Abstract
An abnormal interaction in the brain–gut axis is regarded as the cause of irritable bowel syndrome (IBS). We attempted to determine the association between IBS and subsequent development of epilepsy.
A total of 32,122 patients diagnosed with IBS between 2000 and 2011 were identified from the Longitudinal Health Insurance Database as the study cohort, and 63,295 controls were randomly selected from the insurants without IBS and frequency-matched according to age, sex, and index year as the comparison cohort. Both cohorts were followed up until the end of 2011 to measure the incidence of epilepsy. We analyzed the risks of epilepsy using Cox proportional hazards regression models.
The IBS patients had greater cumulative incidence of epilepsy than the cohort without IBS (log-rank test, P < 0.001 and 2.54 versus 1.86 per 1000 person-years). The IBS cohort had a higher risk of epilepsy after adjusting for age, sex, diabetes, hypertension, stroke, coronary artery disease, head injury, depression, systemic lupus erythematosus, brain tumor, and antidepressants usage (adjusted hazard ratio [aHR]: 1.30, 95% confidence interval [CI]: 1.17–1.45). Stratified by the presence of other risk factors, the relative risk was also greater for patients with (aHR: 1.25, 95% CI: 1.10–1.41) or without other risk factors (aHR: 1.68, 95% CI: 1.35–2.10) in the IBS cohort than for those in the non-IBS cohort. The age-specific relative risk of epilepsy in the IBS cohort was greater than that in the non-IBS cohort for both 35 to 49 age group and 50 to 64 age group (age ≤ 34, aHR:1.31, 95% CI: 0.93–1.85; age 35–49, aHR: 1.43, 95% CI: 1.12–1.83; age 50–64, aHR: 1.56, 95% CI: 1.27–1.91). However, there was no difference between patients > 65 years with IBS and those without IBS (aHR: 1.11, 95% CI: 0.94–1.31).
This population-based cohort study revealed that IBS increases the risk of developing epilepsy. However, IBS may be less influential than other risk factors. Further study is necessary to clarify whether IBS is a risk factor or an epiphenomenon for epilepsy development.
INTRODUCTION
Irritable bowel syndrome (IBS) is characterized by a functional gastrointestinal disorder without structural or organic abnormality. However, patients with IBS have greater risks of comorbidities, higher total medical expenditures, and lower health-related quality of life.1–3 The prevalence of IBS for those at age 20 years and older receiving physical check-up is reported to be 17.5% to 22.1% in Taiwan.4 IBS has caused direct medical expenditures and indirect social costs, particularly after the implementation of the National Health Insurance (NHI) program in Taiwan.4,5 The definition of epilepsy requires the occurrence of at least 1 epileptic seizure, and epilepsy is a brain disorder characterized by an enduring predisposing factor to generate seizures with neurobiological, cognitive, and social consequences.6,7 The prevalence of epilepsy among Taiwan's 23,000,000 people, even with possible underestimation caused by a negative public attitude, reportedly ranges from 0.24% to 0.28%, according to a community-based survey.8–10
The etiology of IBS remains undetermined, even though changes in the microbiota balance of the gastrointestinal tract, dietary allergies, and deregulation of mucosal inflammatory mediators in the gastrointestinal tract have all been proposed as possible biological abnormalities of IBS.11–14 Currently, an abnormal interaction in the brain–gut axis is regarded as the pathophysiologic mechanism of IBS.15 The brain may change the gut motility and the growth, virulence, and gene expression of the microbiota from up to down by releasing stress hormones such as hypothalamic-pituitary-adrenal pathway, by inducing permeability of the gut to the microbiota such as activating a mucosal immune response, or by influencing autonomous nervous system (ANS) such as vagal nerve stimulation.15,16 Furthermore, gut microbiota can communicate with the brain–gut axis from down to up via endocrine messages of mucosa such as 5-hydroxytryptamine from entero-endocrine cells, via cytokines immune messages released from mucosal immune cells such as tumor necrosis factor-α (TNF-α) or interleukin-1β (IL-1β), or via afferent neural messages from the enteric nervous system (ENS) to influence the development and behavior of the brain.14,15 Furthermore, the IBS patients may, thus, become vulnerable to psychological interoception, cognitive impairment, and even epilepsy.15,17–19
It is widely accepted that chronic inflammation plays an important role in the bidirectional dysregulation of brain–gut axis to cause IBS.12,17 In addition to the traditional etiologies of epilepsy such as stroke, head injury, or central nervous system infection, inflammatory cytokines such as TNF-α and IL-1β are also believed to play an important role in epileptogenesis by inducing blood–brain-barrier leakage.20 However, the association between IBS and subsequent risk for epilepsy has not been well established. In our study, we hypothesize that a history of IBS might increase the risk of developing epilepsy.
To examine the association between IBS and subsequent development of epilepsy, a population-based cohort study was conducted, and data from a large medical database, the National Health Insurance Research Database (NHIRD), were analyzed.
METHODS
Data Source
Taiwan's NHI program was from 1995 and including >99% Taiwan's residents.21 In this study, we used the NHIRD (the version was updated in 2011) by application from the Bureau of National Health Insurance (BNHI), which includes the data of 1,000,000 insurants who were randomly selected from NHI beneficiaries (representing 5% of all insurants enrolled in Taiwan's NHI program: Longitudinal Health Insurance Database 2000 [LHID 2000]). The National Health Research Institutes (NHRI) states that no statistical differences in age, sex, and health care costs exist between LHID 2000 and NHIRD. The NHRI has encrypted all patient identification numbers for the protection of privacy. The details of the program have been well written in previous high-quality studies.22,23 The criteria of diseases were defined according to the International Classifications of Disease, 9th Revision, Clinical Modification (ICD-9-CM). The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2–115). The IRB also specifically waived the consent requirement.
Sampled Participants
In this retrospective cohort study, patients age 20 or older with newly diagnosed IBS (ICD-9-CM code 564.1) from 2000 to 2011 were identified as the IBS cohort. The index date of the IBS cohort corresponded to the date of initial IBS diagnosis. Patients age 20 or younger having epilepsy (ICD-9-CM code 345) before the index date or with incomplete age or sex information were excluded. Subjects without IBS were randomly selected from the LHID 2000 as the non-IBS cohort. The non-IBS cohorts were frequency matched with the IBS cohort at an ∼2:1 ratio for age (every 5 years), sex, the presence of epilepsy-associated risk factors such as diabetes, hypertension, stroke, coronary artery disease (CAD), head injury, depression, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), inflammatory bowel disease (IBD), meningitis, encephalitis, brain tumors, human immunodeficiency virus (HIV) infection, and antidepressants usage and the index year of diagnosing IBS.
Outcome and Epilepsy-Associated Risk Factors
The average follow-up period was 6.92 years for the patients in the IBS cohort and 6.80 years for the patients in the non-IBS cohort, respectively. All patients were followed from the index date until the date that epilepsy was diagnosed, withdrawal from the NHI program, loss to follow-up, or the end of 2011. Baseline epilepsy-associated risk factors included age, sex, diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), stroke (ICD-9-CM codes 430–438), CAD (ICD-9-CM codes 410–414), head injury (ICD-9-CM codes 850–854, 959.01), depression (ICD-9-CM codes 296.2, 296.3, 300.4, 311), SLE (ICD-9-CM code 710.0), RA (ICD-9-CM code 714), IBD (ICD-9-CM codes 555, 556), meningitis (ICD-9-CM codes 003.21, 013, 036.0, 047, 049, 053.0, 054.72, 072.1, 090.42, 091.81, 098.82, 100.81, 112.83, 114.2, 115, 117.5, 130, 320, 321, 322, 349.2), encephalitis (ICD-9-CM codes 013.6, 036.1, 046.2, 049.8, 049.9, 052.0, 054.3, 055.0, 056.01, 058.2, 062, 063, 064, 072.0, 090.41, 094.81, 130.0, 139.0, 323, 326), brain tumors (ICD-9-CM codes 191, 192.1, 198.4, 225, 228.02, 237.5, 237.72, 239.6), HIV infection (ICD-9-CM codes 795.71, V08, 042, 079.53), and antidepressants usage.
Statistical Analysis
The distributions of age, sex, and epilepsy-associated risk factors between the 2 cohorts were compared and examined using the chi-square test to examine categorical variables and Student t test to examine continuous variables. The follow-up time in person-years estimated incidence density of epilepsy among different risk factors and stratified by age, sex, and epilepsy-associated risk factors. Univariable and multivariable Cox proportional hazard regression models assessed hazard ratio (HR) and 95% confidence interval (CI) for epilepsy. Variables in the multivariable model included age, sex, diabetes, hypertension, stroke, CAD, head injury, depression, SLE, brain tumors, and antidepressants usage; all of the confounding factors showed a significant difference in the univariable Cox model. The Kaplan–Meier method was used to estimate cumulative incidence of epilepsy and the differences between the curves were tested with 2-tailed log-rank test. All analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC). All statistical tests were 2-sided and P value <0.05 was considered statistically significant.
RESULTS
The cumulative incidence of epilepsy in the IBS cohort was higher than that in the non-IBS cohort (log-rank test, P < .001) (Figure 1). The demographic characteristics and the presence of epilepsy-associated risk factors in the IBS cohort (N = 32,122) and non-IBS cohort (N = 63,295) are shown in Table 1. No statistical difference was found in the distributions of age, sex, diabetes, hypertension, and CAD between the IBS and non-IBS cohorts (mean age was approximately 52 years). Compared with patients in the non-IBS cohort, patients in the IBS cohort tended to have slightly more risks of stroke, head injury, depression, SLE, RA, IBD, meningitis, encephalitis, brain tumors, HIV infection, and antidepressants usage.
FIGURE 1.
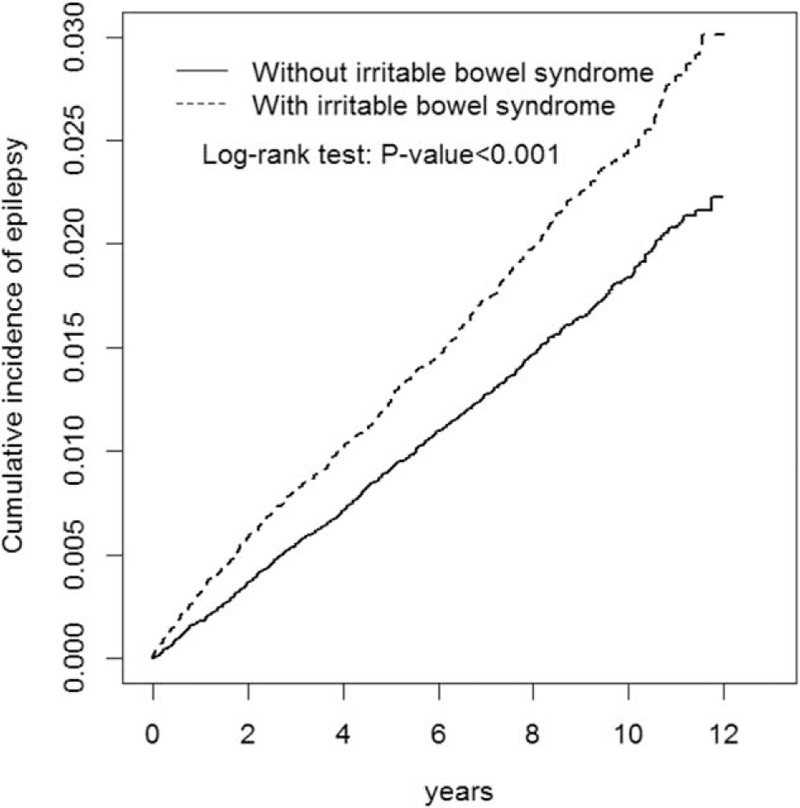
Cummulative incidence comparison of epilepsy for patients with (dashed line) or without (solid line) irritable bowel syndrome.
TABLE 1.
The Demographic Characteristics and the Presence of Epilepsy-Associated Risk Factors in the IBS Cohort and Non-IBS Cohort
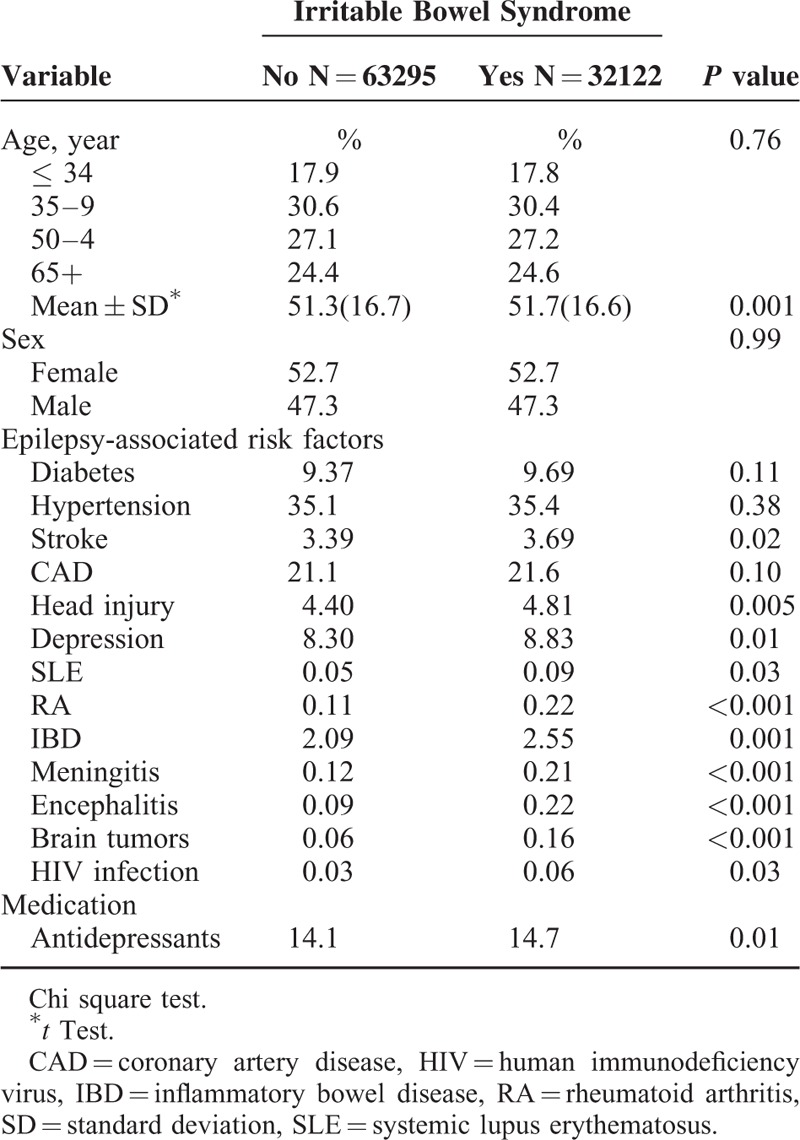
Table 2 shows the incidence of epilepsy and hazard ratio of risk factors for epilepsy. The overall incidence of epilepsy was 1.37-fold higher in the IBS cohort than in the non-IBS cohort (2.54 versus 1.86 per 1000 person-years). Compared with non-IBS cohort, IBS patients had a higher risk of epilepsy (adjusted hazard ratio [aHR]: 1.30, 95% CI: 1.17–1.45) after adjusting for age, sex, diabetes, hypertension, stroke, CAD, head injury, depression, SLE, brain tumors, and antidepressants usage. In the multivariable model, the risk of epilepsy increased with age from 0.99 to 1.84 and was 1.38-fold higher for men than for women (95% CI: 1.24–1.53). The risk of developing epilepsy was higher for patients with diabetes, hypertension, stroke, CAD, head injury, depression, SLE, brain tumors, or antidepressants usage.
TABLE 2.
Incidence of Epilepsy and Hazard Ratio of Epilepsy-Associated Risk Factors
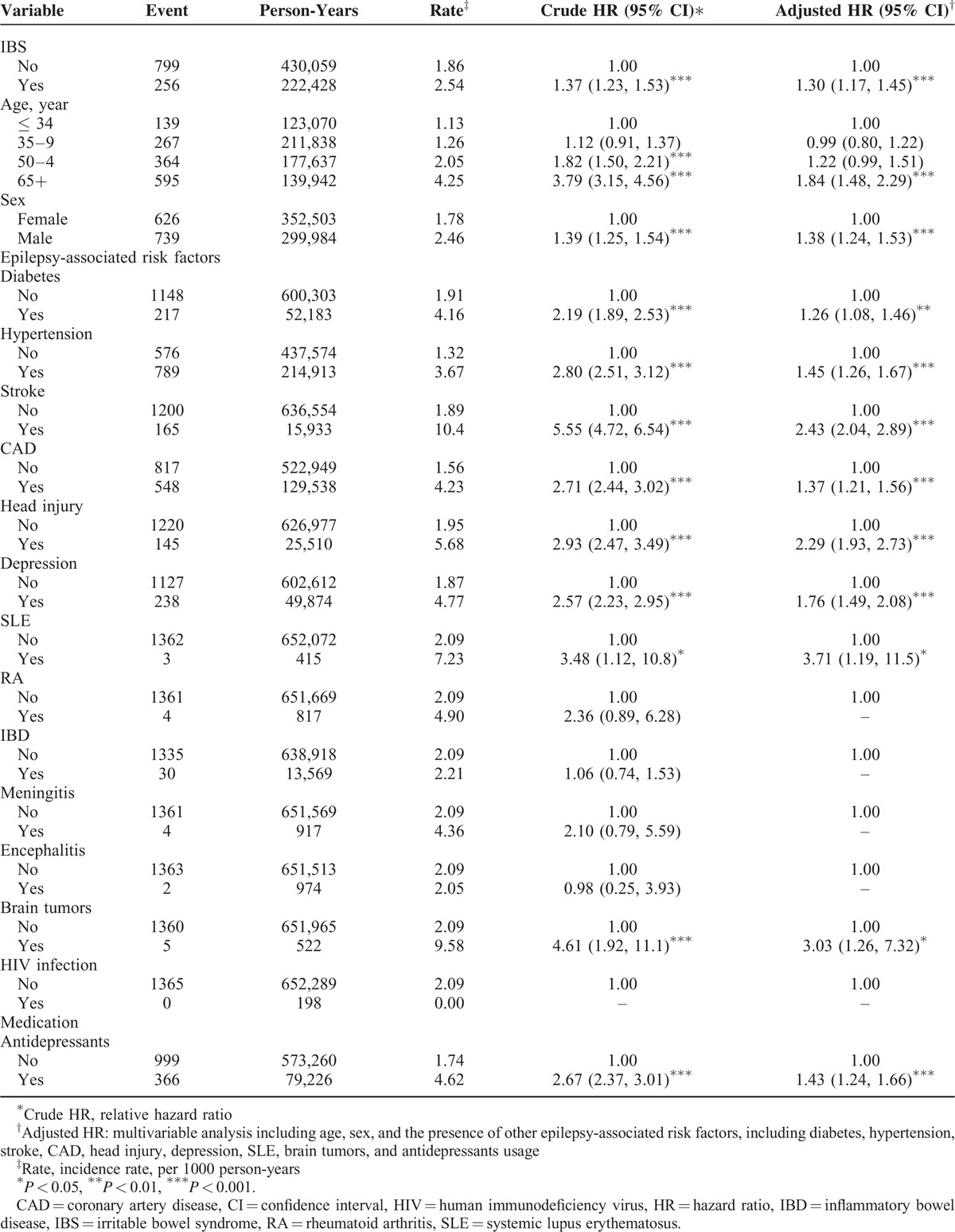
Table 3 shows the comparison of epilepsy incidence and Cox model measured hazards ratio between patients with IBS and without IBS after stratification by age, sex and the presence of other epilepsy-associated risk factors. The incidence of epilepsy increased with age in both cohorts. The age-specific relative risk of epilepsy in the IBS cohort was greater than that in the non-IBS cohort for both 35 to 49 age group and 50 to 64 age group. However, there was no difference between patients >65 years with IBS and those without IBS. The sex-specific relative risk of epilepsy in the IBS cohort was greater than in the non-IBS cohort for men (aHR: 1.48, 95% CI: 1.28–1.71). The relative risk of epilepsy was greater in the IBS cohort than in the non-IBS cohort for both patients with (aHR: 1.25, 95% CI: 1.10–1.41) or without (aHR: 1.68, 95% CI: 1.35–2.10) other epilepsy-associated risk factors.
TABLE 3.
The Comparison of Epilepsy Incidence and Cox Model Measured Hazards Ratio Between Patients With IBS and Without IBS After Stratification by Age, Sex, and the Presence of Other Epilepsy-Associated Risk Factors
DISCUSSION
The prevalence of IBS may be overestimated under the Taiwan NHI program, which provides easily accessible and affordable healthcare facilities. A total of 32,122 IBS patients were selected from 1,000,000 people, so the prevalence was ∼3.2% in our study and was different to that (17.5%–22.1%) reported by Lu et al.4 However, the candidates selected by Lu et al. were from the subjects attending a 2-day self-paid physical check-up and there may be a concern about the socioeconomic differences between the users of self-paid physical check-up and our general population. Furthermore, the prevalence of IBS for Chinese remains extremely variable with 22.8% to 0.82% in Beijing, 6.6% in Hong Kong, and 3.4% in Singapore.4,7,24 The reason for the sex predilection of IBS remains unknown. However, our study supports that various races may exhibit different sex predilections of IBS. In contrast to previous epidemiological studies showing that IBS is more common among females in the West, our study was consistent with the literature to show no sex predilection for IBS patients in the Asian population, including Taiwan, Hong Kong, Singapore, and Japan.4,24
Consistent with Western studies that have shown that IBS is more prevalent in those <50 years, we conclude that the peak age distribution of IBS is 35 to 49 years and then the prevalence of IBS decreased progressively with increasing age based on Table 1.25 However, in our study, the risk of epilepsy after IBS diagnosis increased with age progressively from 1.13 for patients 35 to 49 years to 2.57 for patients > 65 years. The latent time from the diagnosis of IBS to the development of epilepsy has not been ascertained, and further investigation is necessary to clarify the reason for the discordant peak age distribution between IBS and epilepsy. It is noted that our study showed that the age-specific relative risk of epilepsy in the IBS cohort was greater than that in the non-IBS cohort for both 35 to 49 age group and 50 to 64 age group. However, there was no difference between patients > 65 years with IBS and those without IBS. This finding may be explained by that the greater prevalence of other epilepsy-associated risk factors in the older patients decreased the contribution of IBS to the epilepsy risk.
Our study supports that the IBS cohort had more health-care-related illnesses. The reason IBS patients consult physicians more often remains unclear.26–28 Psychosocial factors, severe gastrointestinal symptoms, or physical comorbidities may predict patients’ medical consultation. This current epidemiological study demonstrated that IBS was associated with stroke, head injury, depression, SLE, RA, IBD, meningitis, encephalitis, brain tumor, HIV infection, and antidepressants usage. Furthermore, epilepsy was more common in patients with IBS, age > 65 years, male sex, diabetes, hypertension, stroke, CAD, head injury, depression, SLE, brain tumor, and antidepressants usage. Despite the association between IBS and several traditional risk factors for epilepsy, our study demonstrated that IBS was associated with epilepsy development after adjustment for age, sex, diabetes, hypertension, stroke, CAD, head injury, depression, SLE, brain tumor, and antidepressants usage. However, further study is required to ascertain whether IBS is a risk factor or an epiphenomenon for epilepsy development.
Some studies have suggested that the association between IBS and epilepsy may be due to shared pathophysiological mechanisms. First, Gut microbiota can access the brain and affect host behavior through the cytokines released from the mucosal immune cells, gut neuroendocrine transmitters released from gut endocrine cells, or autonomic nervous system.29,30 Systemic inflammation and peripheral inflammation from the gut mucosa can induce the glial cells and the neurons to synthesize the pro- and anti-inflammatory cytokines. The cytokines, then, can migrate into the brain through the cytokine transporters to change the permeability of blood–brain barrier.20 In addition, morphological changes in gray and white matter have been observed through magnetic resonance imaging in patients with IBS.31,32 Second, cytokines are regarded as a factor linking IBS and epilepsy; Riazi et al have demonstrated that cytokines can increase seizure excitability in animal models of colitis and that postnatal inflammation increases seizure susceptibility in adult rats.33,34 Our finding of higher epilepsy incidence after IBS diagnosis supports the observation in animal models and provides a possible rationale for studying the pathophysiologic mechanism between IBS and epilepsy and providing pharmaceutical choice. Finally, the interactive linkage by stroke and SLE may induce the development of both IBS and epilepsy. Stroke may reflex atherosclerosis caused by endothelial dysfunction, which can stimulate innate immune system to produce cytokines.35,36 Our result, the association between SLE and the development of IBS or epilepsy, was consistent with the literature to support that inflammatory cytokines or cells may play a crucial role in the pathogenesis of IBS and epilepsy; even the inflammatory cytokines or cells levels could not be quantified in our epidemiological study. Furthermore, autoimmune diseases with elevated neuronal autoantibodies in the cerebrospinal fluid (CSF) have been increasingly identified in a subset of epilepsy without previously known disorders.37 Additionally, the synthesis and release of pro-inflammatory cytokines and chemokines were systemically increased in autoimmune diseases.
By contrast, the association between IBS and epilepsy may be due to shared risk factors because the prevalence of important epilepsy risk factors was higher in the patients with IBS compared with the patients without IBS. However, it is reasonable to conclude that the increased risk of epilepsy observed in the patients with IBS was more likely due to the effect of IBS status, because the possible confounding effect of epilepsy risk factors was substantially minimized in our study. The epilepsy risk contributed by IBS has decreased with age because of the greater prevalence of other epilepsy-associated risk factors in the older patients. Furthermore, the results of our subgroup analyses in which we excluded patients with influential risk factors at baseline (Table 3) confirmed the validity of our results. This finding, coupled with the results of the subgroup analyses, confirms the possible causal association between IBS and epilepsy and suggests that IBS is a possible risk factor for epilepsy. Nevertheless, our results showed that IBS may be inferior to the traditional epilepsy-associated risk factors such as age 65 years, male sex, hypertension, stroke, CAD, head injury, depression, SLE, brain tumor, and antidepressants usage, in contributing to the development of epilepsy.
To our knowledge, this is the first population-based study to examine the association between IBS and subsequent development of epilepsy. The national database contains a representative cohort of 1,000,000 citizens covered by the Taiwan NHI program, and the 12-year observation period is beneficial for statistical analysis. The recruited patients were sampled from ‘99% of the residents, a stable population of Taiwan. Our study also offered a longitudinal study, rather than a cross-sectional approach, to evaluate the association between IBS and epilepsy. Moreover, this is the first study of humans to demonstrate an increased incidence of developing epilepsy after IBS diagnosis, even though some risk factors for IBS have been found to be related to the development of epilepsy.
Our study had some limitations. First, we have concerns about the validity of the IBS and epilepsy diagnoses among the patients in our study. However, the Rome criteria and ICD coding are widely used in Taiwan for diagnoses of IBS and epilepsy, and the BNHI organizes medical experts to regularly audit the accuracy of insurance claims codes in Taiwan. Additionally, a bias may have resulted from the fact that patients with IBS seek more medical care than do patients without IBS and are thus more likely to be diagnosed with epilepsy during follow-up. Moreover, the study subjects with IBS diagnosis from 1996 to 1999 were not included at the baseline, and we can only recruit the patients with newly diagnosed IBS from 2000 to 2011. Therefore, some cases of IBS were likely to be excluded in the study cohort. However, the risk of epilepsy remained higher among patients in the IBS cohort than among those in the non-IBS cohort after adjusting for other epilepsy-associated risk factors. Second, we may have overlooked some potential confounding factors because the NHIRD does not offer detailed information about the lifestyle, socioeconomic status, and family history of patients. Third, patients not included in the NHI program were not included in our study population. However, the Taiwan NHI program currently covers > 99% of the Taiwanese population. Finally, ICD-9-CM could not offer the identification of the different subtypes of IBS to assess the incidence of epilepsy in the different subtypes of IBS. Moreover, the data of breath test for small intestinal bacterial overgrowth was unavailable in our study. However, the impact of this classification on treatment response needs validation even there is a good correlation between stool form and colonic transit times. Furthermore, this classification does not take into account the individual susceptibility of visceral sensitivity.24
In conclusion, our population-based cohort study shows that IBS increases the risk of developing subsequent epilepsy. However, IBS may be less influential than other epilepsy-associated risk factors, including age greater than 65 years, male sex, hypertension, stroke, CAD, head injury, depression, SLE, brain tumor, and antidepressants usage, on epilepsy development. The patients with IBS tend to have more health-care-related illnesses. Our study shows no differences in sex between patients with IBS and those without IBS. Further study is necessary to clarify whether IBS is a risk factor or an epiphenomenon for epilepsy development.
Acknowledgments
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Footnotes
Abbreviations: aHR = Adjusted hazard ratio, ANS = Autonomous nervous system, BNHI = Bureau of National Health Insurance, CAD = Coronary artery disease, CI = Confidence interval, CSF = Cerebrospinal fluid, ENS = Enteric nervous system, HIV = Human immunodeficiency virus, HR = Hazard ratio, IBD = Inflammatory bowel disease, IBS = Irritable bowel syndrome, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IL-1β = Interleukin-1β, IRB = Institutional Review Board, LHID 2000 = Longitudinal Health Insurance Database 2000, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, RA = Rheumatoid arthritis, SLE = Systemic lupus erythematosus, TNF-α = Tumor necrosis factor-α.
Author contributions: These authors’ individual contributions were as follows. These authors’ individual contributions were as follows. Conception and design: Chien Hua Chen, Chia-Hung Kao. Administrative support: Chia-Hung Kao. Collection and assembly of data: All authors. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use, and cost: A U.S. managed care perspective. Am J Gastroenterol 2003; 98:600–607. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal N, Spiegel BM. The effect of irritable bowel syndrome on health-related quality of life and health care expenditure. Gastroenterol Clin North Am 2011; 40:11–19. [DOI] [PubMed] [Google Scholar]
- 3.Vandvik PO, Wilhelmsen I, Ihleback C, et al. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implication. Aliment Pharmacol Ther 2004; 20:1195–1203. [DOI] [PubMed] [Google Scholar]
- 4.Lu CL, Chen CY, Lang HC, et al. Current patterns of irritable bowel syndrome in Taiwan: the Rome II questionnaire on a Chinese population. Aliment Pharmacol Ther 2003; 18:1159–1169. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel BM. Burden of illness in irritable bowel syndrome: looking beyond the patient. Clin Gastroenterol Hepatol 2013; 11:156–157. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RS, Boas WVE, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by an International League Bureau for epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005; 46:470–472. [DOI] [PubMed] [Google Scholar]
- 7.Mac TL, Tran DS, Quet F, et al. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet 2007; 6:533–543. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Chen LS, Yen MF, et al. Geographic variation in the age- and gender-specific prevalence and incidence of epilepsy: analysis of national Health Insurance-based data. Epilepsia 2012; 53:283–290. [DOI] [PubMed] [Google Scholar]
- 9.Su CL, Chang SF, Chen ZY, et al. Neuroepidemiological survey in Ilan, Taiwan (NESIT): prevalence of epilepsy. Acta Neurol Taiwanica 1998; 7:75–84. [Google Scholar]
- 10.Chen CC, Chen TF, Hwang YC, et al. Population-based survey on prevalence of adult patients with epilepsy in Tawan (Keelung community-based integrated screening no. 12 Epilepsy Res 2006; 72:67–74. [DOI] [PubMed] [Google Scholar]
- 11.Jones MP, Dilley JB, Drossman D, et al. Bran–gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil 2006; 18:91–103. [DOI] [PubMed] [Google Scholar]
- 12.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010; 7:163–173. [DOI] [PubMed] [Google Scholar]
- 13.Feinle-Bisset C, Vozzo R, Horowitz M, et al. Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol 2004; 99:170–181. [DOI] [PubMed] [Google Scholar]
- 14.Verdu EF, Collins SM. Microbial–gut interactions in health and disease. Irritable bowel syndrome. Best Pract Res Clin Gastroenterol 2004; 18:315–321. [DOI] [PubMed] [Google Scholar]
- 15.Thakur AK, Shakya A, Husain GM, et al. Gut-microbiota and mental health: current and future perspectives. J Pharmacol Clin Toxicol 2013; 2:1016–1030. [Google Scholar]
- 16.Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859. [DOI] [PubMed] [Google Scholar]
- 17.Bashashati M, Rezaei N, Andrews CN, et al. Cytokines and irritable bowel syndrome: where do we stand? Cytokines 2012; 57:201–209. [DOI] [PubMed] [Google Scholar]
- 18.Di Lorenzo Camilleri M. C Brain-gut axis: from basic understanding to treatment of IBS and related disorders. J Pediatr Gastroenterol Nutr 2012; 54:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grodzinsky E, Walter S, Viktorsson L, et al. More negative self-esteem and inferior coping strategies among patients diagnosed with IBS compared with patients without IBS- a case-control study in primary care. BMC Family Practice 2015; 16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Bauer S, Nowak M, et al. Cytokines and epilepsy. Seizure 2011; 20:249–256. [DOI] [PubMed] [Google Scholar]
- 21.Cheng TM. Taiwan's National Health Insurance system: high value for the dollar, in Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan, K.G.H. Okma and L. Crivelli, Editors. 2009, World Scientific: New Jersey pp. 71–204. [Google Scholar]
- 22.Chao CH, Lin CL, Wang HY, et al. Increased subsequent risk of erectile dysfunction in patients with irritable bowel syndrome: a nationwide population-based cohort study. Andrology 2013; 1:793–798. [DOI] [PubMed] [Google Scholar]
- 23.Chen YK, Yeh JH, Lin CL, et al. Cancer risk in patients with cholelithiasis and after cholecystectomy: a nationwide cohort study. J Gastroenterol 2014; 49:923–931. [DOI] [PubMed] [Google Scholar]
- 24.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol 2009; 24:1601–1607. [DOI] [PubMed] [Google Scholar]
- 25.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heaton KW, O’Donnell LJ, Braddon FE, et al. Symptoms of irritable bowel syndrome in a British urban community: consulters and nonconsulters. Gastroenterology 1992; 304:87–90. [DOI] [PubMed] [Google Scholar]
- 27.Drossman DA, Mckee DC, Sandler RS, et al. Psychosocial factors in irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology 1988; 95:701–708. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead WE, Bosmajian L, Zonderman AB, et al. Symptoms of psychological distress associated with irritable bowel syndrome. Comparison of community and medical clinic samples. Gastroenterology 1988; 95:709–714. [DOI] [PubMed] [Google Scholar]
- 29.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 303:G141–G154. [DOI] [PubMed] [Google Scholar]
- 30.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10:735–742. [DOI] [PubMed] [Google Scholar]
- 31.Seminowicz DA, Labus JS, Bueller JA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 2010; 139:e42–e57.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piche M, Chen JI, Roy M, et al. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain 2013; 14:1217–1226. [DOI] [PubMed] [Google Scholar]
- 33.Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res 2010; 89:34–42. [DOI] [PubMed] [Google Scholar]
- 34.Galic MA, Riazi K, Heida JG, et al. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci 2008; 28:6904–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakka HMM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 36.McGovern PG, Jacobs DR, Jr, Shahar E, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation 2001; 104:19–24. [DOI] [PubMed] [Google Scholar]
- 37.Ong MS, Kohane I, Cai T, et al. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol 2014; 7:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]


