytes as compared with OA synoviocytes. The transfection of precursor miR-124a into RA synoviocytes significantly suppressed cell proliferation and arrested the cell cycle at the G1 phase.39 Therefore, miR-34a∗, miR-15a, and miR-124a are all the negative regulators for synovial hyperplasia in patients with RA (Table 2).
Role of miRNAs in Joint Bone Destruction
Joint destruction is a frequent and clinically serious event in patients with RA, and it is closely related to synovial hyperplasia and joint inflammation.35,40,41 T lymphocytes, monocytes, and synovial fibroblast have been identified as the sources of local joint destruction-related signals of osteoclast differentiation in RA patients.40 In addition, MMPs are considered as the markers of joint damage progression of RA.42,43
Over the past years, several miRNAs related to osteoclastogenesis (osteoclast generation) and MMPs release have been defined (Table 2). For example, it is reported that the increased miR-146a inhibited bone destruction through suppressing osteoclastogenesis, and administration of double-stranded miR-146a prevented joint destruction in arthritic mice.22 Because miR-146a is positively correlated with inflammatory responses and disease activity of RA, as stated above, the negative role of miR-146a in joint destruction makes it to be a “double-edged sword” when considering using miR-146a as a diagnostic and therapeutic marker (Figure 1).
FIGURE 1.
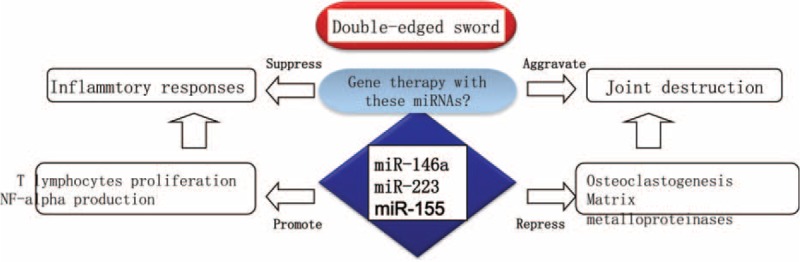
“Double-edged sword” miRNAs in RA. MiR-146a, 155, and 223 have opposite roles in regulating RA pathogenesis. These miRNAs are positively correlated with disease activity through stimulating inflammatory response. On the contrary, they play a role in suppressing joint destruction. The opposite roles of miRNAs give us a lesson to focus more carefully on therapeutic and diagnostic applications of these miRNAs in RA. RA=rheumatoid arthritis.
There is another example for the opposite roles of miRNA in pathogenesis of RA (Table 2). Two research teams focusing on the molecular mechanisms mediated by miR-223, but 2 stories were developed in opposite ways.44 One team demonstrated that miR-223 is intensely expressed in the synovium of patients with RA, and over-expression of miR-223 suppresses osteoclastogenesis in vitro,45 whereas another team showed that lentivirus-mediated silencing of miR-223 can reduce osteoclastogenesis and bone erosion of mice arthritis induced by collagen.46 Therefore, the question raised is why there are opposite roles of 1 miRNA in the pathogenesis of bone destruction of RA? There should be more researches in this field to clarify the complexity of miRNAs functions.
The complexity of miRNAs functions is also supported by the opposite roles of miR-155 in joint destruction of RA (Table 2). It is demonstrated that enforced expression of miR-155 in RA synovial fibroblasts suppressed the induction of MMP-3 and MMP-1 by Toll-like receptor ligands and cytokines,31 indicating a protective role of miR-155 in joint destruction of RA. However, in a model of K/BxN serum-transfer arthritis, the deficiency of miR-155 significantly reduced local bone destruction, attributed to reduced generation of osteoclasts, although the severity of joint inflammation was similar to that in wild-type mice.33
Compared with those miRNAs, roles of miR-19a/b and miR-203 are not paradox in bone destruction pathology of RA (Table 2). RA FLS plays a key role in joint destruction and is believed to spread RA to unaffected joints.14 By using the miRNA microarray analysis, Philippe et al14 demonstrated down-regulation of miR-19b in activated RA FLS. MiR-19b and miR-19a belong to the same cluster. Transfection of RA FLS with miR-19a/b mimics dramatically decreased the release of IL-6 and MMP-3, suggesting a role of miR-19a/b in protecting RA patients from joint inflammation and destruction.14 In addition, it is reported that the expression of miR-203 is higher in RA synovial fibroblasts than in OA synoviocytes or fibroblasts from healthy donors.47 The enforced levels of miR-203 lead to increased secretion of MMP-1 and IL-6 via the nuclear factor (NF)-κB pathway, and thereby contributing to the activated synovial phenotype of RA.47 The opposite effects of miR-19a/b and miR-203 in joint destruction are therefore revealed.
CONCLUSION
In summary, although only a very small fraction of miRNAs is characterized in the pathogenesis of RA (Table 2), evidence in support of the therapeutic potential of miRNA-based strategies is growing, as demonstrated by several therapeutic trials aimed at targeting miRNA in in vivo models of arthritis.5,12,33
Of note, in addition to promoting cytokines production, the expression of miR-146a/b can be markedly up-regulated in RA synovial fibroblasts following stimulation with TNF-α and IL-1β.24 Moreover, expression of miR-155 in RA synovial fibroblasts can also be induced by TNF-α and IL-1β.31 Thus, it appears that not only miRNAs contribute broadly and actively to various aspects of RA pathogenesis, but also the inflammatory milieu may alter miRNAs expression profiles in resident cells of RA joints (Figure 2). Furthermore, there is a recent study demonstrating the roles of target miRNAs in modulating NF-κB activity and restoring transrepression of an NF-κB reporter by dexamethasone,48 suggesting that, as a widely expressed transcription factor, NF-κB may be responsible for the production of cytokines and other inflammatory factors by miRNAs in autoimmune arthritis. Collectively, all the evidence presented here reveals a feedback loop for expression and functions of miRNAs, by which miRNAs contribute to inflammatory reactions, synovial phenotype, and bone destruction in rheumatoid joints (Figure 2).
FIGURE 2.
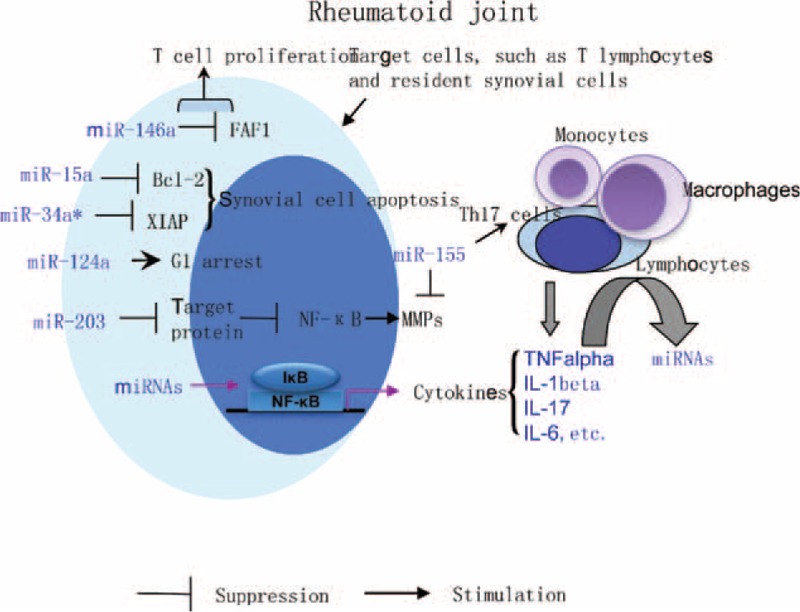
MiRNAs in inflammatory milieu of rheumatoid joint. Roles of several miRNAs in T cell proliferation, apoptosis, and cell cycle of synovial cells and joint destruction are depicted. Importantly, there appears to have a feedback loop by which miRNAs contribute to inflammatory reactions and synovial phenotype in rheumatoid joints. Through NF-κB pathway, some miRNAs such as miR-146a and miR-155 may stimulate the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-17. As the inflammatory mediators, these cytokines can induce lymphocytes, resident synovial cells and other inflammatory cells to produce miRNAs that related to disease activity of RA patients. Bcl-2 = B-cell lymphoma 2, FAF1 = Fas associated factor 1, MMPs = matrix metalloproteinases, NF-κB = nuclear factorκB, XIAP = X-linked inhibitor of apoptosis protein.
Future Perspectives
Although several miRNAs have been found to contribute to various aspects of RA pathogenesis and hold great therapeutic potential, unique miRNA signatures in RA are not found yet. For example, it has been demonstrated that miR-155 has powerful regulatory potential in a wide variety of immune cells through targeting specific mRNAs.30 In addition, miR-323-3p is found to be increased in RA, but it is also a biomarker in immune and inflammatory responses.29 Because pathogenic immune cells and inflammatory responses play a pivotal role in pathogenesis of many types of autoimmune diseases, these miRNAs are not specific for RA pathogenesis, which is further supported by a recent study showing a positive association of a genetic variant in a miRNA-146a target with psoriatic arthritis.49 Therefore, discovery of perfect biomarkers for RA diagnosis and treatment is still a dream of rheumatologists.
It is well known that miRNAs are one of species of small RNA and belong to family of noncoding RNAs. Research in small RNA/noncoding RNA is still in its infancy. Recent reports have described an intricate interplay among diverse RNA species, including protein-coding mRNAs and noncoding RNAs such as long noncoding RNAs, pseudogenes, and circular RNAs.50 These RNA transcripts communicate with and co-regulate each other by competing for binding to shared miRNAs.50,51 Understanding the cross-talk between miRNAs and other noncoding RNAs will lead to the significant insight into gene regulatory networks implicated in the pathogenesis of RA.
Collectively, the identification of specific miRNAs expression patterns in RA as well as a comprehensive understanding of the role of miRNAs in RA pathogenesis offers promise of not only novel molecular diagnostic markers but also new gene therapy strategies for treating RA and other autoimmune arthritis.52
ACKNOWLEDGMENTS
The authors sincerely thank Prof Chuan-Jian Lu (Guangdong Provincial Academy of Chinese Medical Sciences, Guangzhou 510120, China) for her constructive suggestions for this study.
Footnotes
Abbreviations: Bcl-2 = B-cell lymphoma 2, FLS = fibroblast-like synoviocytes, IL-17 = interleukin-17, MiRNAs = micro RNAs, MMPs = matrix metalloproteinases, NF-κB = nuclear factorκB, PBMCs = peripheral blood mononuclear cells, qRT-PCR = quantitative reverse transcription-polymerase chain reaction, RA = rheumatoid arthritis, TNF = tumor necrosis factor.
This study was supported by China Postdoctoral Science Foundation (No. 2014M562163), National Natural Science Foundation of China (No. 81473681), as well as Chinese Medical Science and Technology research funding from Guangdong Provincial Hospital of Chinese Medicine (No. YN2014ZH04).
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Torres A, Torres K, Maciejewski R, et al. MicroRNAs and their role in gynecological tumors. Med Res Rev 2011; 31:895–923. [DOI] [PubMed] [Google Scholar]
- 2.Xu WD, Lu MM, Pan HF, et al. Association of microRNA-146a with autoimmune diseases. Inflammation 2012; 35:1525–1529. [DOI] [PubMed] [Google Scholar]
- 3.Zisoulis DG, Kai ZS, Chang RK, et al. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature 2012; 486:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada S, Asahara H. Current strategies for microRNA research. Mod Rheumatol 2012; 22:645–653. [DOI] [PubMed] [Google Scholar]
- 5.Nagata Y, Nakasa T, Mochizuki Y, et al. Induction of apoptosis in the synovium of mice with autoantibody-mediated arthritis by the intraarticular injection of double-stranded microRNA-15a. Arthritis Rheum 2009; 60:2677–2683. [DOI] [PubMed] [Google Scholar]
- 6.Wang MJ, Huang Y, Huang RY, et al. Determination of role of thromboxane A2 in rheumatoid arthritis. Discov Med 2015; 19:23–32. [PubMed] [Google Scholar]
- 7.Korczowska I. Rheumatoid arthritis susceptibility genes: an overview. World J Orthop 2014; 5:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 9.Huang RY, Huang QC, Burgering BM. Novel insight into the role of alpha-actinin-1 in rheumatoid arthritis. Discov Med 2014; 17:75–80. [PubMed] [Google Scholar]
- 10.Salemi S, Biondo MI, Fiorentino C, et al. Could early rheumatoid arthritis resolve after periodontitis treatment only?: case report and review of the literature. Medicine (Baltimore) 2014; 93:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ursini F, Russo E, Letizia Hribal M, et al. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore) 2015; 94:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakasa T, Nagata Y, Yamasaki K, et al. A mini-review: microRNA in arthritis. Physiol Genomics 2011; 43:566–570. [DOI] [PubMed] [Google Scholar]
- 13.Karouzakis E, Gay RE, Gay S, et al. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat Rev Rheumatol 2009; 5:266–272. [DOI] [PubMed] [Google Scholar]
- 14.Philippe L, Alsaleh G, Suffert G, et al. TLR2 expression is regulated by microRNA miR-19 in rheumatoid fibroblast-like synoviocytes. J Immunol 2012; 188:454–461. [DOI] [PubMed] [Google Scholar]
- 15.Miao CG, Yang YY, He X, et al. New advances of microRNAs in the pathogenesis of rheumatoid arthritis, with a focus on the crosstalk between DNA methylation and the microRNA machinery. Cell Signal 2013; 25:1118–1125. [DOI] [PubMed] [Google Scholar]
- 16.Baxter D, McInnes IB, Kurowska-Stolarska M. Novel regulatory mechanisms in inflammatory arthritis: a role for microRNA. Immunol Cell Biol 2012; 90:288–292. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez SA, Piera-Velazquez S. Potential role of human-specific genes, human-specific microRNAs and human-specific non-coding regulatory RNAs in the pathogenesis of systemic sclerosis and Sjogren's syndrome. Autoimmun Rev 2013; 12:1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang D, Shen N. MicroRNA involvement in lupus: the beginning of a new tale. Curr Opin Rheumatol 2012; 24:489–498. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med 2012; 18:1077–1086. [DOI] [PubMed] [Google Scholar]
- 20.Niimoto T, Nakasa T, Ishikawa M, et al. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet Disord 2010; 11:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauley KM, Satoh M, Chan AL, et al. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther 2008; 10:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakasa T, Shibuya H, Nagata Y, et al. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum 2011; 63:1582–1590. [DOI] [PubMed] [Google Scholar]
- 23.Chatzikyriakidou A, Voulgari PV, Georgiou I, et al. A polymorphism in the 3’-UTR of interleukin-1 receptor-associated kinase (IRAK1), a target gene of miR-146a, is associated with rheumatoid arthritis susceptibility. Joint Bone Spine 2010; 77:411–413. [DOI] [PubMed] [Google Scholar]
- 24.Nakasa T, Miyaki S, Okubo A, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 2008; 58:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Wan Y, Guo Q, et al. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther 2010; 12:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alizadeh S, Kaviani S, Soleimani M, et al. Mir-55 inhibition can reduce cell proliferation and induce apoptosis in Jurkat (Acute T cell Leukemia) cell line. Iran J Ped Hematol Oncol 2014; 4:141–150. [PMC free article] [PubMed] [Google Scholar]
- 27.Abou-Zeid A, Saad M, Soliman E. MicroRNA 146a expression in rheumatoid arthritis: association with tumor necrosis factor-alpha and disease activity. Genet Test Mol Biomarkers 2011; 15:807–812. [DOI] [PubMed] [Google Scholar]
- 28.Iliopoulos D, Kavousanaki M, Ioannou M, et al. The negative costimulatory molecule PD-1 modulates the balance between immunity and tolerance via miR-21. Eur J Immunol 2011; 41:1754–1763. [DOI] [PubMed] [Google Scholar]
- 29.Xu T, Huang C, Chen Z, et al. MicroRNA-323-3p: a new biomarker and potential therapeutic target for rheumatoid arthritis. Rheumatol Int 2014; 34:721–722. [DOI] [PubMed] [Google Scholar]
- 30.Leng RX, Pan HF, Qin WZ, et al. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev 2011; 22:141–147. [DOI] [PubMed] [Google Scholar]
- 31.Stanczyk J, Pedrioli DM, Brentano F, et al. Altered expression of microRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 2008; 58:1001–1009. [DOI] [PubMed] [Google Scholar]
- 32.Kurowska-Stolarska M, Alivernini S, Ballantine LE, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A 2011; 108:11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bluml S, Bonelli M, Niederreiter B, et al. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum 2011; 63:1281–1288. [DOI] [PubMed] [Google Scholar]
- 34.Singh RP, Massachi I, Manickavel S, et al. The role of miRNA in inflammation and autoimmunity. Autoimmun Rev 2013; 12:1160–1165. [DOI] [PubMed] [Google Scholar]
- 35.Weitoft T, Ronnelid J, Knight A, et al. Outcome predictors of intra-articular glucocorticoid treatment for knee synovitis in patients with rheumatoid arthritis—a prospective cohort study. Arthritis Res Ther 2014; 16:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jie LG, Huang RY, Sun WF, et al. Role of cysteinerich angiogenic inducer 61 in fibroblastlike synovial cell proliferation and invasion in rheumatoid arthritis. Mol Med Rep 2015; 11:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang QC, Huang RY. The cyclooxygenase-2/thromboxane A2 pathway: a bridge from rheumatoid arthritis to lung cancer? Cancer Lett 2014; 354:28–32. [DOI] [PubMed] [Google Scholar]
- 38.Niederer F, Trenkmann M, Ospelt C, et al. Down-regulation of microRNA-34a∗ in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis Rheum 2012; 64:1771–1779. [DOI] [PubMed] [Google Scholar]
- 39.Nakamachi Y, Kawano S, Takenokuchi M, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 2009; 60:1294–1304. [DOI] [PubMed] [Google Scholar]
- 40.Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther 2011; 13:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung SM, Kim KW, Yang CW, et al. Cytokine-mediated bone destruction in rheumatoid arthritis. J Immunol Res 2014; 2014:263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchetverikov I, Kraan MC, van El B, et al. Leflunomide and methotrexate reduce levels of activated matrix metalloproteinases in complexes with alpha2 macroglobulin in serum of rheumatoid arthritis patients. Ann Rheum Dis 2008; 67:128–130. [DOI] [PubMed] [Google Scholar]
- 43.Koenders MI, Marijnissen RJ, Devesa I, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum 2011; 63:2329–2339. [DOI] [PubMed] [Google Scholar]
- 44.Chen SY. MicroRNA-223: a double-edged sword in rheumatoid arthritis. Rheumatol Int 2014; 34:285–286. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya H, Nakasa T, Adachi N, et al. Overexpression of microRNA-223 in rheumatoid arthritis synovium controls osteoclast differentiation. Mod Rheumatol 2013; 23:674–685. [DOI] [PubMed] [Google Scholar]
- 46.Li YT, Chen SY, Wang CR, et al. Brief report: amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum 2012; 64:3240–3245. [DOI] [PubMed] [Google Scholar]
- 47.Stanczyk J, Ospelt C, Karouzakis E, et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum 2011; 63:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen L, Huang F, Ye L, et al. Circulating microRNA predicts insensitivity to glucocorticoid therapy in Graves’ ophthalmopathy. Endocrine 2015. [DOI] [PubMed] [Google Scholar]
- 49.Chatzikyriakidou A, Voulgari PV, Georgiou I, et al. The role of microRNA-146a (miR-146a) and its target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scand J Immunol 2010; 71:382–385. [DOI] [PubMed] [Google Scholar]
- 50.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014; 505:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Necsulea A, Soumillon M, Warnefors M, et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 2014; 505:635–640. [DOI] [PubMed] [Google Scholar]
- 52.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res 2011; 157:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]


