Abstract
The aim of this study was to evaluate the imaging features of testicular adrenal rest tumors (TARTs) on baseline ultrasound (BUS).
The imaging features of 30 TART lesions pathologically or clinically confirmed in 15 patients who had undergone BUS were evaluated, and the sonographic characteristics of the lesions were analyzed.
All 15 cases were bilateral and located near the testicular mediastinum. Approximately 56.7% (17/30) of the TART lesions exhibited homogeneous hypoechogenicity, 36.7% (11/30) of the lesions exhibited heterogeneous hypoechogenicity, and 6.6% (2/30) of the lesions exhibited heterogeneous isoechogenicity. In addition, 76.7% (23/30) of the lesions exhibited a rich blood supply, whereas 23.3% (7/30) of the lesions exhibited a scarce blood supply.
The sonographic characteristics of the TARTs were bilateral growth, location adjacent to the testicular mediastinum, hypoechogenicity, and rich blood supply, which may play important roles in early clinical diagnosis.
INTRODUCTION
Testicular adrenal rest tumors (TARTs) are benign intratesticular masses that occur in male patients with congenital adrenal hyperplasia (CAH), with more than 90% of cases caused by a deficiency of 21-α-hydroxylase.1,2 TARTs originate from aberrant adrenal cells in the testes and can impair both spermatogenesis and endocrine testicular function. Studies have indicated that TARTs may be present in childhood, with an increasing prevalence after the onset of puberty.3,4 Therefore, the early detection of TARTs is important to preserve testicular function, and scrotal baseline ultrasound (BUS) screening is recommended beginning in early childhood. Although several reports of TART sonographic features have been described previously, the reports are limited by a small numbers of cases5–7 or the old age of the patients.8,9 This situation prompted us to investigate the sonographic features of TARTs during puberty, evaluating patients no older than 18 years.
PATIENTS AND METHODS
Patients
From June 2009 to January 2015, 79 male patients had been diagnosed as CAH and screened for testicular evaluation in our institute, and only 15 patients had TARTs in this study. The prevalence of TARTS in our patient population was 19% (15/79), which was all bilateral, without unilateral. Written informed consent was obtained from all patients, and the study was approved by the first affiliated hospital of Sun Yat-Sen University Institutional Review Board.
Ultrasound Equipment and BUS Examination
Two ultrasound machines were used in this study depending on their availability. The first machine was an High Density Interconnector 5000 US machine (Phillips Healthcare, Tokyo, Japan) equipped with a transducer with a frequency range of 7.0 to12.0 MHz. The other was an Aplio XV machine (Toshiba Medical Systems, Tokyo, Japan) equipped with a transducer with a frequency range of 7.5 to 10.0 MHz.
All BUS examinations were performed by 1 of 2 experienced radiologists who had more than 3 years of experience in diagnosing andrological diseases. The entire testicle was scanned thoroughly using BUS, and the target lesions were identified. The location, size, shape, boundary, and echogenicity of the lesion, as well as the testicular size and the presence or absence of the testicular mediastinum were recorded. Afterwards, the transducer was maintained in a stable position and shifted to the color Doppler imaging mode, and the blood supply of the lesions was recorded.10
RESULTS
Patient Characteristics
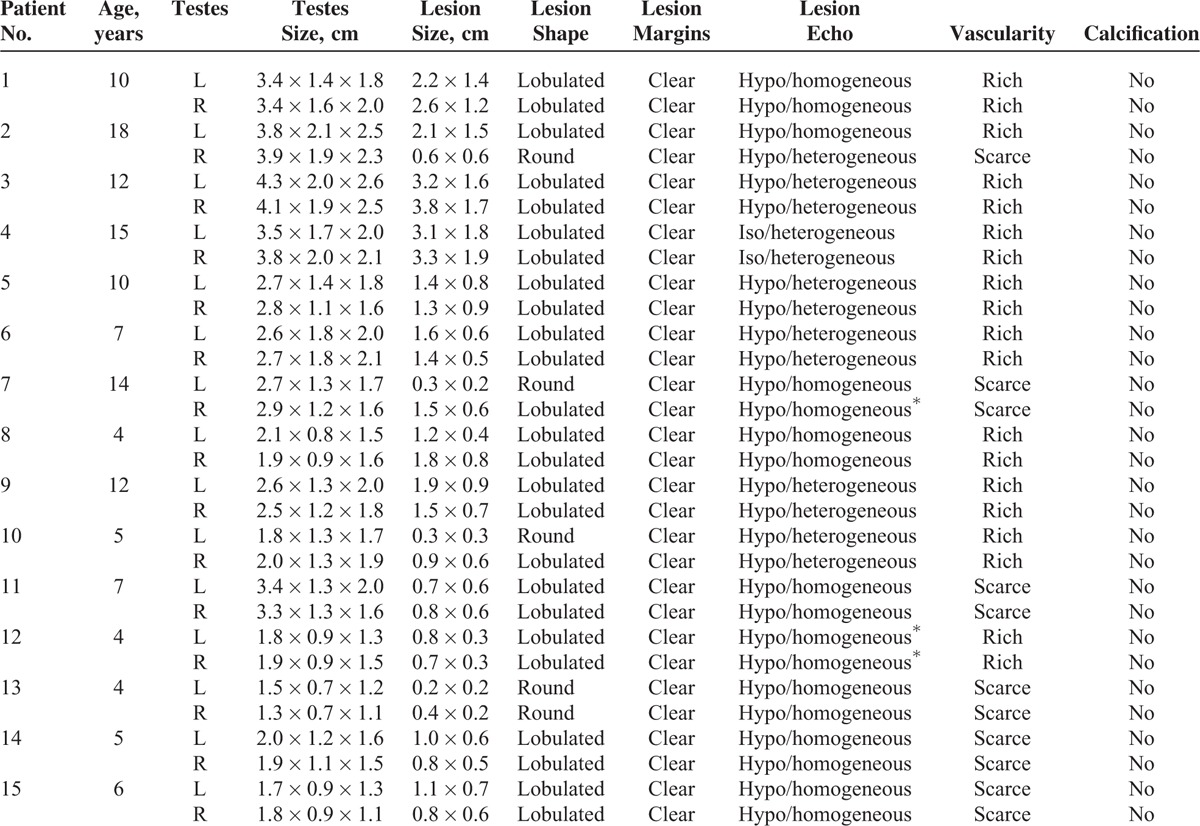
Fifteen male patients involved in this study were aged 4 to 17 years (mean age, 8.8 ± 3.8 years), height 104.6 to 172.5 cm (mean height, 138.7 ± 22.5 years), and weighted 15 to 57 kg (mean weight, 36.5 ± 14.0 kg). All of the patients had been diagnosed with CAH for at least 1 year; the blood pressure and blood glucose of the cases were all at normal levels; 33.3% (5/15) of the cases exhibited normal bone age, and nearly 66.7% (10/15) of the cases exhibited advanced bone age;11,12 and the andrological evaluation of these cases were listed in Table 1. In addition, 80% (12/15) of the cases were confirmed by pathological examination using surgical specimens, and immunohistochemistry showed that CD56, inhibin, and vimentin were all positive, which were the pathological characteristics of adrenal gland (Figure 1). The remaining 3 cases were clinically confirmed (diagnostic criteria: the testicular lesions near the mediastinum were gradually diminishing with an increasing amount of hormone therapy in patients who had been diagnosed with CAH). The hormone levels of these 15 patients with TARTs before and after operation and hormone therapy were showed in Table 2.
TABLE 1.
Basic Characteristics of Pubescent Patients With Congenital Adrenal Hyperplasia and Testicular Adrenal Rest Tumors
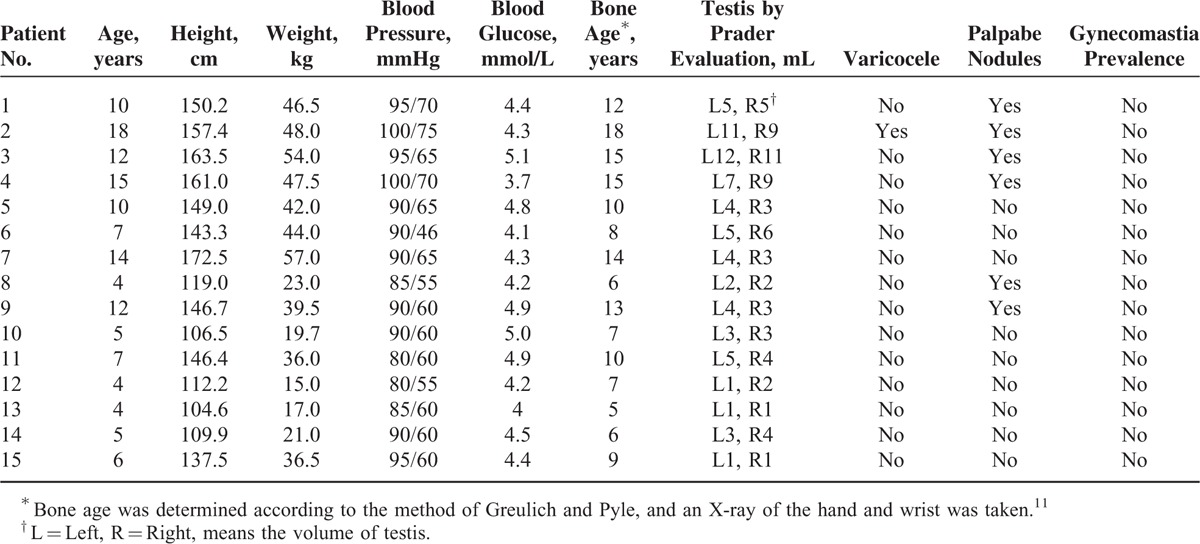
FIGURE 1.
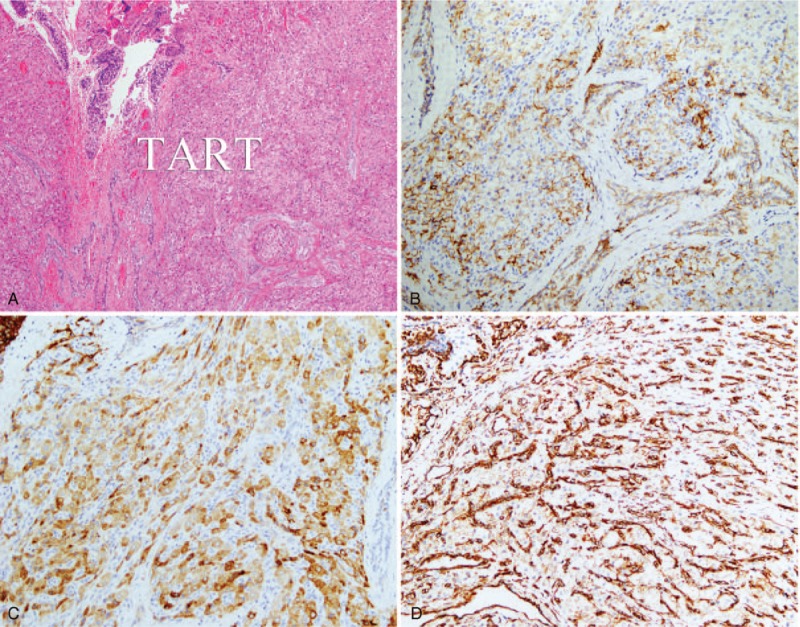
Testicular adrenal rest tumor was growing into testis (A). And immunohistochemistry showed that CD56 (B), inhibin (C), and vimentin (D) were all positive, which was the pathological characteristics of adrenal gland.
TABLE 2.
The Basic Hormone Levels of Pubescent Patients With Congenital Adrenal Hyperplasia and Testicular Adrenal Rest Tumors Before and After Operation and Hormone Therapy
Sonographic Findings of the Lesions
All 15 cases were bilateral, and there were 30 lesions in total. The lesions were 1.4 ± 0.8 cm (range: 0.2–3.8 cm) in diameter on average. In 93.3% of the patients (14/15), the testicular mediastinum were clearly visualized, and only 1 mediastinum exhibited a fuzzy appearance. All of the lesions had clear boundaries and were located near the testicular mediastinum or the testicular hilum; 83.3% (25/30) of the lesions had an irregular shape, whereas 5 (5/30, 16.7%) lesions were round. Furthermore, 56.7% (17/30) of the TART lesions exhibited homogeneous hypoechogenicity (Figure 2), 36.7% (11/30) of the TART lesions exhibited heterogeneous hypoechogenicity (Figure 3), and 6.6% (2/30) of the TART lesions exhibited heterogeneous isoechogenicity (Figure 4A). Color Doppler revealed that 76.7% (23/30) of the lesions had a rich blood supply and that 23.3% (7/30) of the lesions had a scarce blood supply. Neither testicles nor TART lesions were with calcifications (Table 3).
FIGURE 2.
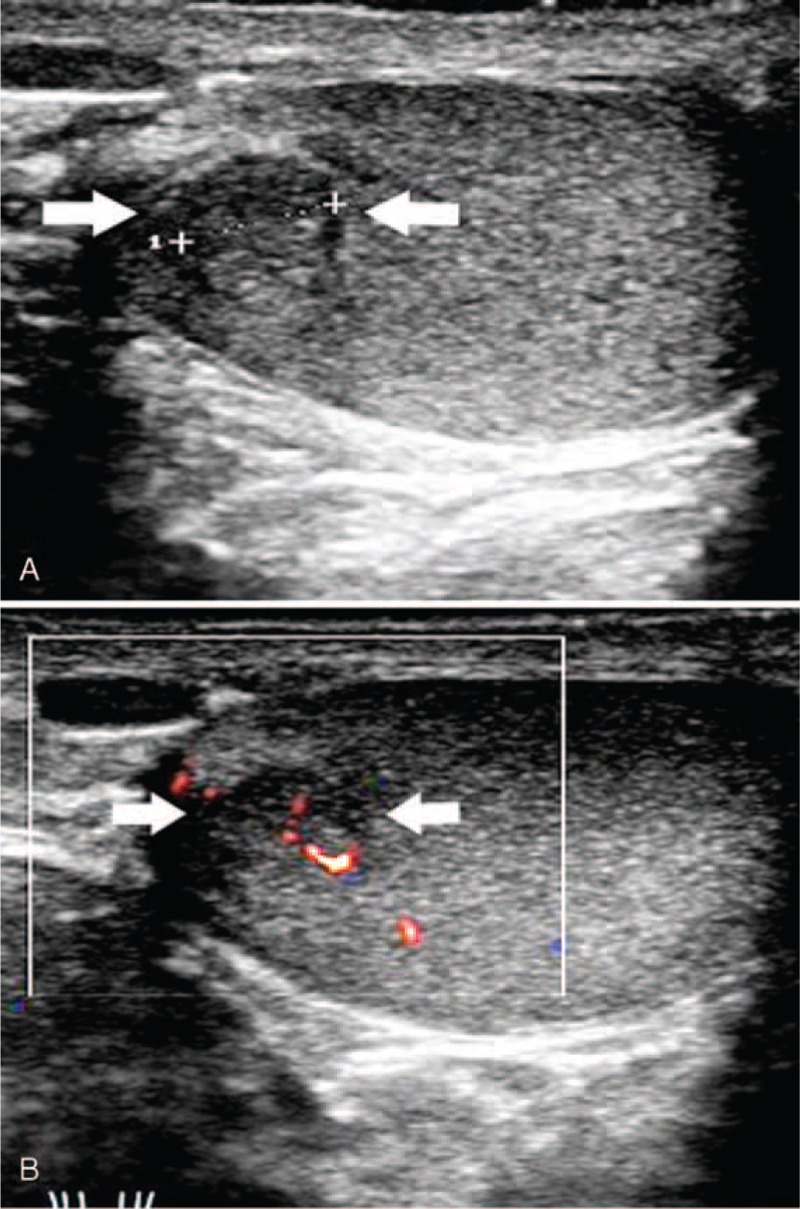
Testicular adrenal rest tumor in a 4-year-old patient with congenital adrenal hyperplasia (patient 13). Sonographic examination of the left testis revealed a homogeneous hypoechoic lesion (arrow) with a round clear boundary, 4 mm in diameter, adjacent to the mediastinum of the testis (A, B).
FIGURE 3.
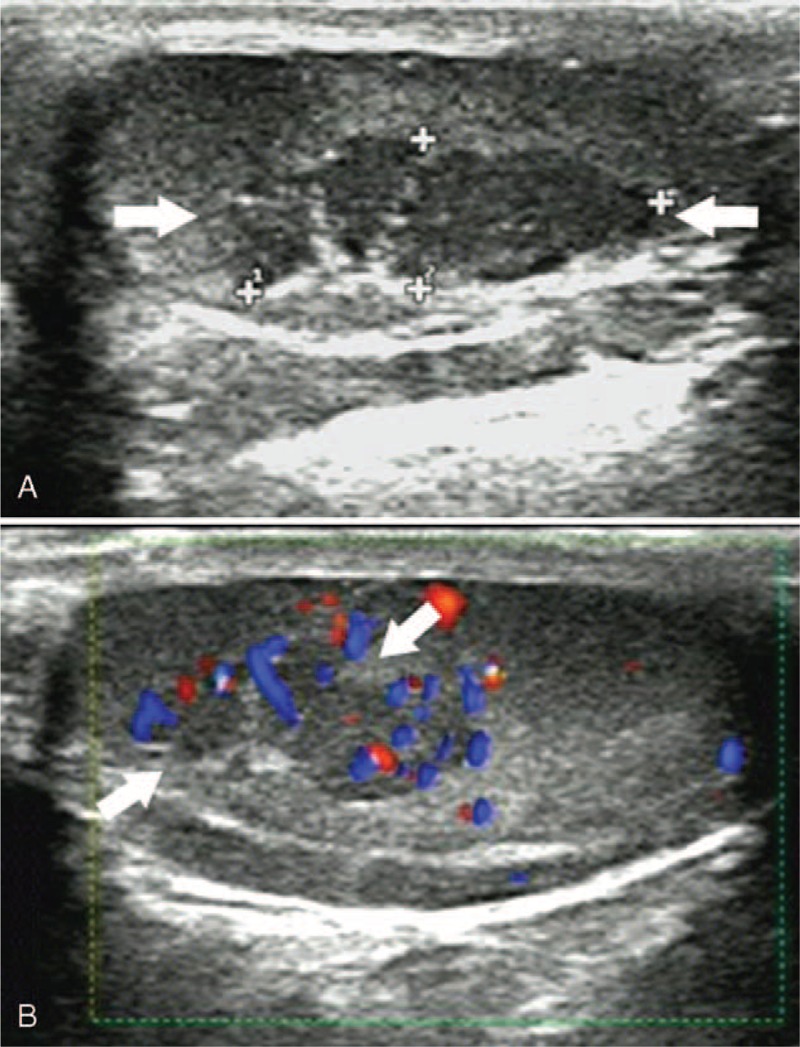
Testicular adrenal rest tumor in a 10-year-old patient with congenital adrenal hyperplasia (patient 5). (A) Sonographic examination of the right testis revealed a clearly defined heterogeneous hypoechoic lesion (arrow), 14 mm in diameter, adjacent to the mediastinum of testis. (B) Color Doppler demonstrated marked vascularization compared with the normal testicular parenchyma (arrow).
FIGURE 4.
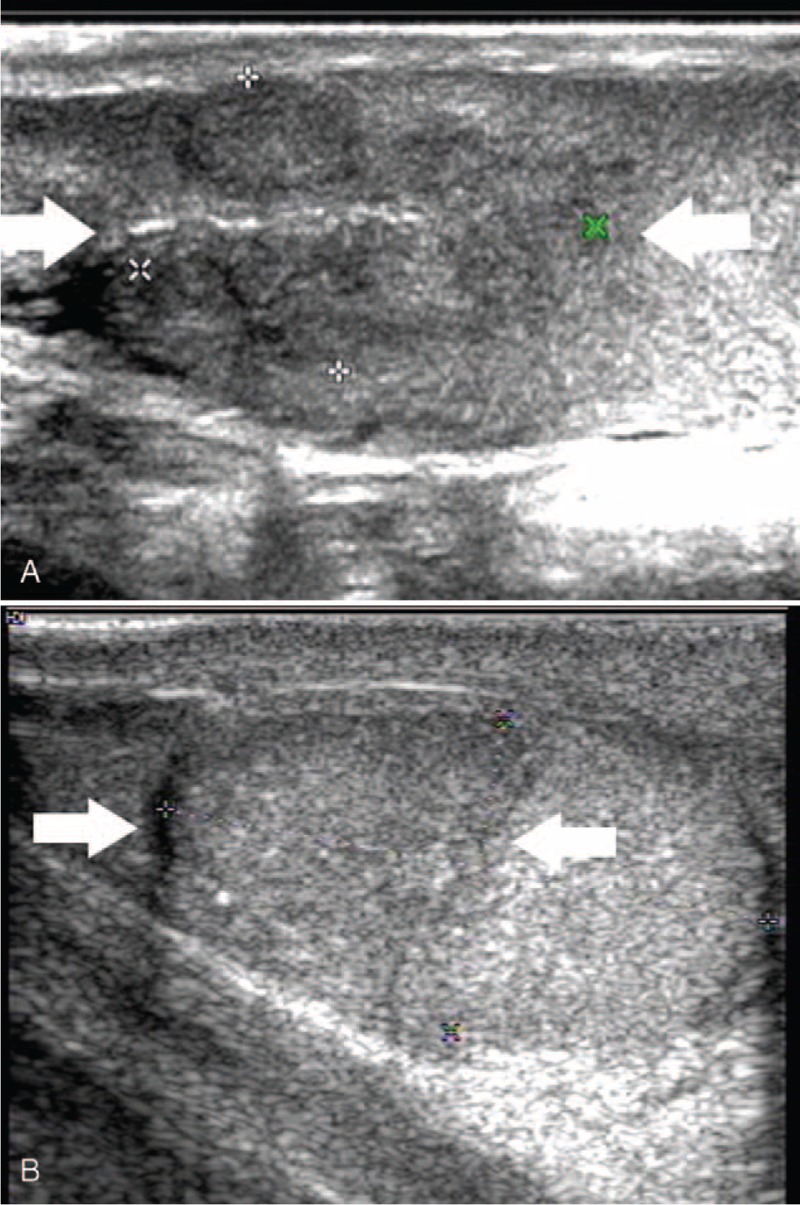
Testicular adrenal rest tumor in a 15-year-old patient with congenital adrenal hyperplasia (patient 4). (A) Sonographic examination of the left testis revealed a heterogeneous isoechoic lesion with a lobulated boundary (arrow), 31 mm in diameter, adjacent to the mediastinum of testis. (B) Sonographic examination of the left testis half a year later after operation revealed no TART lesion in the heterogeneous echoic testis caused by operation (arrow).
TABLE 3.
Sonographic Features of Pubescent Patients With Congenital Adrenal Hyperplasia and Testicular Adrenal Rest Tumors
The follow-up period was from 3 to 12 months, and the TART lesions were almost disappeared after operation and hormone therapy. The testis exhibited heterogeneous echogenicity, which might be caused by operation (Figure 4B).
DISCUSSION
In male patients with CAH, TARTs are relatively common, especially in adult males, with a prevalence of nearly 94%.8 In recent years, it has become evident that some of these lesions can be detected in childhood, with a prevalence ranging from 18.3% to 29% in studies performed on children.3,13 A similar prevalence of 19% (15/79) was found in our patient population. To the best of our knowledge, few reports have described the imaging characteristics of TARTs in puberty and adolescent;7,14 the present study is the first to evaluate the sonographic features of TARTs in a large pediatric CAH population from 0 to 18 years of age.
We retrospectively analyzed the imaging features of 30 TART lesions that had been pathologically or clinically confirmed within the past 5 years. Our results revealed that all 15 cases were bilateral in onset, similar to previous reports.5,8 Although the results of Delfino et al16 indicated that 2 of 11 cases were unilateral, we believe that this result might be influenced by lesions too small to detect; in our series, the size of the smallest lesion was 2 mm in diameter, and it is sometimes difficult to detect lesions smaller than 2 mm.
Ultrasonography revealed that all 30 lesions in our series were located adjacent to the testicular mediastinum and had clear boundaries. The location in the mediastinum has previously been described and is considered a typical feature of TARTs in CAH.6 The results of Stikkelbroeck et al8 revealed blurring of the margins in 36% of lesions, but all of our lesions had clearly defined margins, which may be due to the advanced ultrasonic technology compared with that used a decade ago.
In our study, 83.3% (25/30) of the lesions had an irregular, lobulated shape, and ranged from 0.7 to 3.8 cm in size. Five (5/30, 16.7%) lesions were round, ranging from 0.2 to 0.6 cm in size. We believe the TARTs begin as small, distinct lesions that could grow and coalesce to form 1 large, lobulated lesion. The histopathological hypothesis of the development of these lesions was that they originated from aberrant adrenal rest cells; when stimulated by adrenocorticotropic hormone, these adrenal rest cells in the testicular tissue were activated and became hyperplastic, subsequently appearing as confluent lesions.15
The TARTs in CAH in our group exhibited hypoechogenicity compared with normal testicular tissue except in 6.6% (2/30) of the lesions, which exhibited isoechogenicity. Our results are consistent the findings from most of the previously reported studies.6,16,17 By contrast, with respect to lesion size, Stikkelbroeck et al8 reported that 17 of 20 lesions <2 cm were hypoechoic, whereas all lesions >2 cm were hypoechoic with hyperechoic reflections. The significant difference in the echogenicity of TARTs was likely due to the selection of patients, who were all adults in Stikkelbroeck's report and might have had a longer disease course compared with the pubescent patients; furthermore, hyperechogenicity might indicate fibrotic changes or calcifications in the lesions.
Color Doppler revealed that 76.7% (23/30) of the lesions exhibited a rich blood supply, whereas 23.3% (7/30) of the lesions exhibited a scarce blood supply. In addition, the vessels coursing through the lesions did not deviate or change in caliber. The latter observation has also been made by Avila et al6 and Stikkelbroeck et al,8 who suggested that this appearance may be useful in distinguishing TARTs because it has not been described with other testicular tumors.
Therefore, TART screening by scrotal BUS beginning from early childhood is important in male patients with CAH to prevent the development of future complications, especially infertility. Notably, the high TART prevalence in adult patients is due to failure of detection during childhood.
Our study still has a limitation, which is the uncertain relationship between TARTs size and abnormal hormone levels. So, a well designed clinical study is needed to get the relationship between them.
In conclusion, the sonographic characteristics of TARTs are bilateral growth, location adjacency to the testicular mediastinum, hypoechogenicity, and a rich blood supply. We recommend that ultrasound be performed from the onset of puberty in all boys with classic CAH to avoid the risk of infertility.
Acknowledgments
The authors thank funding from the National Nature Science Foundation of China (No: 30901384 and No: 81271576), the Guangdong Province Medical Research Foundation (No: A2013194), and the Guangdong Province S&T Plan Foundation (No: 2013B060500044).
Footnotes
Abbreviations: TART = testicular adrenal rest tumor, BUS = baseline ultrasound, CAH = congenital adrenal hyperplasia.
This study is funded by the National Nature Science Foundation of China (No: 30901384 and No: 81271576), the Guangdong Province Medical Research Foundation (No: A2013194), and the Guangdong Province S&T Plan Foundation (No: 2013B060500044).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med 2003; 349:776–788. [DOI] [PubMed] [Google Scholar]
- 2.der Grinten HLC, Dehzad F, Kamphuis-van UK, et al. Increased prevalence of testicular adrenal rest tumours during adolescence in congenital adrenal hyperplasia. Horm Res Paediatr 2014; 82:238–244. [DOI] [PubMed] [Google Scholar]
- 3.Aycan Z, Bas VN, Cetinkaya S, et al. Prevalence and long-term follow-up outcomes of testicular adrenal rest tumours in children and adolescent males with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2013; 78:667–672. [DOI] [PubMed] [Google Scholar]
- 4.Bouman A, de Kaa CH, der Grinten HLC. Prevalence of testicular adrenal rest tissue in neonates. Horm Res Paediatr 2011; 75:90–93. [DOI] [PubMed] [Google Scholar]
- 5.Dogra V, Nathan J, Bhatt S. Sonographic appearance of testicular adrenal rest tissue in congenital adrenal hyperplasia. J Ultrasound Med 2004; 23:979–981. [DOI] [PubMed] [Google Scholar]
- 6.Avila NA, Premkumar A, Shawker TH, et al. Testicular adrenal rest tissue in congenital adrenal hyperplasia: findings at Gray-scale and color Doppler US. Radiology 1996; 198:99–104. [DOI] [PubMed] [Google Scholar]
- 7.Lee D, Rodgers SK. Testicular adrenal rests. Ultrasound Q 2008; 24:105–107. [DOI] [PubMed] [Google Scholar]
- 8.Stikkelbroeck NM, Suliman HM, Otten BJ, et al. Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: sonographic and MR features. Eur Radiol 2003; 13:1597–1603. [DOI] [PubMed] [Google Scholar]
- 9.Nagamine WH, Mehta SV, Vade A. Testicular adrenal rest tumors in a patient with congenital adrenal hyperplasia: sonographic and magnetic resonance imaging findings. J Ultrasound Med 2005; 24:1717–1720. [DOI] [PubMed] [Google Scholar]
- 10.Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update 2015; 21:56–83. [DOI] [PubMed] [Google Scholar]
- 11.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr 1952; 40:423–441. [DOI] [PubMed] [Google Scholar]
- 12.Martin DD, Heil K, Heckmann C, et al. Validation of automatic bone age determination in children with congenital adrenal hyperplasia. Pediatr Radiol 2013; 43:1615–1621. [DOI] [PubMed] [Google Scholar]
- 13.Cakir ED, Mutlu FS, Eren E, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia. J Clin Res Pediatr Endocrinol 2012; 4:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avila NA, Shawker TS, Jones JV, et al. Testicular adrenal rest tissue in congenital adrenal hyperplasia: serial sonographic and clinical findings. AJR Am J Roentgenol 1999; 172:1235–1238. [DOI] [PubMed] [Google Scholar]
- 15.Rich MA, Keating MA. Leydig cell tumors and tumors associated with congenital adrenal hyperplasia. Urol Clin North Am 2000; 27:519–528.x. [DOI] [PubMed] [Google Scholar]
- 16.Delfino M, Elia J, Imbrogno N, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: prevalence and sonographic, hormonal, and seminal characteristics. J Ultrasound Med 2012; 31:383–388. [DOI] [PubMed] [Google Scholar]
- 17.Fitoz S, Atasoy C, Adiyaman P, et al. Testicular adrenal rests in a patient with congenital adrenal hyperplasia: US and MRI features. Comput Med Imaging Graph 2006; 30:465–468. [DOI] [PubMed] [Google Scholar]


