Abstract
Recent studies suggest that statin may benefit cancer prognosis, especially through its radiosensitization effect. But controversy exists in other studies. Hence, we performed a meta-analysis of results from 35 studies to evaluate the effect of statin use on urologic cancers.
We conducted computerized search from PubMed, Embase, and ISI Web of Knowledge through May 2015, screened the retrieved references, and collected and evaluated relevant information. We extracted and synthesized corresponding hazard ratios (HR) and confidence interval (CI) by using Review Manager 5.3 and STATA 13. This review was registered at PROSPERO with registration No. CRD42015020171.
We selected total 35 retrospective studies and conducted a meta-analysis of results from these studies. The pooled results suggested no benefit of statin use to bladder cancer and renal cell carcinoma, except overall survival [HR = 0.81, 95% CI: 0.69–0.96]. However, significant improvement of prostate cancer prognosis including overall survival [HR = 0.82, 95% CI: 0.70–0.97] and cancer-specific survival [HR = 0.70, 95% CI: 0.59–0.83] was indicated, but not including tumor progression [HR = 0.84, 95% CI: 0.62–1.14]. Statin use improved biochemical recurrence of prostate cancer in radiotherapy patients [HR = 0.68, 95% CI: 0.54–0.85] but not in radical prostatectomy patients [HR = 0.97, 95% CI: 0.82–1.15].
Current evidence suggests no benefit of statin use to bladder cancer and renal cell carcinoma, except in overall survival. While statin use benefited prostate cancer patients in overall survival, cancer-specific survival but not in tumor progression; it also improved biochemical recurrence in radiotherapy patients but not in radical patients. To verify these results, randomized controlled trials are necessary.
INTRODUCTION
Statin is a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor and widely used for hypercholesterolemia patients. Recent studies prompt to indicate statin as a panacea because of its effects in treating variant diseases. A previous study showed that statin use was a protective factor for cancer incidence risk.1 To date, statin is known as a pleiotropic drug rather than cholesterol-lowering medication. A retrospective survey of Shared Equal Access Regional Cancer Hospital (SEARCH) database indicated that triglycerides and low-density lipoproteins were associated with increased risk of prostate cancer recurrence. In the contrary, high-density lipoproteins were associated with decreased risk of prostate cancer in dyslipidemia.2 It is compatible with the conclusion of another study that statin use significantly reduces breast cancer mortality.3 Similar outcomes were also observed in numerous urological cancer treatment studies. However, controversy of real effect existed in variant studies.4,5 Hence, we aimed to conduct a meta-analysis of well selected observational studies to evaluate prognostic effect of statin use in urinary cancer treatment, limited in renal cell carcinoma, bladder cancer, and prostate cancer. This study was registered in PROSPERO with registration number CRD42015020171.6
METHODS
Search and Screen Strategy
We performed a systematic literature search of PubMed, Embase, and ISI Web of Knowledge to retrieve urologic cancer clinical studies using statin through May 3, 2015. We used search key words including statin, renal cell carcinoma, bladder cancer, prostate cancer, survival and mortality, etc. The detailed search strategy was described in the supplement 1. The citations in the retrieved articles were also screened for any relevant studies. The initial screen was conducted by reviewing the title and abstract by 2 independent investigators (YL and DLS) to eliminate the irrelevant articles. Then, the full-text articles were reviewed according to eligibility criteria. Any clinical study comprising the evaluation of statin use on urologic cancer prognosis was eligible. In this article, we only include results of renal cell carcinoma (RCC), bladder cancer (BC), and prostate cancer (PCa). Articles that has abstract only, duplicated literature, overlapping patients or duplicated data presented in conferences; or does not study RCC, BC or PCa; or has no data available, were excluded. In this study, all data and analyses were based on the previous published studies, and thus no ethical approval and patient consent are required.
Data Extraction and Quality Assessment
Before data collection, a spreadsheet was designed for the key information. Data extraction was independently performed by 2 researchers (YL and DLS) and cross-checked. Meanwhile, any disagreement or uncertainty was resolved by group discussion. Data extracted from the articles included the name of the first author and publication year, country, cancer type, recruitment period, number of patients, age, main treatment, follow-up, prognostic outcomes, definition of outcomes, and adjusted factors. The data were extracted from the original articles. During data extraction, multivariate outcomes were prior to univariate outcomes when both were provided, while if no multivariate results were presented, univariate outcomes were used instead. If there was no exact time to event survival data, we either estimated HR and 95% CI by the methods that were provided by Tierney et al7 using the given survival or mortality curve or other available data, or referred previous study outcomes8 or contacted the corresponding author for the original data. The extracted data from studies which have potential overlapping patients were removed before meta-analysis to avoid over-analysis. The quality assessment was carried out by using Newcastle–Ottawa Scale (NOS) for cohort study that comprised 3 domains with 8 items to evaluate bias risk.9 Above 5 stars of total 9 stars was deemed as good quality.
Statistical Analysis
Review Manager 5.3 (The Cochrane Collaboration, Copenhagen) was used to perform quantitative synthesis. Hazard ratio (HR) and its 95% confident interval were used to evaluate the survival outcome. First, Cochran's Q test and Higgins I2 statistic were calculated for heterogeneity detection.10P ≥ 0.1 and I2 ≤ 50% were deemed to no significant heterogeneity, and fixed effects model was used. Otherwise, random effects model was used. The inverse variance method was used to calculate the pooled hazard ratio. Sensitivity analysis was conducted by using the method of leave-one-out to test the feasibility of the pooled results. Publication bias was detected with Egger's regression intercept test and was only performed in outcomes comprised more than 10 studies by using STATA 13 (Stata Corp LP, College Station, TX).10,11 A 2-tailed P < 0.05 was considered statistically significant.
RESULTS
Eligible Studies and Quality Assessment
In total, 526 abstracts were retrieved by the initial search strategy. After screening, 35 studies4,5,12–44 including a France article31 were included in the qualitative and quantitative synthesis. The screening diagram was shown in Figure 1. The characteristics of included studies and the Newcastle–Ottawa Scale (NOS) quality assessment were shown in Table 1 . Outcomes included overall survival, cancer-specific survival, recurrence-free survival, progression-free survival, and biochemical recurrence.
FIGURE 1.
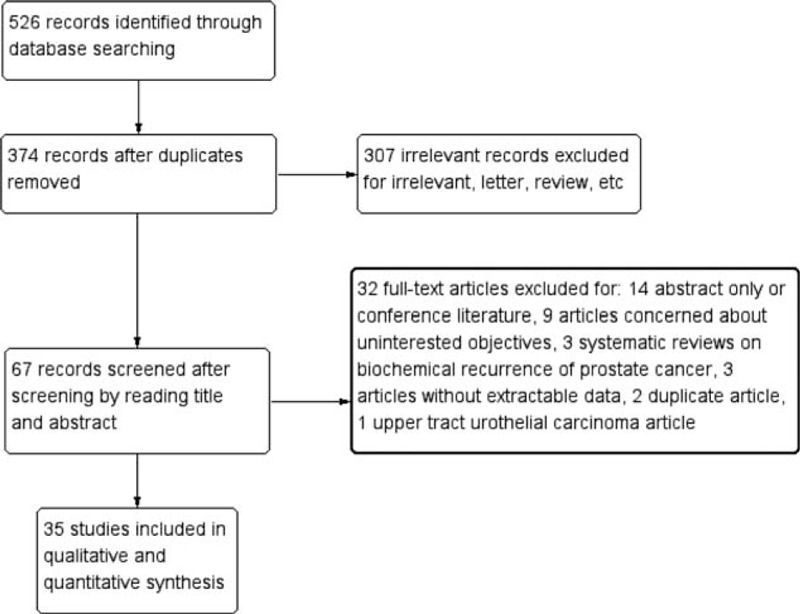
Literature screen diagram.
TABLE 1.
Characteristics of Included Studies
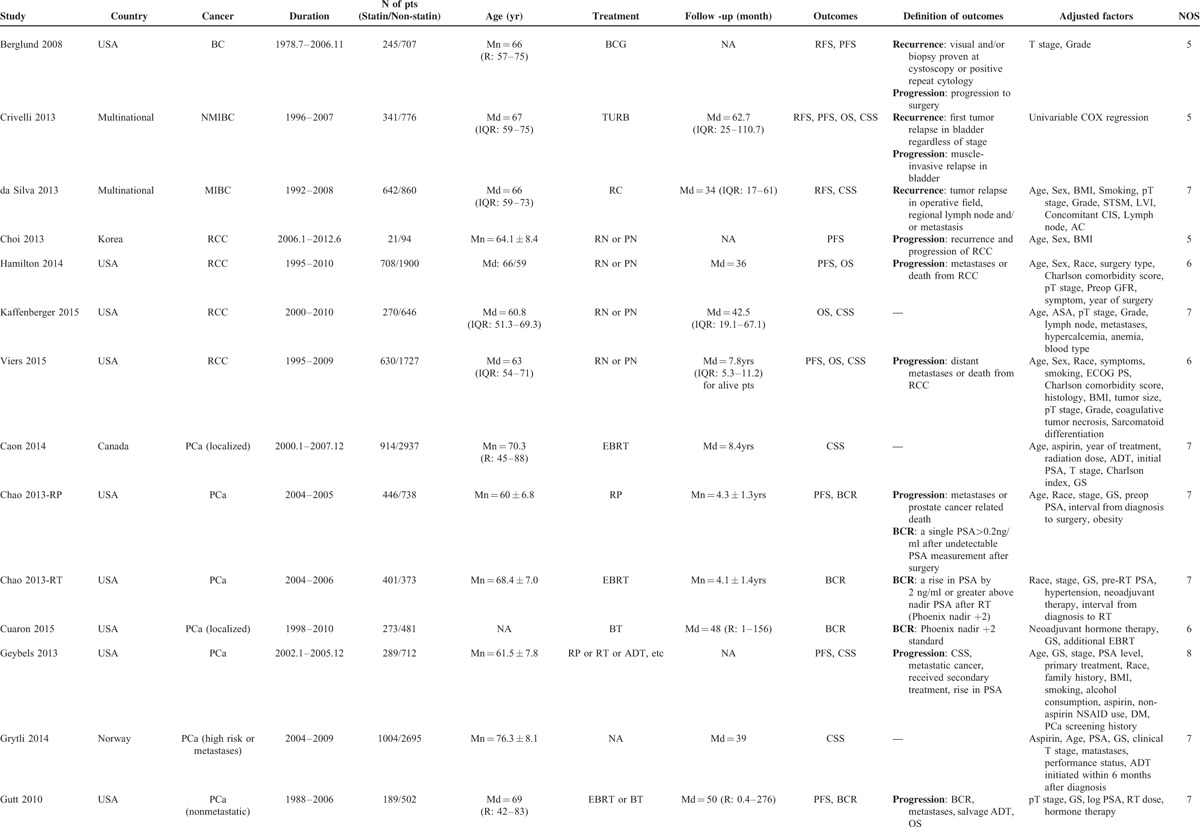
TABLE 1 (Continued).
Characteristics of Included Studies
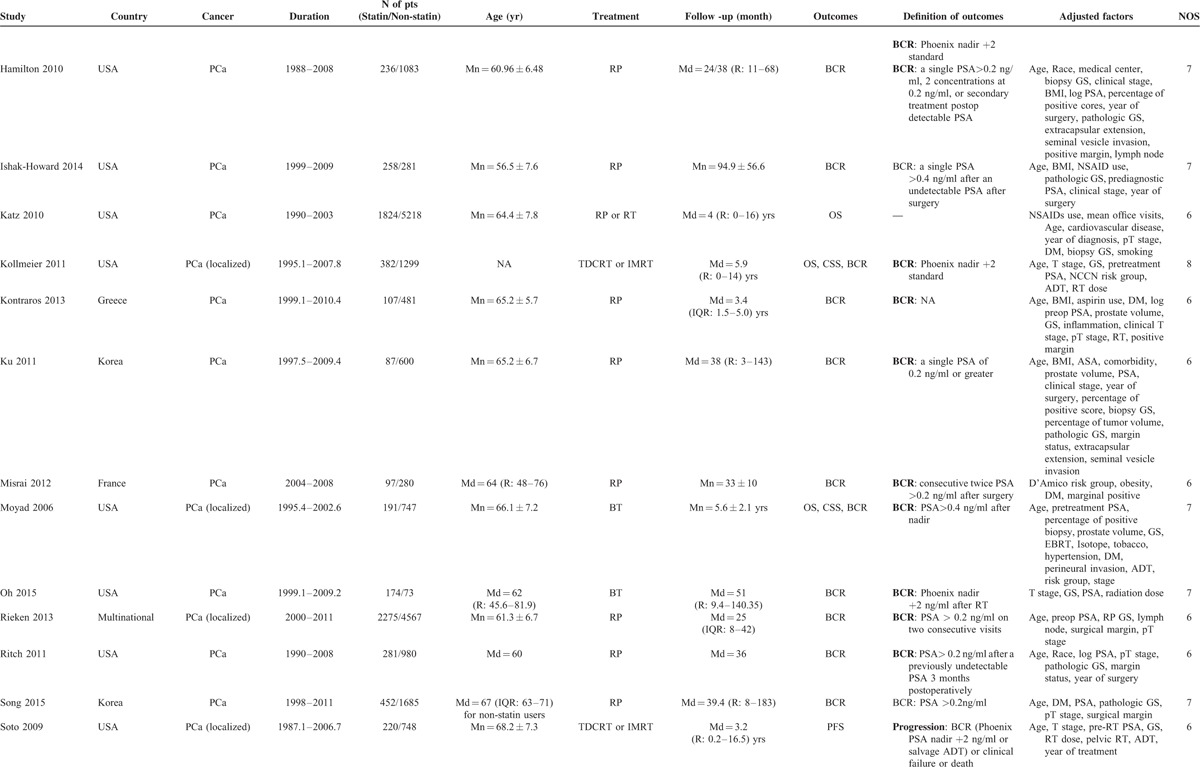
TABLE 1 (Continued).
Characteristics of Included Studies
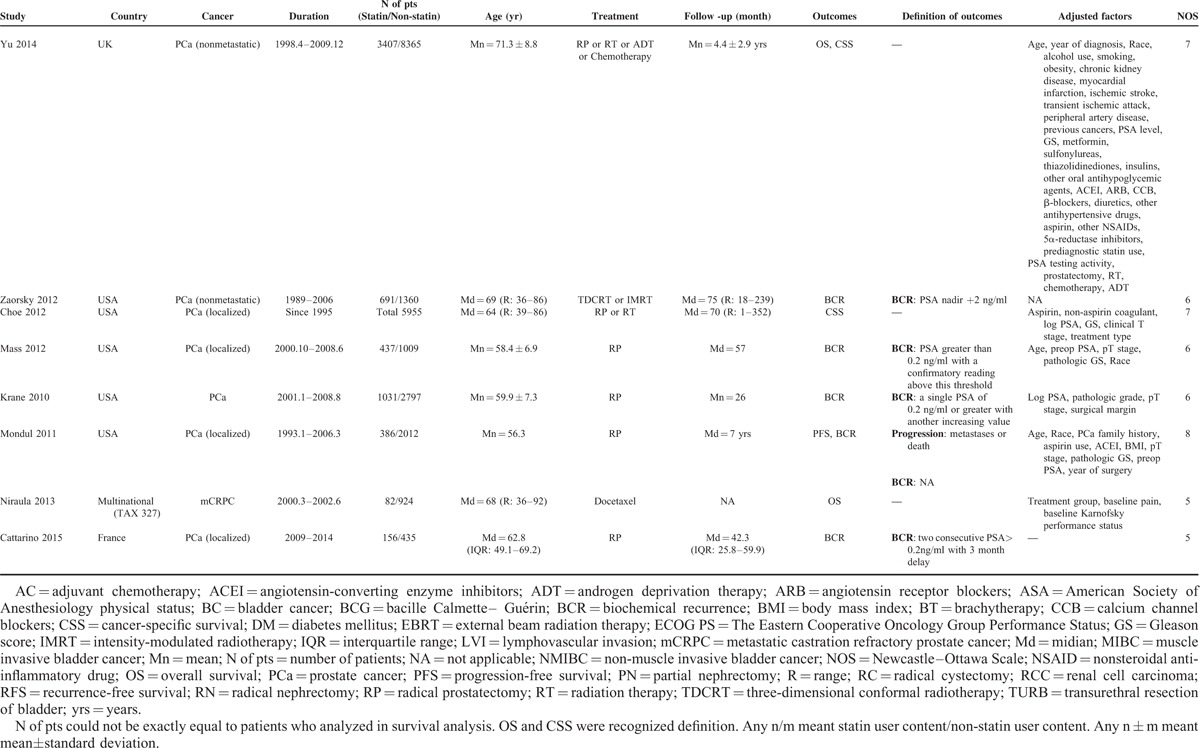
Survival Outcomes
In renal cell carcinoma, 4 studies were included.15–18 Among them, 3 reported overall survival, 2 reported cancer-specific survival, and 3 reported tumor progression status. As shown in Figure 2, the pooled results of statin use in overall survival, cancer-specific survival, and tumor progression of renal cell carcinoma were HR = 0.81 (95% CI: 0.69–0.96), 0.72 (0.35–1.50), and 0.91 (0.54–1.55), respectively.
FIGURE 2.
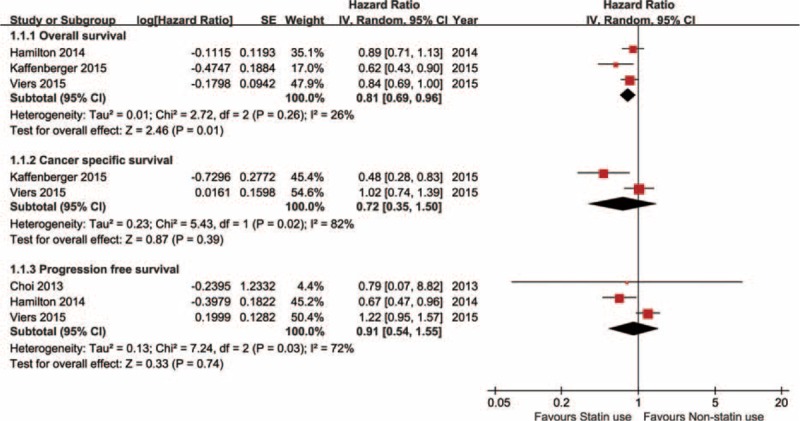
Statin use on survival outcomes of renal cell carcinoma (RCC).
Three bladder cancer studies reported oncological prognosis.12–14 One study reported overall survival with HR = 1.14 (0.89–1.44). Two studies reported cancer-specific survival and the pooled result was HR = 1.06 (0.87–1.29). Three studies reported recurrence-free survival with pooled HR = 1.05 (0.94–1.18). Two studies reported tumor progression and the pooled HR was 0.87 (0.65–1.15). All results were calculated by applying fixed effect model and shown in Figure 3.
FIGURE 3.
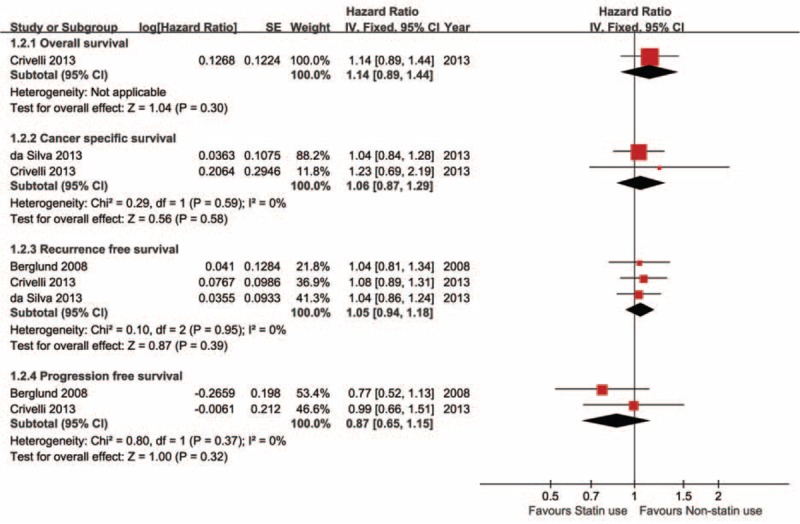
Statin use on survival outcomes of bladder cancer (BC).
Among prostate cancer studies, 5 reported overall survival outcomes and the pooled HR of statin use versus nonstatin use was 0.82 (0.70–0.97). Accordingly, 6 studies presented cancer-specific survival outcomes, the pooled result was HR = 0.70 (0.59–0.83). Tumor progression was reported in 5 studies and the pooled risk was 0.84 (0.62–1.14). All the above 3 clinical outcomes were analyzed by using the randomized effect model shown in Figure 4. Additionally, biochemical recurrence of prostate cancer became important in statin anticancer research. All prostate cancer studies subgroup were stratified by major treatment method and a subgroup analysis was conducted considering its radiosensitization effect. In radical prostatectomy subgroup, 13 studies presented biochemical result and the pooled hazard ratio of statin use versus nonstatin use was 0.97 (0.82–1.15), P = 0.73. However, in radiotherapy subgroup, 7 references reported biochemical recurrence, the pooled HR was 0.68 (0.54–0.85), P = 0.0009. Figure 5 shows the forest plot of biochemical recurrence of prostate cancer.
FIGURE 4.
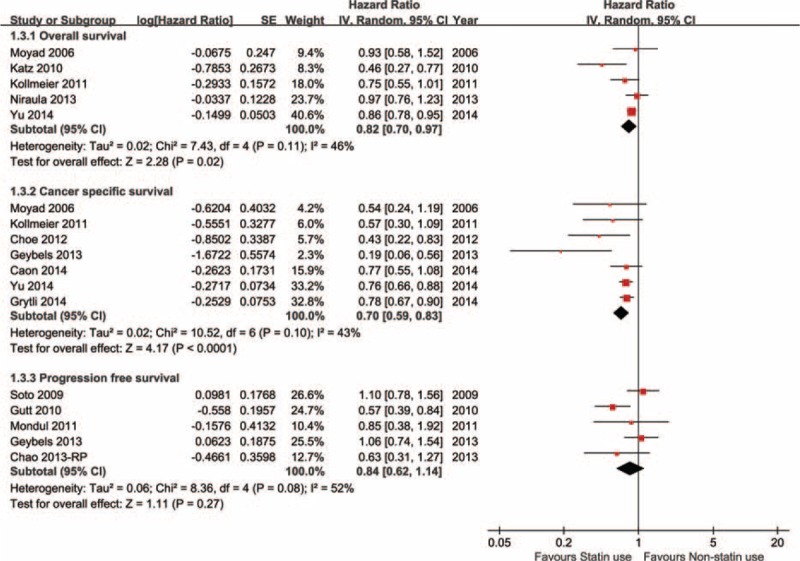
Statin use on survival outcomes of prostate cancer (PCa).
FIGURE 5.
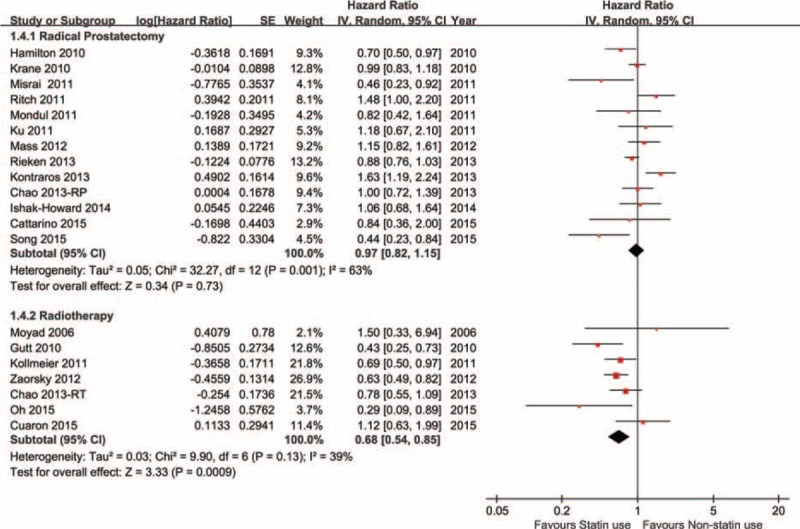
Stain use on biochemical recurrence (BCR) of prostate cancer (PCa).
Publication Bias and Sensitivity Analysis
Publication bias detection was conducted by Egger's asymmetric test only in biochemical recurrence. P value of the linear regression was 0.803 for radical prostatectomy subgroup, 0.977 for radiotherapy subgroup, and 0.463 for the entire group of prostate cancer. The results show that no significant publication bias was observed and the funnel plot is shown in Figure 6. A sensitivity analysis was conducted in prostate cancer. There is no significant change observed in cancer-specific survival, tumor progression, and biochemical recurrence after removing any included study (results were omitted).
FIGURE 6.
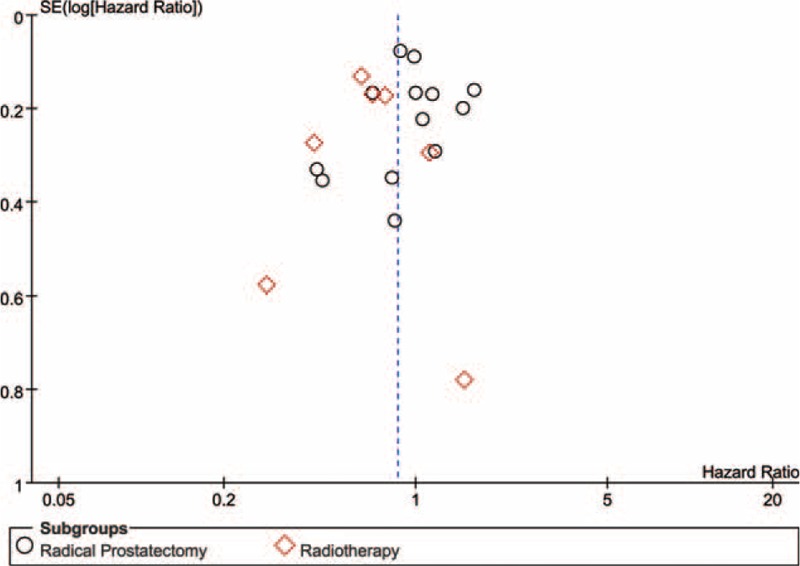
Funnel plot of included studies concerning biochemical recurrence (BCR).
DISCUSSION
Among medical studies, there is a great controversy on the effect of statin. Antitumor and tumor promotion effect are both presented in variant studies.45,46 However, the mechanism of antitumor or tumor promotion effect of statin has not yet been clearly elucidated. Hindler et al47 summarized the role of statin in cancer therapy as 4 aspects: First, statin inhibits tumor cell growth by inhibiting dolichol, geranylpyrophosphate, and farnesylpyrophosphate that are regulators of cell cycle, by inhibiting Ras and Rho that mediate cell proliferation, and by stabilizing the cell cycle kinase inhibitors p21 and p27. Second, inhibition of angiogenesis: statin has pros and cons for angiogenesis. High dose statin has an antiangiogenesis effect by inhibiting capillary tube formation and reducing vascular endothelial growth factor release. However, low-dose statin has a proangiogenesis effect by stimulating protein kinase B and activating endothelial nitric oxide synthase. Third, statin induces cell apoptosis by upregulating proapoptotic proteins and reducing antiapoptotic proteins. Fourth, statin suppresses tumor metastasis by reducing the expression of endothelial leukocyte adhesion molecule E-selectin and matrix metalloproteinase, inhibiting epithelial growth factor induced tumor cell invasion. Sun et al48 demonstrated that cholesterol increases Ca2+ entry via the TRPM7 channel, which promotes proliferation of prostate cells by inducing the activation of the AKT and/or the ERK pathway. Additionally, cholesterol-mediated Ca2+ entry induces an increase of calpain activity that represses E-cadherin expression, which could lead to migration of prostate cancer cells. Bañez et al49 reported that statin use significantly reduced the risk of inflammatory infiltration in prostate cancer, which was proved to be associated with cancer development and prognosis.50–53 All the above studies attend to elucidate the possible anti-cancer mechanism of statin. While in clinical studies, there is also a great controversy on the effect of statin use for cancer patients’ prognosis. Hoffmann et al54 reported that non-muscle-invasive bladder cancer patients, who were treated with bacille Calmette–Guérin (BCG) immunotherapy and were exposed to statin use, had worsening prognosis. This study was subsequently questioned by Kamat et al.55 They reported no significant difference in the tumor recurrence, progression, or deaths in their cohort with 156 patients treated with BCG. A large cross-sectional study reported that statin use was associated with a reduction in the probability that older men would have an abnormal screening PSA result regardless of the PSA threshold (PSA > 2.5, > 4.0, or > 6.5 ng/mL). This reduction is more pronounced with higher statin dose, longer statin duration, and higher statin potency.56 It revealed the anti-cancer effect of statin. Additionally, statin has a radiosensitization effect for prostate cancer both in vitro and in vivo.25,28,57 However, some studies do not support this synergism.21,22 Our analysis shows significant effect of statin use in prostate cancer patients underwent radiotherapy but not in patients with radical prostatectomy.
In our study, we investigated 4 clinical outcomes and 1 biochemical outcome in 3 major urologic cancers. In renal cell carcinoma treatment, statin use was associated with improvement of overall survival but not in cancer-specific survival and tumor progression. The improvement of overall survival in statin use patients was not stable and probably was derived from the protection from cardiovascular-related death.58 In bladder cancer, no significance improvement was observed in overall survival, cancer-specific survival, or tumor recurrence and progression. A possible explanation for these results is that the inherent poor prognosis of bladder cancer overcomes the anticancer effect of statin use, or because of its intracavity. The biological behavior of bladder cancer could also be a risk for drug effect because even though in chemotherapy, numerous drug resistant or insensitive bladder cancer existed.59 In prostate cancer, significant improvements of overall survival and cancer-specific survival, not tumor progression, were observed. Meanwhile, biochemical recurrence of PSA was intensively analyzed. Referred to previous study,8 stratification by major treatment methods was performed. In radical prostatectomy subgroup, no difference was observed between statin use and non-use. However, statin use significantly improved biochemical recurrence in prostate cancer patients treated with radiotherapy. Sensitivity analysis described as above did not alter the results. It is compatible with the radiosensitization effect of statin use. There seemed to be a paradox that statin use did not improve biochemical recurrence in radical prostatectomy patients but improve overall survival and cancer-specific survival. A hypothesis was that these benefits derived from radiation therapy patients. But it has not been verified. Additionally, as to clinical outcomes, small study effect was an obvious risk for the pooled results. Generally, statin use seemed to benefit prostate cancer, especially prostate cancer patients underwent radiotherapy.
Additionally, all studies included or excluded were obviously biased. Statin, unlike chemotherapeutic drugs, is a gentle medication for cancer, if it has the anticancer effect. However, the accumulative effect of statin use is unclear yet. On the other hand, the definition of statin use has not been clearly elaborated. The statin category is also different from each type. Additionally, statin use was a time-dependent covariate in these survival cohorts. Stratifying statin users by records of pre or at cancer diagnosis or treatment is not appropriate. First, the duration of statin use is volatile. A man consumed statin for 5 years is different from the man with 5-month statin consumption. Second, the statin use status of included patients was also volatile during the follow-up. For example, a statin use patient could discontinue statin consumption. Mostly, those nonstatin users could consume statin after diagnosis or treatment of cancer. This would obviously confound the survival analysis. Based on thus, randomized controlled trials would help verify this benefit. Additionally, it is too early to apply statin medication to urologic cancer patients. Adverse effect, dose, and economic factors were important obstacles that must be overcome before application though significant benefit was verified by randomized controlled trials in the future.
CONCLUSION
Our meta-analysis summarized the published literature with statin use exposure and the pooled results suggested no benefit of statin use to bladder cancer and renal cell carcinoma, except in overall survival. However, significant improvement of prostate cancer prognosis including overall survival and cancer-specific survival was indicated, but not including tumor progression. Statin use improved biochemical recurrence of prostate cancer in radiotherapy patients but not in radical prostatectomy patients. Randomized controlled trials would help verify these results.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BC = bladder cancer, BCG = bacille Calmette–Guérin, BCR = biochemical recurrence, CSS = cancer-specific survival, HR = hazard ratio, NOS = Newcastle–Ottawa Scale, OS = overall survival, PCa = prostate cancer, PFS = progression-free survival, RCC = renal cell carcinoma, RFS = recurrence-free survival.
YL and D-LS contributed equally to this work.
The authors have no conflicts of interest to disclose.
S-JF and LY conceived and designed this research. YL and D-LS performed the literature search, bias assessment of included studies, and data extraction. S Zhang and HX conducted data analysis. S Zhang, D-LS, HX, and YL coauthored the manuscript. LY and S-JF gave methodological guidance during the research and made the final revision of the manuscript.
REFERENCES
- 1.Kuoppala J, Lamminpää A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer 2008; 44:2122–2132. [DOI] [PubMed] [Google Scholar]
- 2.Allott EH, Howard LE, Cooperberg MR, et al. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH Database. Cancer Epidemiol Biomarkers Prev 2014; 23:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardwell CR, Hicks BM, Hughes C, et al. Statin use after diagnosis of breast cancer and survival. Epidemiology 2015; 26:68–78. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton RJ, Banez LL, Aronson WJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer 2010; 116:3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritch CR, Hruby G, Badani KK, et al. Effect of statin use on biochemical outcome following radical prostatectomy. BJU Int 2011; 108:E211–E216. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y, She D, Hu X, et al. Statin use in the treatment of urologic cancer patients: a systematic review and meta-analysis. Prospero 2015; CRD42015020171.http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42015020171, access date: May 2015. [Google Scholar]
- 7.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HS, Schoenfeld JD, Mailhot RB, et al. Statins and prostate cancer recurrence following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. Ann Oncol 2013; 24:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Access date: May 2015. [Google Scholar]
- 10.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011.]. http://www.cochrane-handbook.org Access date: May 2015. [Google Scholar]
- 11.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berglund RK, Savage CJ, Vora KC, et al. An analysis of the effect of statin use on the efficacy of bacillus Calmette–Guerin treatment for transitional cell carcinoma of the bladder. J Urol 2008; 180:1297–1300. [DOI] [PubMed] [Google Scholar]
- 13.Crivelli JJ, Xylinas E, Kluth LA, et al. Effect of statin use on outcomes of non-muscle-invasive bladder cancer. BJU Int 2013; 112:E4–E12. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva RD, Xylinas E, Kluth L, et al. Impact of statin use on oncological outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy. J Urol 2013; 190:1427. [DOI] [PubMed] [Google Scholar]
- 15.Choi SK, Min GE, Jeon SH, et al. Effects of statins on the prognosis of local and locally advanced renal cell carcinoma following nephrectomy. Mol Clin Oncol 2013; 1:365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton RJ, Morilla D, Cabrera F, et al. The association between statin medication and progression after surgery for localized renal cell carcinoma. J Urol 2014; 191:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaffenberger SD, Lin-Tsai O, Stratton KL, et al. Statin use is associated with improved survival in patients undergoing surgery for renal cell carcinoma. Urol Oncol 2015; 33:21e11–21e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viers BR, Houston Thompson R, Psutka SP, et al. The association of statin therapy with clinicopathologic outcomes and survival among patients with localized renal cell carcinoma undergoing nephrectomy. Urol Oncol Semin Orig Investig 2015; 33: 388e11-18. doi:10.1016/j.urolonc.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Caon J, Paquette M, Hamm J, et al. Does statin or ASA affect survival when prostate cancer is treated with external beam radiation therapy? Prostate Cancer 2014; 2014:184297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao C, Jacobsen SJ, Xu L, et al. Use of statins and prostate cancer recurrence among patients treated with radical prostatectomy. BJU Int 2013; 111:954–962. [DOI] [PubMed] [Google Scholar]
- 21.Chao C, Williams SG, Xu L, et al. Statin therapy is not associated with prostate cancer recurrence among patients who underwent radiation therapy. Cancer Lett 2013; 335:214–218. [DOI] [PubMed] [Google Scholar]
- 22.Cuaron J, Pei X, Cohen GN, et al. Statin use not associated with improved outcomes in patients treated with brachytherapy for prostate cancer. Brachytherapy 2015; 14:179–184. [DOI] [PubMed] [Google Scholar]
- 23.Geybels MS, Wright JL, Holt SK, et al. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. Prostate 2013; 73:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grytli HH, Fagerland MW, Fossa SD, et al. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 2014; 65:635–641. [DOI] [PubMed] [Google Scholar]
- 25.Gutt R, Tonlaar N, Kunnavakkam R, et al. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol 2010; 28:2653–2659. [DOI] [PubMed] [Google Scholar]
- 26.Ishak-Howard MB, Okoth LA, Cooney KA. Statin use and the risk of recurrence after radical prostatectomy in a cohort of men with inherited and/or early-onset forms of prostate cancer. Urology 2014; 83:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz MS, Carroll PR, Cowan JE, et al. Association of statin and nonsteroidal anti-inflammatory drug use with prostate cancer outcomes: results from CaPSURE. BJU Int 2010; 106:627–632. [DOI] [PubMed] [Google Scholar]
- 28.Kollmeier MA, Katz MS, Mak K, et al. Improved biochemical outcomes with statin use in patients with high-risk localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys 2011; 79:713–718. [DOI] [PubMed] [Google Scholar]
- 29.Kontraros M, Varkarakis I, Ntoumas K, et al. Pathological characteristics, biochemical recurrence and functional outcome in radical prostatectomy patients on statin therapy. Urol Int 2013; 90:263–269. [DOI] [PubMed] [Google Scholar]
- 30.Ku JH, Jeong CW, Park YH, et al. Relationship of statins to clinical presentation and biochemical outcomes after radical prostatectomy in Korean patients. Prostate Cancer Prostatic Dis 2011; 14:63–68. [DOI] [PubMed] [Google Scholar]
- 31.Misrai V, Do C, Lhez J-MM, et al. Is statin use associated with D’Amico risk groups and biochemical recurrence after radical prostatectomy? Prog Urol 2012; 22:273–278. [DOI] [PubMed] [Google Scholar]
- 32.Moyad MA, Merrick GS, Butler WM, et al. Statins, especially atorvastatin, may improve survival following brachytherapy for clinically localized prostate cancer. Urol Nurs 2006; 26:298–303. [PubMed] [Google Scholar]
- 33.Oh DS, Koontz B, Freedland SJ, et al. Statin use is associated with decreased prostate cancer recurrence in men treated with brachytherapy. World J Urol 2014; 33:93–97. [DOI] [PubMed] [Google Scholar]
- 34.Rieken M, Kluth LA, Xylinas E, et al. Impact of statin use on biochemical recurrence in patients treated with radical prostatectomy. Prostate Cancer Prostatic Dis 2013; 16:367–371. [DOI] [PubMed] [Google Scholar]
- 35.Song C, Park S, Park J, et al. Statin use after radical prostatectomy reduces biochemical recurrence in men with prostate cancer. Prostate 2015; 75:211–217. [DOI] [PubMed] [Google Scholar]
- 36.Soto DE, Daignault S, Sandler HM, et al. No effect of statins on biochemical outcomes after radiotherapy for localized prostate cancer. Urology 2009; 73:158–162. [DOI] [PubMed] [Google Scholar]
- 37.Yu O, Eberg M, Benayoun S, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol 2014; 32:5–11. [DOI] [PubMed] [Google Scholar]
- 38.Zaorsky NG, Buyyounouski MK, Li T, et al. Aspirin and statin nonuse associated with early biochemical failure after prostate radiation therapy. Int J Radiat Oncol Biol Phys 2012; 84:e13–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mass AY, Agalliu I, Laze J, et al. Preoperative statin therapy is not associated with biochemical recurrence after radical prostatectomy: our experience and meta-analysis. J Urol 2012; 188:786–791. [DOI] [PubMed] [Google Scholar]
- 40.Krane LS, Kaul SA, Stricker HJ, et al. Men presenting for radical prostatectomy on preoperative statin therapy have reduced serum prostate specific antigen. J Urol 2010; 183:118–125. [DOI] [PubMed] [Google Scholar]
- 41.Mondul AM, Han M, Humphreys EB, et al. Association of statin use with pathological tumor characteristics and prostate cancer recurrence after surgery. J Urol 2011; 185:1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choe KS, Cowan JE, Chan JM, et al. Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy. J Clin Oncol 2012; 30:3540–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niraula S, Pond G, de Wit R, et al. Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study. Can Urol Assoc J 2013; 7:E74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cattarino S, Seisen T, Drouin SJ, et al. Influence of statin use on clinicopathological characteristics of localized prostate cancer and outcomes obtained after radical prostatectomy: a single center study. Can J Urol 2015; 22:7703–7708. [PubMed] [Google Scholar]
- 45.Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med 2000; 6:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999; 284:1994–1998. [DOI] [PubMed] [Google Scholar]
- 47.Hindler K, Cleeland CS, Rivera E, et al. The role of statins in cancer therapy. Oncologist 2006; 11:306–315. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Sukumaran P, Varma A, et al. Cholesterol-induced activation of TRPM7 regulate cell proliferation, migration, and viability of human prostate cells. Biochim Biophys Acta 2014; 1843:1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bañez LL, Klink JC, Jayachandran J, et al. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev 2010; 19:722–728. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Mantovani A, Allavena P, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 51.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7:211–217. [DOI] [PubMed] [Google Scholar]
- 53.Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother 2014; 63:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann P, Roumeguère T, Schulman C, van Velthoven R. Use of statins and outcome of BCG treatment for bladder cancer. vol. 355. United States: 2006. doi:10.1056/NEJMc062714. [DOI] [PubMed] [Google Scholar]
- 55.Orsola A, Cecchini L, Bellmunt J. Statins and the effect of BCG on bladder cancer. N Engl J Med 2007; 356:1276. [PubMed] [Google Scholar]
- 56.Shi Y, Fung KZ, Freedland SJ, et al. Statin medications are associated with a lower probability of having an abnormal screening prostate-specific antigen result. Urology 2014; 84:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He Z, Mangala LS, Theriot CA, et al. Cell killing and radiosensitizing effects of atorvastatin in PC3 prostate cancer cells. J Radiat Res 2012; 53:225–233. [DOI] [PubMed] [Google Scholar]
- 58.Bruckert E, Ferrieres J. Evidence supporting primary prevention of cardiovascular diseases with statins: gaps between updated clinical results and actual practice. Arch Cardiovasc Dis 2014; 107:188–200. [DOI] [PubMed] [Google Scholar]
- 59.Hayden A, Douglas J, Sommerlad M, et al. The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol Oncol 2014; 32:806–814. [DOI] [PubMed] [Google Scholar]


