Abstract
Antihypertensives have been linked to new-onset diabetes (NOD) and different classes of antihypertensives may alter the risk for the development of NOD; however, the effect of different antihypertensives on the development of NOD in women with hypertension and coronary artery disease (CAD) has not been well studied. The purpose of this study is to investigate the association between usage of different antihypertensive drugs and the development of NOD in female patients with hypertension and CAD.
Data in this retrospective cohort study were obtained from claim forms submitted to the Taiwan Bureau of National Health Insurance in central Taiwan during the period 2006–2011. We estimated the odds ratios (OR) to approximate the relative risk of NOD development associated with antihypertensive drug use.
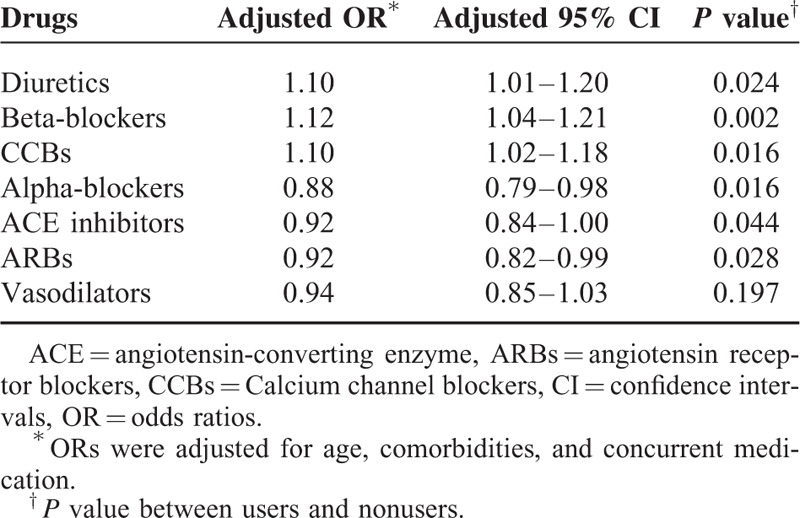
Of the 20,108 female patients with CAD at baseline, 2288 patients developed NOD during the 6-year follow-up. Subjects treated with angiotensin-converting enzyme (ACE) inhibitors (OR, 0.92; 95% confidence interval [CI], 0.84–1.00), angiotensin receptor blockers (OR, 0.92; 95% CI, 0.82–0.99), and alpha-blockers (OR, 0.88; 95% CI, 0.79–0.98) in the adjusted analyses had greater reductions of the risk than among nonusers. Patients who took diuretics (OR, 1.10; 95% CI, 1.01–1.20), beta-blockers (OR, 1.12; 95% CI, 1.04–1.21), and calcium channel blockers (OR, 1.10; 95% CI, 1.02–1.18) were at high risk of developing NOD than nonusers. Vasodilators were not associated with risk of NOD.
We conclude that women with hypertension who take ACE inhibitors, angiotensin receptor blockers, and alpha-blockers are at lower risk of NOD and that use of diuretics, beta-blockers, and calcium channel blockers was associated with a significantly increased risk of developing NOD during the 6-year follow-up.
INTRODUCTION
Diabetes mellitus is a major risk factor for coronary heart disease and contributes significantly to cardiovascular morbidity and mortality both in men and women.1,2 Each year more women than men die from coronary artery diseases (CAD) including myocardial infarction and sudden cardiac death. Studies have shown that the prevalence of diabetes, especially new-onset diabetes (NOD), is increasing in women worldwide.3,4 A number of prospective trials on antihypertensive drug use have investigated whether these agents are associated with the development of NOD in hypertensive patients.5–10 Although the majority of studies found that cardiovascular risk is higher when diabetes and hypertension coexist than when the two conditions stand alone in women, data from these studies are limited because the majority of epideminological studies on NOD have focused on men or on Caucasian populations.10–12 In addition, most studies have investigated only a single class of antihypertensive agent, with angiotensin receptor blockers (ARBs) being the most commonly studied.12,13 Thus, it is not completely clear whether certain antihypertensive drug classes are associated with higher risk for NOD than other antihypertensive drug classes in female patients with CAD.
In this retrospective cohort study, we explored the relationship between antihypertensive drugs (diuretics, beta-blockers, calcium channel blockers [CCBs], alpha-blockers, vasodilators, angiotensin converting enzyme [ACE] inhibitors, ARBs) and the development of NOD in female hypertensive patients with CAD.
METHODS
Subjects
Data were obtained from claim forms provided to the central regional branch of the Bureau of National Health Insurance (BNHI) in Taiwan during the period 2006 through 2011. The BHNI stores information from claim forms in 2 tables: a visit table and a prescription table. Visit tables contain information regarding patient identification numbers, sex, age, 3 diagnostic codes, and medical expenditures, as well as information pertaining to the medical institutions and attending physicians. The prescription table lists the quantity and expenditure for all drugs, operations, and treatments. We summarized the claim records of each patient into 1 record.
Study Design
At baseline (January 1, 2006), we excluded 638 hypertensive patients (International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) codes 401–405) and CAD (ICD-9-CM codes 410–414) because they had diabetes diagnosis (ICD-9-CM code 250) or prescription for antidiabetic drugs between January 1, 2004 and January 1, 2006. A total of 20,293 hypertensive patients without diabetes were included in the study at baseline. Patients were followed-up from study entry until the NOD diagnosis, death, or end of follow-up, whichever occurred first. The end of the follow-up period was December 31, 2011. The primary study outcome was the development of NOD, which was defined as the first time that a diabetes code or antidiabetic prescription appeared in the outpatient claim records. During the 6-year follow-up, we excluded 165 patients who were lost to follow-up or died. Finally, 20,128 patients were enrolled in the analysis (Figure 1). Patients were grouped into 1 of the following 7 mutually exclusive exposure groups defined by ever use of (1) diuretics, (2) beta-blockers, (3) CCBs, (4) alpha-blockers, (5) ACE inhibitors, (6) ARBs, and (7) vasodilators.14 In Taiwan, these antihypertensive drugs are available only by prescription. This study was approved by the Institutional Review Board of the Armed Forces Taichung General Hospital (No. 97018).
FIGURE 1.
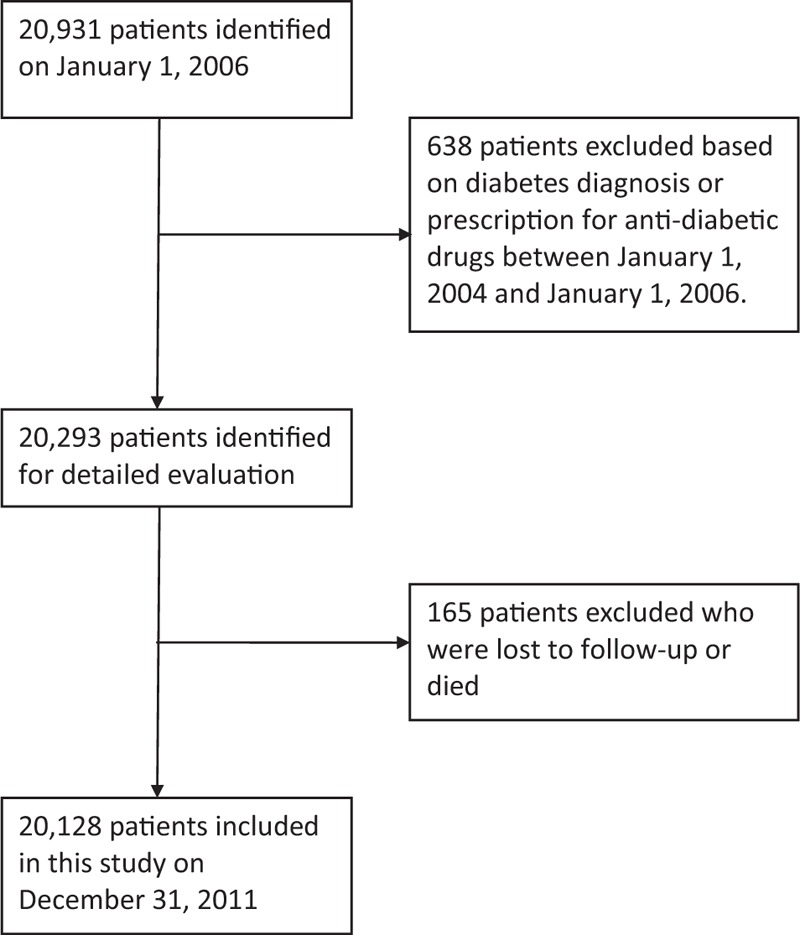
Flowchart of selection of patients for the inclusion in this study.
Statistical Analysis
Data were described with means and standard deviation for normally distributed variables and with frequencies and percentages for categorical variables. The unpaired Student t test or the chi square test were examined for the differences between the NOD group and the non-NOD group in the distribution of demographic characteristics, comorbidities, and concurrent medications. We compared both the drug use and nonuse subjects in order to find out which drug classes might increase or decrease the probability of developing NOD with the Cox regression model, adjusting for age, comorbidities, and concurrent medication.15 All data management and OR calculations were done using the Statistical Analysis System (SAS) software for Windows (version 9.1; SAS Institute, Cary, NC). A P value < 0.05 was considered statistically significant.
RESULTS
Population Characteristics
Of the 20,128 eligible subjects, 2288 (11.4%) developed NOD during the period 2006–2011. The mean age of NOD patients was 64.8 + 13.4 years and that of non-NOD patients was 64.3 ± 13.1 years. There were no significant differences in age between the 2 groups of patients (P = 0.93) (Table 1).
TABLE 1.
Baseline Characteristics of All Patients
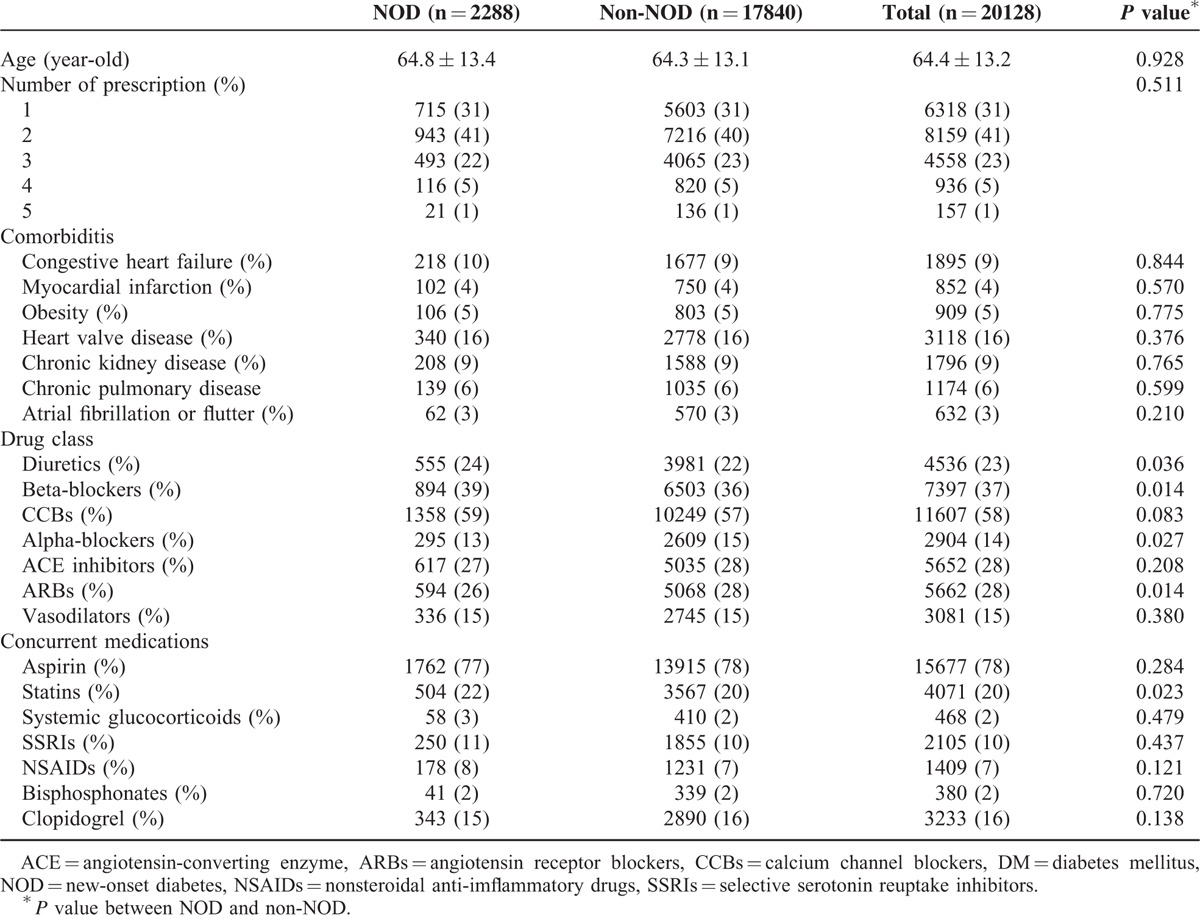
In addition, 31% (6318) of the patients took only 1 drug class, 41% (8159) took 2 drug classes, 23% (4558) took 3 drug classes, 5% (936) took 4 drug classes, and 1 % (157) of patients took 5 drug classes (Table 1). At baseline, there were no significant differences in prevalence of congestive heart failure, myocardial infarction, obesity, heart valve disease, chronic kidney disease, chronic pulmonary disease, and atrial fibrillation or flutter between the 2 groups of patients. Nearly 58% of subjects took CCBs, 37% of subjects took beta-blockers, 28% of subjects took ACE inhibitors and ARBs, 23% of subjects took diuretics, 15% of subjects took vasodilators, and 14% of subjects took an alpha-blocker. NOD subjects took more statins than subjects without NOD, but there were no significant difference in usage of aspirin, systemic glucocorticoids, selective serotonin reuptake inhibitors, nonsteroidal anti-inflammatory drugs, bisphosphonates, or clopidogrel between the 2 groups.
Cox Survival Analysis Adjusted for Age, Comorbidities, and Concurrent Medication
Users of diuretics (OR, 1.10; 95% confidence interval [CI], 1.01–1.20), beta-blockers (OR, 1.12; 95% CI, 1.04–1.21), and CCBs (OR, 1.10; 95% CI, 1.02–1.18) were at significantly higher risk of developing NOD than nonusers after adjusting for age, comorbidities, and concurrent medication usage (P < 0.05). Users of Alpha-blockers (OR, 0.88; 95% CI, 0.79–0.98), ACE inhibitors (OR, 0.92; 95% CI, 0.84–0.90), and ARBs (OR, 0.92; 95% CI, 0.82–0.99) were at a lower risk of developing NOD than nonusers. Vasodilators were not associated with risk of developing NOD (P > 0.05) (Table 2).
TABLE 2.
Incidence of ORs with 95% CIs for New-Onset Diabetes According to Prescriptions for Antihypertensive Drugs Compared with Nonuser Subjects
DISCUSSION
In this population-based longitudinal study, we found that ACE inhibitors, ARBs, and alpha-blockers were independently associated with a decreased risk of developing NOD and that diuretics, beta-blockers, and CCBs were independently associated with an increased risk of developing NOD in women with hypertension and CAD in central Taiwan. Vasodilator usage was not associated with NOD development.
Previous studies have demonstrated that diuretics accelerate the development of NOD in patients with hypertension.16,17 It has been suggested that diuretic therapy has been associated with impaired insulin release through depletion of serum potassium and increase hepatic insulin resistance, resulting in continued hepatic glucose production despite high insulin levels.18,19 Our data are consistent with the results from a large randomized clinical trial showing an increased risk for NOD in individuals taking a diuretic as compared to placebo.20 Similarly, some observational studies have indicated that women taking diuretics have a 10% to 30% higher risk of developing NOD than those not taking diuretic drugs.21,22 Taylor et al reported a significant 20% increased risk of developing NOD in older women and a 45% increased risk of developing NOD in younger women after diuretic treatment respectively.22 In contrast, Padwal et al found no association between the use of thiazide diuretics and NOD.23 However, their study had a mean follow-up period of <1 year and may have lacked statistical power.24
Beta-blockers may worse insulin resistance through reduced cardiac output and peripheral glucose ulitization.19 Therefore, recent evidence suggests that long-term use may increase the risk of NOD.19 Our finding that beta-blocker usage is associated with an increased risk of developing new onset diabetes is similar to that reported in previous studies.22,25 However, other studies have reported that beta-blockers have a neutral effect on risk of NOD in patients with hypertension.20,23 In the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial, which included 9306 patients, the authors reported that there was no association between beta-blocker use and NOD.20 The high risk for diabetes mellitus (impaired glucose intolerance) and the relatively small sample size (5640 patients) in that study may partially explain the discrepancy between our findings and the findings reported in the NAVIGATOR trial.
Calcium channel blockers are generally considered to have a neutral effect on the development of NOD.22,26,27 Many studies have indicated that CCBs are associated with a greater risk of NOD than ACE inhibitors and ARBs but a lower risk of NOD than beta-blockers and thiazide diuretics. Our finding that calcium channel blockers increased the risk of NOD is similar to that reported in the Nurses’ Health Study (NHS) I, which found that older women who took oral calcium channel blockers were at higher risk of developing NOD than women taking placebo.22
ACE inhibitors or angiotensin receptor blockers may improve insulin sensitivity secondary to kinin, prostaglandins or nitric oxide accumulation, and increased peripheral blood flow to skeletal muscle.19 Thus, many studies have shown that blockers of the renin angiotensin system (ACE inhibitors and ARBs) reduce the risk of developing NOD when compared to placebo.28–31 In the present study, both ACE inhibitors and ARBs were found to have protective effects against developing NOD compared to placebo during antihypertensive therapy. A similar finding was reported in the Heart Outcomes Prevention Evaluation (HOPE)28 and NAVIGATOR20 trials. However, the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial failed to show a statistically significant reduction in NOD with the ACE inhibitor ramipril versus placebo in patients with impaired fasting glucose.29 The lack of hypertension as an inclusion criterion and the relatively short follow-up period (3 years) in the DREAM trial might explain why no significant differences in NOD were detected between the 2 groups.
We found that the incidence of NOD was significantly lower among patients who took Alpha-blockers. Previous studies have consistently demonstrated that alpha-blocker classes of antihypertensive medications have protective effects on carbohydrate and lipid metabolism because alpha-blockers may promote peripheral vasodilation and improve insulin sensitivity and glucose uptake.32 However, to the best of our knowledge, no studies have investigated the relationship between alpha-blocker usage and risk of developing NOD in women with hypertension and CAD.
In the present study, vasodilators were found not to be associated with NOD in patients with hypertension. To the best of our knowledge, no studies have evaluated the relationship between vasodilators and NOD.17,22
Our study also has some limitations. First, our data were derived from a health insurance database. Therefore, actual blood sugar levels and some important confounding variables such as body mass index of patients, family history, and smoking status were not available. However, because the data we used were population-based data, we assumed that there were no differences among the 7 antihypertensive groups. Second, the process of insulin resistance in this study of patients who developed NOD must have started many years before the diagnosis and it might have coexisted with the process of hypertension for which antihypertensives were used. The exposure and follow-up period of our study was relatively long and the patients were not new users but current users. In this situation, the cause and effect relationship between antihypertensive agents and NOD development cannot be determined in this study. Third, all diagnoses of diabetes mellitus were based on physician reporting in central Taiwan only; therefore, it is not clear how our findings can be generalized to patients in different areas.
CONCLUSIONS
Our results suggest that ACE inhibitors, ARBs, and alpha-blockers reduce the risk of developing NOD. Our findings could have practical clinical applications for strategies to prevent adverse outcomes in women with hypertension and CAD.
Acknowledgments
This study was supported by the central regional branch of the Bureau of National Health Insurance.
Footnotes
Abbreviations: ACE = angiotensin converting enzyme, ARBs = angiotensin receptor blockers, BNHI = Bureau of National Health Insurance, CAD = coronary artery diseases, CCBs = calcium channel blockers, CI = confidence interval, DM = Diabetes mellitus, DREAM = diabetes reduction assessment with ramipril and rosiglitazone medication, HOPE = heart outcomes prevention evaluation, ICD-9-CM = international classification of diseases, ninth revision clinical modification, NAVIGATOR = nateglinide and valsartan in impaired glucose tolerance outcomes research, NHS = Nurses’ Health Study, NOD = new-onset diabetes, NSAIDs = nonsteroidal anti-imflammatory drugs, OR = odds ratios, SSRIs = Selective serotonin reuptake inhibitors.
Y-SL and H-YC equally contributed to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Park GM, An H, Lee SW, et al. Risk score model for the assessment of coronary artery disease in asymptomatic patients with Type 2 diabetes. Medicine 2015; 94:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalyani RR, Lazo M, Ouyang P, et al. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care 2014; 37:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efird JT, O’Neal WT, Griffin WF, et al. Increased coronary artery disease severity in black women undergoing coronary bypass surgery. Medicine 2015; 94:e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Hera JM, García-Ruiz JM, Martínez-Camblor P, et al. Real incidence of diabetes mellitus in a coronary disease population. Am J Cardiol 2012; 111:333–338. [DOI] [PubMed] [Google Scholar]
- 5.Rajala U, Qiao Q, Laakso M, et al. Antihypertensive drugs as predictors of type 2 diabetes among subjects with impaired glucose tolerance. Diabetes Res Clin Pra 2000; 50:231–239. [DOI] [PubMed] [Google Scholar]
- 6.Liou YS, Ma T, Tien L, et al. The relationship between antihypertensive combination therapies comprising diuretics and/or beta-blockers and the risk of new-onset diabetes: a retrospective longitudinal cohort study. Hypert Res 2009; 32:231–239. [DOI] [PubMed] [Google Scholar]
- 7.ALLHAT officers and coordinators for the ALLHAT collaborative research group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 8.Pepine CJ, Handberg M, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): A randomized controlled trial. JAMA 2003; 290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 9.Dahlof BE, Devereux RB, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906. [DOI] [PubMed] [Google Scholar]
- 10.Vardeny O, Uno H, Braunwald E, et al. Opposing effects of β blockers and angiotensin-converting enzyme inhibitors on development of new-onset diabetes mellitus in patients with stable coronary artery disease. Am J Cardiol 2011; 107:1705–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogihara T, Fujimoto A, Nakao K, et al. ARB candesartan and CCB amlodipine in hypertensive patients: the CASE-J trial. Expert Rev Cardiovasc Ther 2008; 6:1195–1201. [DOI] [PubMed] [Google Scholar]
- 12.Weycker D, Edelsberg J, Vincze G, et al. Risk of diabetes in a real-world setting among patients initiating antihypertensive therapy with valsartan or amlodipine. J Human Hypert 2007; 21:374–380. [DOI] [PubMed] [Google Scholar]
- 13.Chang CH, Chang YC, Wu LC, et al. Different angiotensin receptor blockers and incidence of diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol 2014; 13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liou YS, Ma T, Tien L, et al. The relationship between antihypertensive combination therapies comprising diuretics and/or beta-blockers and the risk of new-onset diabetes—A retrospective longitudinal cohort study. Hypert Res 2009; 32:496–499. [DOI] [PubMed] [Google Scholar]
- 15.Jong GP, Chang MH, Tien L, et al. Antihypertensive drugs and new-onset diabetes: a retrospective longitudinal cohort study. Cardiovasc Ther 2009; 27:159–163. [DOI] [PubMed] [Google Scholar]
- 16.Zidek W, Schrader J, Lüders S, et al. Ramipril-based versus diuretic-based antihypertensive primary treatment in patients with pre-diabetes (ADaPT) study. Cardiovasc Diabetol 2012; 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott WJ. Differential effects of antihypertensive drugs on new-onset diabetes. Curr Hypertens Rep 2005; 7:249–256. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee R, Yeh HC, Edelman D, et al. Potassium and risk of Type 2 diabetes. Expert Rev Endocrinol Metab 2011; 6:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn DF, Wilson TW. Antihypertensive medications and blood sugar: theories and implications. Can J Cariol 2006; 22:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen L, Shah BR, Reyes EM, et al. Role of diuretics, β blockers, and statins in increasing the risk of diabetes in patients with impaired glucose tolerance: reanalysis of data from the NAVIGATOR study. BMJ 2013; 347:f6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barzilay JI, Davis BR, Cutler JA, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Int Med 2006; 166:2191–2201. [DOI] [PubMed] [Google Scholar]
- 22.Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care 2006; 29:1065–1070. [DOI] [PubMed] [Google Scholar]
- 23.Padwal R, Mamdani M, Alter DA, et al. Antihypertensive therapy and incidence of type 2 diabetes in an elderly cohort. Diabetes Care 2004; 27:2458–2463. [DOI] [PubMed] [Google Scholar]
- 24.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003. [DOI] [PubMed] [Google Scholar]
- 25.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007; 369:201–207. [DOI] [PubMed] [Google Scholar]
- 26.Padwal R, Laupacis A. Antihypertensive Therapy and incidence of type 2 diabetes: a systematic review. Diabetes Care 2004; 27:247–255. [DOI] [PubMed] [Google Scholar]
- 27.Fukao K, Shimada K, Hiki M, et al. Effects of calcium channel blockers on glucose tolerance, inflammatory state, and circulating progenitor cells in non-diabetic patients with essential hypertension: a comparative study between azeldipine and amlodipine on glucose tolerance and endothelial function—a crossover trial (AGENT). Cardiovasc Diabetol 2011; 10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stohr R, Marx N. Renin-angiotensin-aldosterone system antagonists and the prevention of type 2 diabetes mellitus. Curr Pharm Design 2012; 18:958–962. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb SS. Selection of antihypertensive agents in patients at risk for diabetes. Curr Hypertens Rep 2005; 7:461–465. [DOI] [PubMed] [Google Scholar]
- 30.Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. New Engl JMed 2006; 355:1551–1562. [DOI] [PubMed] [Google Scholar]
- 31.Barzilay JI, Gao P, Rydén L, et al. Effects of telmisartan on glucose levels in people at high risk for cardiovascular dsease but free from diabetes: the TRANSCEND study. Diabetes Care 2011; 34:1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heran BS, Galm BP, Wright JM. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database Syst Rev 2012; 15:004643. [DOI] [PMC free article] [PubMed] [Google Scholar]


