Abstract
Peripheral neuropathy and inflammatory reactions of the central nervous system may accompany rheumatoid arthritis (RA). Inflammatory processes play a critical role in epilepsy. Therefore, we conducted this study to determine the risk of epilepsy in patients with RA.
The RA cohort comprised patients ages 20 years and older who were newly diagnosed with RA between 2000 and 2011, with data obtained from the Registry of Catastrophic Illnesses Patient Database. Patients without RA were frequency matched with an RA cohort at a 1:1 ratio according to age, sex, and year of RA diagnosis.
The overall crude hazard ratio (HR) for epilepsy was 1.27-fold higher in the RA cohort compared with that in the controls. After adjustment for age, sex, comorbidities, and medications, the patients with RA were associated with an increased risk of epilepsy compared with those without RA (adjusted HR [aHR] = 1.52, 95% confidence interval [CI] = 1.12–2.07). Compared with the RA patients with ≤ 560 days of nonsteroidal anti-inflammatory drug (NSAID) use, the RA patients with 1181 to 2145 and >2145 days of NSAID use had a significantly lower risk of epilepsy (aHR = 0.35, 95% CI = 0.24–0.52 and aHR = 0.15, 95% CI = 0.09–0.24, respectively).
This study provides compelling evidence of an increased risk of epilepsy in patients with RA. The period of NSAID treatment is negatively associated with the risk of epilepsy in RA patients.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease that may cause deformed joints, synovial damage, and severe disability.1,2 In the United States, RA affects 1.3 million adults with physical disabilities.3,4 The prevalence of RA in Taiwan is similar to that reported in Caucasian patients.5 Several studies have indicated that peripheral neuropathy and inflammatory reactions of the central nervous system (CNS) may accompany RA, such as autoimmune, neurodegenerative, and carpal tunnel syndromes.6,7 Furthermore, increasing evidence has shown that inflammatory processes play a critical role in epilepsy,8–10 which is one of the most common and chronic neurological disorders and causes severe physical and psychological complications.11–13 There were more than 50 million people worldwide with epilepsy.14–16 Both RA and epilepsy have been associated with an increased risk of stroke, ischemic heart disease, and hypertension.17–20 Nevertheless, evidence of a direct association between RA and epilepsy remains lacking. Therefore, we conducted this study to determine the risk of epilepsy in patients with RA.
METHODS
Data Sources
Data were obtained from the National Health Insurance Research Database (NHIRD), which contains claims data from 1996 to 2011 related to inpatient care, ambulatory care, dental care, prescription drugs, and costs. Taiwan implemented the compulsory single-payer National Health Insurance (NHI) program in 1995, and it now covers over 99% of the 23.75 million residents of Taiwan.21 All of the data are confidential and all patients are anonymous and unidentified, thus ensuring that the NHI reimbursement data are suitable for public research. Similar identification numbers are encrypted to all data files for linking data according to privacy protocols. For this retrospective cohort study, we used a subset of the NHIRD including files from the Registry of Catastrophic Illnesses Patient Database (RCIPD) and Longitudinal Health Insurance Database 2000 (LHID2000). International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to define diseases in the NHIRD. The Ethical Review Board of China Medical University approved this study (CMU-REC-101-012).
Sampled Patients
The RA cohort comprised patients ages 20 years and older who were newly diagnosed with RA (ICD-9-CM Code 714) between 2000 and 2011 from the RCIPD. The RCIPD was established to track patients with major or catastrophic illnesses, including cancer, end-stage renal disease, mental illness, congenital illness, and several autoimmune diseases such as RA. To validate the diagnosis of the patients, the Bureau of NHI routinely reviews the original medical charts of all patients who apply for catastrophic illness registration. The RA diagnosis date was defined as the index date. Patients with a history of epilepsy (ICD-9-CM Code 345) before the index date or with incomplete medical information were excluded. All patients without a history of RA were randomly selected from the LHID2000. Patients without RA were frequency matched with the RA cohort at a 1:1 ratio according to age (in 5-year bands), sex, and year of RA diagnosis by using the same exclusion criteria.
Outcome
Each patient was followed from the index date until epilepsy diagnosis, withdrawal from the NHI program, censoring because of death, or the end date of the database (December 31, 2011).
Comorbidities and Medications
Baseline comorbidities were analyzed as follows: diabetes (ICD-9-CM Code 250), hypertension (ICD-9-CM Codes 401–405), hyperlipidemia (ICD-9-CM Code 272), head injury (ICD-9-CM Codes 310.2, 800, 801, 803, 804, 850, 851, 853, and 854), coronary artery disease (CAD) (ICD-9-CM Codes 410–414), chronic obstructive pulmonary disease (COPD) (ICD-9-CM Codes 491, 492, and 496), stroke (ICD-9-CM Codes 430–438), and autoimmune disease (ICD-9-CM 710.0, 710.1, 710.2, 710.3, 710.4, and 696.0). In addition, nonsteroidal anti-inflammatory drug (NSAID) use, steroidal drug use, aspirin use, opioid use, and various biologic therapies (including azathioprine, cyclosporin, immunoglobulin, mycophenolate, and tacrolimus) were analyzed between the RA patients and the controls.
Statistical Analysis
The distributions of categorical characteristics between the RA cohort and the controls were described and compared using Pearson's chi-squared test for categorical variables and a 2-sample t test for continuous variables. The cumulative incidence of epilepsy between the RA and control cohorts was assessed using the Kaplan–Meier method, and the differences were assessed using a log-rank test. The incidence densities (per 1000 person-year) of epilepsy were estimated according to various risk factors. Univariate and multivariate Cox-proportional hazard regression models were adopted to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of developing epilepsy. In addition, the Cox model was used to assess the risk of epilepsy development in the RA cohort compared with the control cohort. In further analysis, we evaluated the effect of NSAID use duration according to the quartile of cumulative use per day of NSAIDs (≤560, 561–1180, 1181–2145, and >2145 days) on the risk of epilepsy in the patients with RA. All of the data analyses were performed using SAS for Windows (Version 9.4; SAS Institute, Inc., Cary, NC). A 2-tailed P value of 0.05 was considered statistically significant.
RESULTS
Demographic Characteristics, Comorbidities, and Medications in RA and Control Cohorts
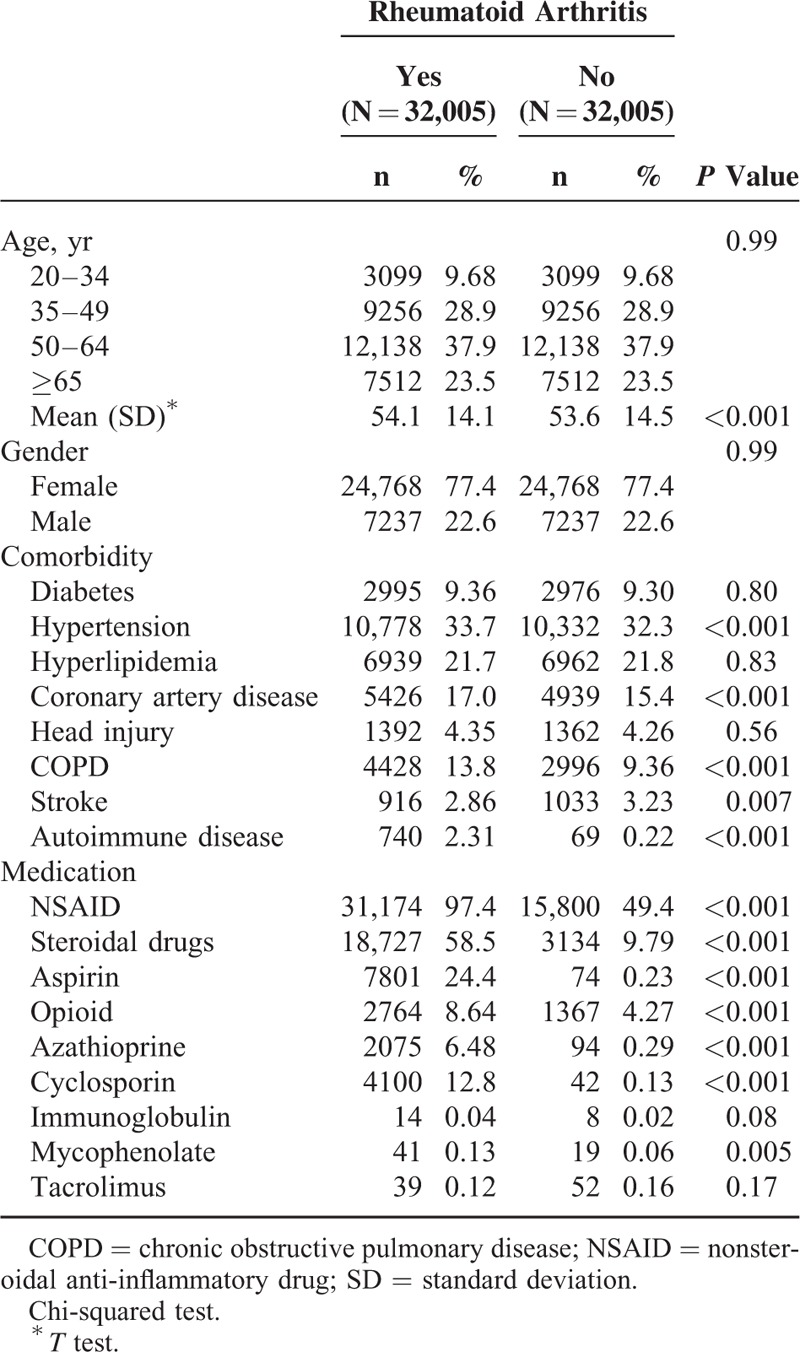
The demographic characteristics and comorbidities of the RA and control cohorts are shown in Table 1. No statistical difference was observed in the distributions of age and sex between the RA cohort and controls (mean age of approximately 54 years). Among the RA patients, 61.4% were older than 50 years, and 77.4% were women. Compared with the control cohort, the RA patients exhibited a higher prevalence of hypertension, hyperlipidemia, CAD, COPD, and autoimmune disease (P < 0.001). Most medications were more prevalent in the RA cohort at the baseline, compared with the control cohort (P < 0.05), except immunoglobulin use and tacrolimus use.
TABLE 1.
Characteristics of Patients Between Patients With and Patients Without Rheumatoid Arthritis
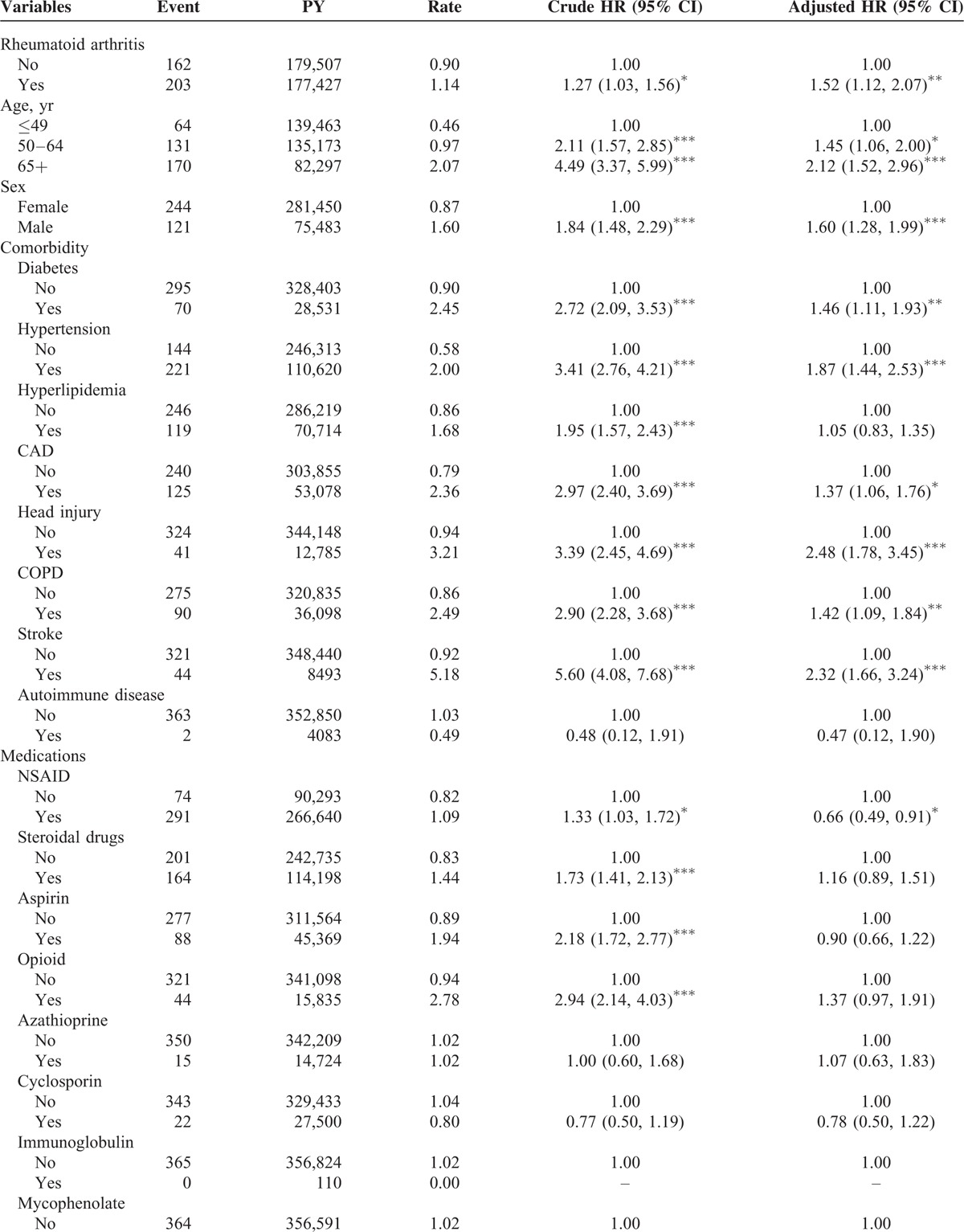
Cox Model Analysis of Risk Factors Affecting Epilepsy Development
The overall incidence of epilepsy was 1.27-fold higher in the RA cohort compared with that in the controls (1.14 vs 0.90 per 1000 person-year, 95% CI = 1.03–1.56) (Table 2 ). After adjustment for age, sex, comorbidities, and medications, the patients with RA were associated with an increased risk of epilepsy compared with those without RA (adjusted HR [aHR] = 1.52, 95% CI = 1.12–2.07). Epilepsy incidence increased with age. Compared with the patients ages 49 years and younger, the risk of epilepsy development was 1.45-fold higher in the patients ages 50 to 64 years (95% CI = 1.06–2.00) and 2.12-fold higher in those ages 65 years and older (95% CI = 1.52–2.96), after adjustment for potential risk factors. Men had a higher incidence of epilepsy than that of women (1.60 vs 0.87 per 1000 person-year, respectively). The risk of developing epilepsy was 1.60-fold higher for the men compared with that for the women (95% CI = 1.28–1.99). The incidence of epilepsy was increased in the patients with comorbidity. A higher risk of epilepsy was present for the patients with comorbidities of diabetes (aHR = 1.46, 95% CI = 1.11–1.93), hypertension (aHR = 1.87, 95% CI = 1.44–2.53), CAD (aHR = 1.37, 95% CI = 1.06–1.76), head injury (aHR = 2.48, 95% CI = 1.78–3.45), COPD (aHR = 1.42, 95% CI = 1.09–1.84), and stroke (aHR = 2.32, 95% CI = 1.66–3.24). The risk of developing epilepsy was 0.66-fold for the patients with NSAID use compared with the patients without NSAID use (95% CI = 0.49–0.91).
TABLE 2.
Incidence and Hazard Ratio for Epilepsy and Epilepsy-Associated Risk Factor
Incidence and HRs of Epilepsy Stratified by Sex, Age, Comorbidity, and Medications: Comparison Between RA and Control Cohorts
When the analyses were stratified according to sex, the relative risk of epilepsy development was significantly higher in the female RA patients compared with the female patients without RA (aHR = 1.59, 95% CI = 1.10–2.31) (Table 3). In the RA cohort with NSAID use, the patients had a 46% higher risk of epilepsy compared with the control cohort with NSAID use (95% CI = 1.06–2.03). In the RA cohort with aspirin use, the patients had 0.26-fold risk of epilepsy compared with the control cohort with aspirin use (95% CI = 0.08–0.87). Among the nonmedication patients, the RA patients still had a greater risk of epilepsy than that of the controls except for non-NSAID use.
TABLE 2 (Continued).
Incidence and Hazard Ratio for Epilepsy and Epilepsy-Associated Risk Factor
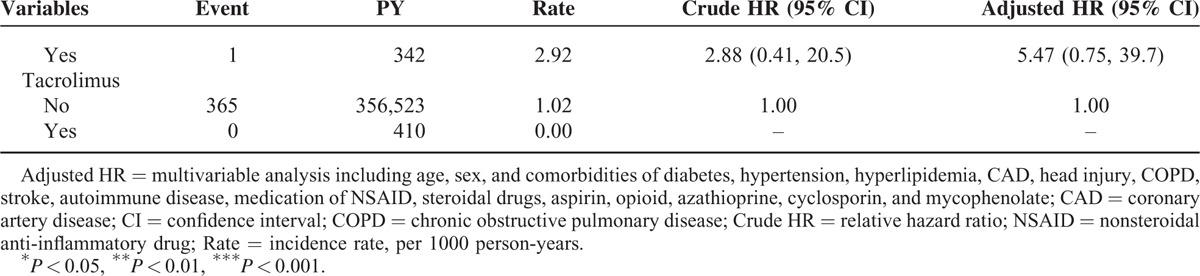
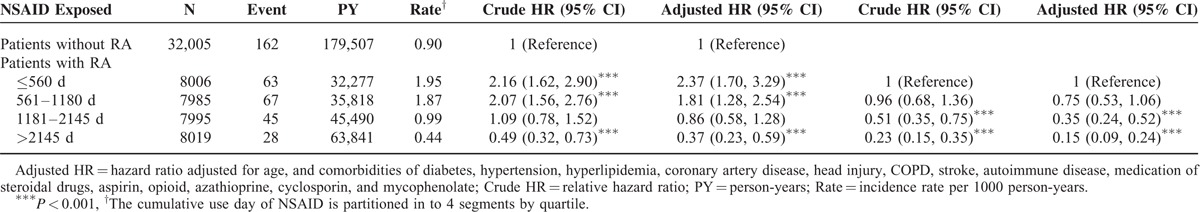
Incidence and HRs of Epilepsy Stratified by Duration of NSAID: Comparison Between RA and Control Cohorts
Compared with the patients without RA, the RA patients with ≤560 days of NSAID use demonstrated a significantly higher risk of epilepsy (aHR = 2.37, 95% CI = 1.70–3.29), followed by the RA patients with 561 to 1180 days of NSAID use, who exhibited an aHR of 1.81 (95% CI = 1.28–2.54). Specifically, the risk of epilepsy was significantly lower (aHR = 0.37, 95% CI = 0.23–0.59) in the RA patients with >2145 days of NSAID use than in the control cohort. The risk of epilepsy was markedly lower when the RA patients had a longer NSAID treatment duration. Compared with the RA patients with ≤560 days of NSAID use, the RA patients with 1181 to 2145 and >2145 days of NSAID use had a significantly lower risk of epilepsy (aHR = 0.35, 95% CI = 0.24–0.52 and aHR = 0.15, 95% CI = 0.09–0.24, respectively).
Cumulative Incidence of Epilepsy in the RA and Control Cohorts
For the mean follow-up period of 5.54 years for the RA cohort and 5.61 years for the control cohort, the Kaplan–Meier analysis results showed that the cumulative incidence curves for epilepsy were significantly higher for the RA cohort than for the control cohort (log-rank test P < 0.001; Figure 1).
FIGURE 1.
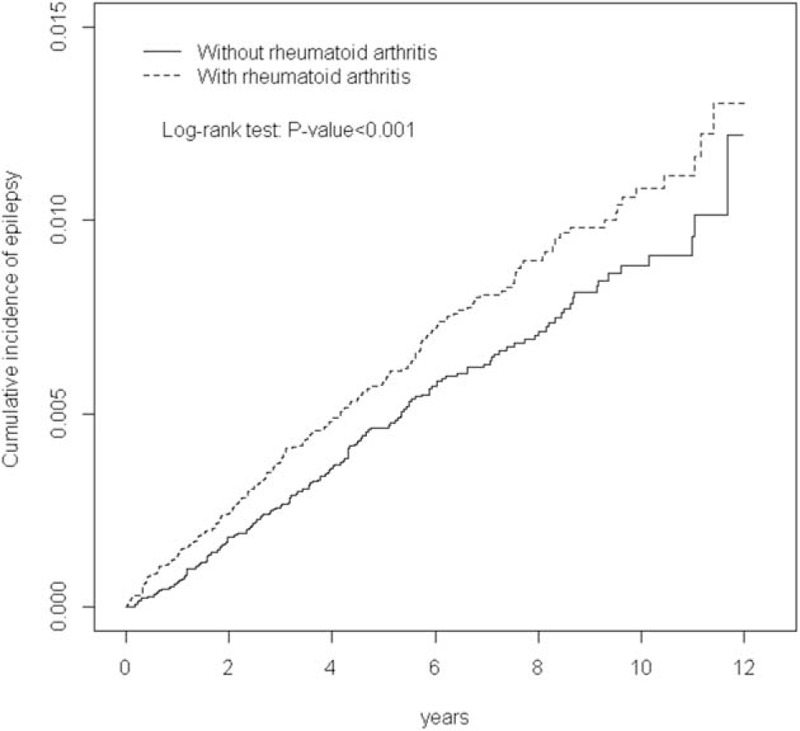
Cumulative incidence of epilepsy compared between patients with and without rheumatoid arthritis.
DISCUSSION
The major findings indicate that the RA patients exhibited a considerably higher risk of epilepsy occurrence and that the risk of epilepsy and duration of NSAID treatment for RA are negatively associated. The processes and mechanisms leading to the generation of epilepsy are not fully understood; however, some patients develop epilepsy because of stroke, brain tumor, and traumatic brain injury.22–24 Several studies have indicated that chronic inflammation is a possible mechanism increasing the risk of epilepsy. RA is an autoimmune disease that entails chronic inflammation. Cytokines play a crucial role in the activation of innate immunity and the transition to adaptive immunity, and they are released by immunocompetent and endothelial cells, or by glia and neurons in the CNS.25,26 Evidence indicates that inflammation in the CNS is associated with epilepsy.27
Another possible linkage is pain, which is a major symptom in patients with RA.28 In the past few decades, several studies have indicated that the cerebral cortex and its extensive cortical network are involved in pain processing.29–31 Studies have revealed that the mechanism of seizures involves variation in the circuitry between the thalamus and cerebral cortex.29,32 The pain in RA patients may induce seizures caused by unusual electrical activity in the brain. As shown in Table 4, we found a negative association between the duration of NSAID use and the risk of epilepsy. This dose response association is consistent with our hypotheses.
TABLE 3.
Incidence and Hazard Ratio of Epilepsy Between Patients With and Without Rheumatoid Arthritis
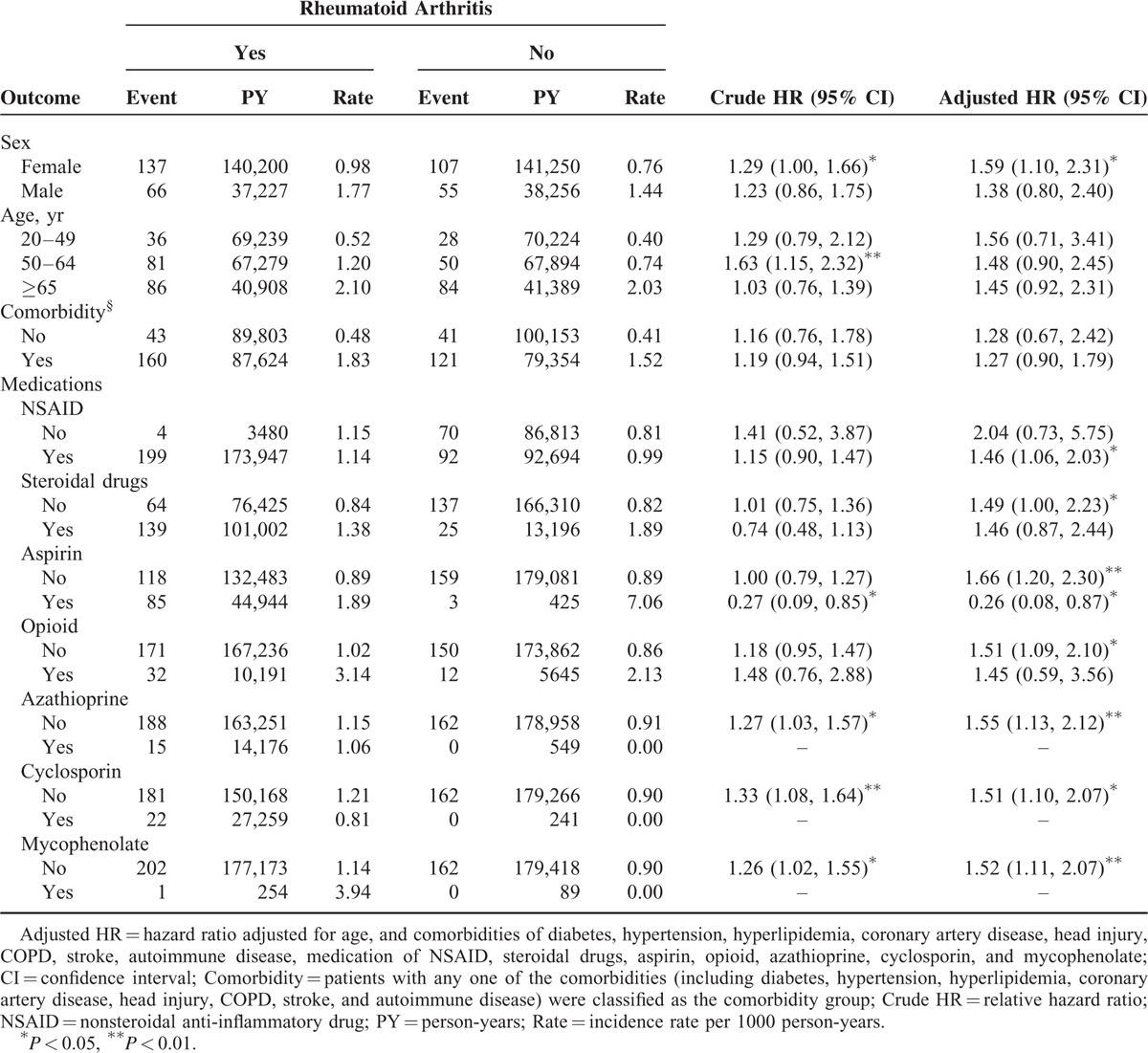
TABLE 4.
Incidence and Adjusted Hazard Ratio of Epilepsy Stratified by Duration of NSAID Between Patients With and Without Rheumatoid Arthritis
However, whether these two mechanisms increase the risk of epilepsy in patients with RA could not be determined because of the limitations of the NHIRD used in this study. Therefore, additional clinical and experimental studies are warranted.
In this study, all RA patients in Taiwan were included in the current databases. The prevalence of RA was approximately 1%, and the RA prevalence in women was 3-fold higher than that in the men. The results are similar to those of other studies.33–36 Furthermore, the RA patients had a significantly higher frequency of hypertension, hyperlipidemia, CAD, COPD, and stroke compared with the healthy control group. These findings are consistent with those of previous studies.19,20,37
In addition to age and sex, we considered the influences of covariates, such as diabetes, hypertension, hyperlipidemia, stroke, head injury, and stroke, which were considered to be associated with both the risk of RA and epilepsy.38–40 Furthermore, evidence has suggested that cigarette smoking increases both the risk of RA and epilepsy.41,42 Data on health behavior are limited in the NHIRD. In other NHIRD studies, the general approach has been to use COPD as a proxy predictor of cigarette smoking.43,44
This epidemiological study on epilepsy in Taiwan was limited. This was the first nationwide study to examine the association regarding the increasing risk of epilepsy in RA patients. First, the strength of this study was that all RA patients in Taiwan were included from current databases that contain data such as those related to inpatient care, ambulatory care, dental care, prescription drugs, and costs. Taiwan has only one compulsory social insurance program. Approximately 99% of the 23.74 million residents of Taiwan are enrolled in the NHI program. Second, based on the low prevalence of RA, we included a large number of RA patients. Another national study showed that the total number of RA patients in Taiwan was 30,504 between 1996 and 200829; therefore, in this study, we included 32,005 RA patients. Third, we conducted this study with a long follow-up period (1996–2011), thus enabling possible epilepsy occurrence to be assessed. In Taiwan, 99% of patients with RA were available in the RCPID. They might have had more clinical visits than did those without RA, thus resulting in a higher prevalence of comorbidity for RA patients. In this study, the potential bias due to the unequal chances of clinical visits might not occur with a long follow-up period.
Furthermore, we determined the potential limitations of this study. Blood tests, electroencephalograms, and neuroimaging are generally performed in RA and epilepsy diagnoses. Although the clinical data in the NHIRD are limited, we ascertained RA and epilepsy according to ICD-9-CM codes, which are determined by fully trained physicians; the RA and epilepsy diagnosis data in the NHIRD are reliable and have been widely used in previous studies. Finally, one of the major limitations of the NHIRD is the lack of information on cigarette smoking and alcohol consumption. We believe that the confounding effects of cigarette smoking and alcohol consumption were minimal because RA is a gender-specific disease, and the prevalence of cigarette smoking and alcohol consumption in women is significantly lower than that in men in Taiwan.45 In addition, other risk factors that might be associated with risk of epilepsy, such as diet, medications, environment, and familiar risks, were not available in the NHIRD.
In conclusion, this study provides compelling evidence and the possible mechanisms for the increased risk of epilepsy in patients with RA. RA patients are encouraged to seek treatment for pain relief as early as possible to help lower the risk of epilepsy, thereby slowing epilepsy progression with a longer period of NSAID use. Based on the limitations, additional clinical studies are warranted to investigate the underlying mechanisms of the association between RA and epilepsy.
Footnotes
Abbreviations: BMD = bone mineral density, CIs = confidence intervals, CO = carbon monoxide, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, HRs = hazard ratios, HT = hypertension, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IHD = ischemic heart disease, IRR = incidence rate ratio, NHIRD = National Health Insurance Research Database.
Author contributions: All authors have contributed significantly, and all authors are in agreement with the content of the manuscript. Study concept and design: K-HC and C-HK; acquisition of data: all authors; analysis and interpretation of data: K-HC, C-LL, and C-HK; drafting of the manuscript: all authors; critical revision of the manuscript for intellectual content: K-HC, C-LL, and C-HK; statistical analysis: C-LL; obtained funding: C-HK; administrative, technical, or material support: all authors; study supervision: C-HK.
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for the Top University Plan of the Ministry of Education, Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005; 4:130–136. [DOI] [PubMed] [Google Scholar]
- 2.Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med 2007; 120:936–939. [DOI] [PubMed] [Google Scholar]
- 3.Margaretten M, Barton J, Julian L, et al. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011; 63:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum 2008; 58:15–25. [DOI] [PubMed] [Google Scholar]
- 5.Chou CT, Pei L, Chang DM, et al. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol 1994; 21:302–306. [PubMed] [Google Scholar]
- 6.Bathon JM, Moreland LW, DiBartolomeo AG. Inflammatory central nervous system involvement in rheumatoid arthritis. Semin Arthritis Rheum 1989; 18:258–266. [DOI] [PubMed] [Google Scholar]
- 7.Thomson CA, McColl A, Cavanagh J, et al. Peripheral inflammation is associated with remote global gene expression changes in the brain. J Neuroinflammation 2014; 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis N, Auvin S. Inflammation and epilepsy in the developing brain: clinical and experimental evidence. CNS Neurosci Ther 2015; 21:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amhaoul H, Staelens S, Dedeurwaerdere S. Imaging brain inflammation in epilepsy. Neuroscience 2014; 279:238–252. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox KS, Vezzani A. Does brain inflammation mediate pathological outcomes in epilepsy? Adv Exp Med Biol 2014; 813:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med 2003; 349:1257–1266. [DOI] [PubMed] [Google Scholar]
- 12.Vezzani A. Inflammation and epilepsy. Epilepsy Curr 2005; 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer HM, Mula M, Sander JW. The global burden and stigma of epilepsy. Epilepsy Behav 2008; 12:540–546. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Epilepsy in the WHO Africa Region, Bridging the Gap: The Global Campaign Against Epilepsy “Out of the Shadows.” Geneva: WHO; 2004. [Google Scholar]
- 15.Ngugi AK, Bottomley C, Kleinschmidt I, et al. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010; 51:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011; 52 Suppl 7:2–26. [DOI] [PubMed] [Google Scholar]
- 17.Jetter GM, Cavazos JE. Epilepsy in the elderly. Semin Neurol 2008; 28:336–341. [DOI] [PubMed] [Google Scholar]
- 18.Lopinto-Khoury C, Mintzer S. Antiepileptic drugs and markers of vascular risk. Curr Treat Options Neurol 2010; 12:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon DH, Goodson NJ, Katz JN, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 2006; 65:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liou TH, Huang SW, Lin JW, et al. Risk of stroke in patients with rheumatism: a nationwide longitudinal population-based study. Sci Rep 2014; 4:5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Database NHIR. Taiwan, http://nhird.nhri.org.tw/en/index.html (cited in 2015). [Google Scholar]
- 22.Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol 2005; 4:21–31. [DOI] [PubMed] [Google Scholar]
- 23.Ngugi AK, Bottomley C, Kleinschmidt I, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case–control studies. Lancet Neurol 2013; 12:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osakwe C, Otte WM, Alo C. Epilepsy prevalence, potential causes and social beliefs in Ebonyi State and Benue State, Nigeria. Epilepsy Res 2014; 108:316–326. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 2002; 3:216–227. [DOI] [PubMed] [Google Scholar]
- 26.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344:907–916. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox KS, Vezzani A. Does brain inflammation mediate pathological outcomes in epilepsy? Adv Exp Med Biol 2014; 813:169–183. 10.1007/978-94-017-8914-1_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenna F, Wright V. Pain and rheumatoid arthritis. Ann Rheum Dis 1985; 44:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie YF, Huo FQ, Tang JS. Cerebral cortex modulation of pain. Acta Pharmacol Sin 2009; 30:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones AK, Derbyshire SW. Reduced cortical responses to noxious heat in patients with rheumatoid arthritis. Ann Rheum Dis 1997; 56:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crofford LJ, Sano H, Karalis K, et al. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol 1993; 151:1587–1596. [PubMed] [Google Scholar]
- 32.Schrader J, Wahl M, Kuschinsky W, et al. Increase of adenosine content in cerebral cortex of the cat during bicuculline-induced seizure. Pflugers Arch 1980; 387:245–251. [DOI] [PubMed] [Google Scholar]
- 33.Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritis—underscoring the problem. Rheumatology (Oxford) 2006; 45:1325–1327. [DOI] [PubMed] [Google Scholar]
- 34.Symmons D, Turner G, Webb R, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford) 2002; 41:793–800. [DOI] [PubMed] [Google Scholar]
- 35.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 2009; 11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang WK, Chiou MJ, Kuo CF, et al. No overall increased risk of cancer in patients with rheumatoid arthritis: a nationwide dynamic cohort study in Taiwan. Rheumatol Int 2014; 34:1379–1386. [DOI] [PubMed] [Google Scholar]
- 37.Fischer LM, Schlienger RG, Matter C, et al. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol 2004; 93:198–200. [DOI] [PubMed] [Google Scholar]
- 38.De Reuck J, Goethals M, Vonck K, et al. Clinical predictors of late-onset seizures and epilepsy in patients with cerebrovascular disease. Eur Neurol 2005; 54:68–72. [DOI] [PubMed] [Google Scholar]
- 39.Ohman J. Hypertension as a risk factor for epilepsy after aneurysmal subarachnoid hemorrhage and surgery. Neurosurgery 1990; 27:578–581. [DOI] [PubMed] [Google Scholar]
- 40.Isojärvi JI, Laatikainen TJ, Knip M, et al. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 1996; 39:579–584. [DOI] [PubMed] [Google Scholar]
- 41.Cassano PA, Koepsell TD, Farwell JR. Risk of febrile seizures in childhood in relation to prenatal maternal cigarette smoking and alcohol intake. Am J Epidemiol 1990; 132:462–473.discussion 474–478. [DOI] [PubMed] [Google Scholar]
- 42.Hazes JM, Dijkmans BA, Vandenbroucke JP, et al. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis 1990; 49:980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang KH, Chung CJ, Lin CL, et al. Increased risk of dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age (Dordr) 2014; 36:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang KH, Chang MY, Muo CH, et al. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS ONE 2014; 9:e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuang YC, Chuang KY. Gender differences in relationships between social capital and individual smoking and drinking behavior in Taiwan. Soc Sci Med 2008; 67:1321–1330. [DOI] [PubMed] [Google Scholar]


