Abstract
Several epidemiologic studies in Western countries have examined the association between asthma and prostate cancer risk, but the results have been inconclusive. We investigated this association in a large, nationwide, population-based case-cohort study. Using the Taiwan National Health Insurance Research Database from 1997 to 2008, we collected data from 12,372 men, including 4124 with asthma and 8248 age-, residence-, and insurance premium-matched control subjects, who were never diagnosed with asthma. Competing risk-adjusted Cox proportional hazards regression was used to calculate the hazard ratios (HRs) and 95% confidence interval (CI) for determining the association between prostate cancer and asthma. During a mean follow-up of 5.05 years (standard deviation, 2.10), there were 74 cases of prostate cancer. The incidence of prostate cancer was 163.0/100,000 person-years (95% CI: 113.0–228.0) in the asthma patients. Asthma was significantly associated with prostate cancer (HR: 2.36; 95% CI: 1.22–4.57; P = 0.011) after adjusting for age, residential area, insurance premium, hypertriglyceridemia, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, duration of hospitalization, and mortality. In the subgroup analysis, independent risk factors for prostate cancer among men with asthma were age (HR: 1.09; 95% CI: 1.05–1.21; P < 0.001) and hypertension (HR: 2.75; 95% CI: 1.24–7.80; P = 0.047). The results of our study suggest that men with asthma have an increased risk of prostate cancer.
INTRODUCTION
Asthma is one of the most common allergic diseases worldwide. Clinically, asthma is a reversible form of bronchial hypersensitivity characterized by chronic airway inflammation and repeated episodes of wheezing, breathlessness, cough, and chest tightness.1 The increasing global prevalence of asthma and the long-term consequences of the affected patients is worthy of attention. There have recently been increasing numbers of epidemiologic studies to investigate the relationship between asthma and cancer. However, the results remain controversial. Asthma may be associated with lower risks of ovarian cancer2 and brain tumor3–5; positive associations with the risk of lung cancer,6,7 stomach cancer,8 colon cancer,8 and pancreatic cancer have also been reported.9
Allergy-related carcinogenesis is a topic of interest but has generated considerable controversy. Two hypotheses that attempt to explain the possible mechanism between allergic disease and cancer are immune surveillance and the antigenic stimulation theory.10 Allergy might enhance the human immune system to recognize and eliminate cancer cells. In contrast, the antigenic stimulation hypothesis proposes that hyperactive immune conditions trigger chronic cellular inflammation, resulting in DNA mutation in dividing cells and inevitably leading to cancer development.10
Prostate cancer is one of most common cancer in Western countries and remains a major public health problem.11 Some causative factors have been implicated in prostate carcinogenesis, including androgen, diet, environmental factors, obesity, and chronic prostate inflammation.12–14 There are also some studies addressing systemic inflammation disease conditions that might affect prostate tumorigenesis. Kazma et al15 investigated the association of inflammation and prostate cancer risk by comparing individual single-nucleotide polymorphisms between advanced prostate cancer patients and normal controls. The study showed that 4 genes (TLR1, TLR6, OAS1, and OAS2) involved in the innate inflammation pathways were nominally related to advance prostate cancer risk. The other study by Toriola et al16 showed that elevated leukocyte count, a marker of systemic inflammation, was highly associated with increasing prostate cancer risk. Because asthma is a chronic inflammatory disease, several studies have examined the association between asthma and prostate cancer. Severi et al17 conducted a large-scale cohort study in Melbourne, which demonstrated a small increase in prostate cancer risk among asthma patients.17 In contrast, a recent large study by Platz et al18 showed that men who were never diagnosed with asthma were unlikely to develop lethal prostate cancer. The incidence and biologic characteristic of prostate cancer is quite different in Asian men than Caucasian men.19,20 To the best of our knowledge, there is no study that has investigated the association of asthma with prostate cancer risk in an Asian population.
Given the previous inconclusive results and lack of Asian data, we conducted a large-scale, population-based, case-cohort study to evaluate the relationship between asthma and prostate cancer in Taiwan.
METHODS
Source Population and Data
Longitudinal data during the period from 1997 to 2008 were collected from the National Health Insurance Research Database (NHIRD) of 1 million Taiwanese residents, which is approximately 5% of the total number of beneficiaries covered by the Taiwan National Health Insurance (NHI) program. The NHI program was launched on March 1, 1995, and provides universal, compulsory, and nationally administered health insurance that covers nearly all health care services for >99% of the Taiwanese population. The NHIRD includes patient identification number, sex, date of birth, diagnostic codes, medications, and the dates of hospitalizations and/or medical visits. The NHI Bureau also requires the registration of patients with serious disabling disease (SDD) such as end-stage renal disease, congenital abnormalities, and cancer. The final approval of SDD certificates requires strict evaluation by experts in the NHI Bureau. Patients with SDD certificates are eligible for exemption from insurance premiums and copayments. Because of the universal coverage of the island population and detailed medical information, the NHIRD has been used in many epidemiologic studies.21–23
Study Sample
The NHI diagnosis coding follows the system used in the International Classification of Diseases, Ninth Revision (ICD-9). Because this was a case-cohort study, we assigned an entrance date or so-called index date for our study patients. The index date represents the date of the first diagnosis of asthma; this index date was also assigned to the nonasthma control. The study subjects were men >15 years who were newly diagnosed with asthma (ICD-9 codes, 493.XX) between January 1, 2000, and December 31, 2008. Medical claims data from 1997 to 1999 were used to confirm that all asthma cases were newly diagnosed asthma patients. That meant that the asthma patients in this study were not diagnosed with asthma at least 3 years preceding their index date in our database. This process, essentially, certified the asthma first diagnosis date (index date) of the subjects more precisely. An asthma case is defined as any inpatient diagnosis of asthma and/or an outpatient diagnosis of asthma within at least 1 year. All asthma diagnoses were confirmed by at least one pulmonary function test after the date of asthma diagnosis.
Similarly, prostate cancer claim records were collected for patients with ICD-9 code 185.XX. A diagnosis of prostate cancer is defined on the basis of the histopathologic findings or significantly elevated prostate-specific antigen with radiologic evidence of metastasis. All prostate cancer patients had been issued SDD certificates after expert review in the NHI Bureau, as per protocol. The control group consisted of age-, sex-, residence-, and insurance premium-matched patients without a history of asthma in a 1:2 ratio. The primary study endpoint was the diagnosis of prostate cancer, corroborated by the SDD certificate.
Ethics Statement
The Institutional Review Board (IRB) of the Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, approved this study. Written consent from the study patients was not required because data were collected from the NHI database, which consists of deidentified secondary data used for research purposes, and the IRB gave a formal written waiver of the need for consent.
Measurement of Covariates
The diseases that were previously identified as risk factors for prostate cancer included covariates, such as hypertriglyceridemia (ICD-9 codes 272.1, 272.2, 272.3), hypertension (ICD-9 codes 401–405), diabetes mellitus (DM; ICD-9 code 250), and chronic obstructive pulmonary disease (COPD; ICD-9 codes 491, 492, 494, 496).
Statistical Analysis
The Student t test and the χ2 test were used to compare baseline characteristics. Death before prostate cancer was considered a competing risk event. Death-adjusted cumulative incidences of prostate cancer were calculated using the Fine and Gray24 method. Each person's first presentation within the study period was used to calculate outcome risk over given time intervals. The risks of prostate cancer during follow-up were calculated by survival analysis, with the time function calculated as the number of years from the index date to the end of follow-up, death, or migration, whichever occurred earlier. As mentioned, the index date was the first date of asthma diagnosis and was assigned to their matched controls. Competing risk-adjusted Cox regression models24 were fitted to estimate the association of asthma with prostate cancer after adjusting for covariates, including age, residential area, insurance premium, hypertriglyceridemia, hypertension, DM, COPD, duration of hospitalization, and mortality. Because residential area, insurance premium, and duration of hospitalization might affect the screen rate of prostate cancer, we included these factors in the adjusted analysis. Competing risk-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Statistical analyses and data management was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC). All tests were 2-sided and P values <0.05 were considered statistically significant. The cumulative incidences and Cox models in the competing risk analysis were calculated using the R package “cmprsk.”25
RESULTS
Characteristics of Patients
We analyzed data from 4124 male asthma patients and 8248 age-, sex-, residence-, and insurance premium-matched control patients. The mean (standard deviation, SD) age was 54.87 (18.69) years. Table 1 summarizes the characteristics of both the groups. Compared with the controls, asthma patients had higher prevalences of hypertriglyceridemia (P < 0.001), hypertension (P < 0.001), COPD (P < 0.001), and more days of hospitalization (P < 0.001). The defined daily dose (DDD) of steroid was significantly higher in the asthma group than in the controls (P < 0.001). The prevalence of DM was similar between asthma and control patients (P = 0.073).
TABLE 1.
Characteristics of Patients With and Without Asthma in the Present Study

Incidence of Prostate Cancer
After a mean (SD) duration of follow-up of 5.05 (2.10) years, there were 74 cases of prostate cancer: 34 (0.82%) had a history of asthma and 40 (0.48%) did not (Table 1). The incidence of prostate cancer was 163.0/100,000 person-years (95% CI: 113.0–228.0) in the asthma patients (study group) and 96.0/100,000 person-years (95% CI: 68.6–130.7) in the control group. Kaplan–Meier analysis of the cumulative incidence of prostate cancer showed that the rate of prostate cancer was significantly higher among patients with asthma than among the control group (P < 0.001, by modified log-rank test) (Figure 1).
FIGURE 1.
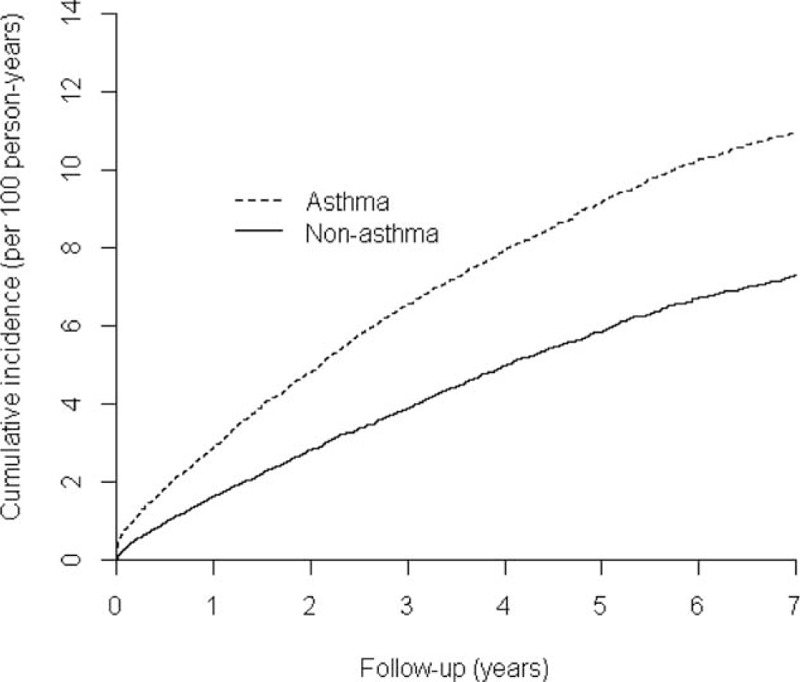
Cumulative incidence on prostate by study groups (asthma vs nonasthma, modified log-rank test; P < 0.001).
Multivariate Analysis of Risk Factors for Prostate Cancer
Table 2 shows the results of competing risk-adjusted Cox regression analysis for prostate cancer. Patients with a history of asthma were significantly associated (HR: 2.36; 95% CI: 1.22–4.57; P = 0.011) with prostate cancer after adjusting for age, residential area, insurance premium, hypertriglyceridemia, hypertension, DM, COPD, duration of hospitalization, and mortality. Age was also independently associated with a prostate cancer diagnosis (HR: 1.08; 95% CI: 1.06–1.10; P < 0.001). In stratified analysis of patients aged 15 to 64 years and ≥65 years, the association between asthma and prostate cancer was more obvious among those aged ≥65 years (HR: 1.85; 95% CI: 1.08–3.16; P = 0.025). The association between asthma and prostate cancer among those aged 15 to 64 years was not significant (HR: 1.40; 95% CI: 0.48–4.07; P = 0.536).
TABLE 2.
Independent Predictors of Prostate Cancer Among Enrolees Identified by Competing Risk-Adjusted Cox Regression Analysis

Risk Factors for Prostate Cancer Among Asthma Patients
We performed subgroup analysis to identify factors that predisposed men with asthma to prostate cancer. The results are shown in Table 3. In competing risk-adjusted Cox regression analysis, the risk of prostate cancer was not associated with residence, insurance premium, use of steroids, hypertriglyceridemia, DM, COPD, or duration of hospitalization. However, age (HR: 1.09; 95% CI: 1.05–1.12; P < 0.001) and diagnosis of hypertension (HR: 2.75; 95% CI: 1.27–7.80; P = 0.047) were independently associated with prostate cancer risk in patients with asthma.
TABLE 3.
Risk Factors for Prostate Cancer in Asthma Patients
DISCUSSION
In this population-based case-cohort study, history of asthma was associated with higher risk of prostate cancer among Taiwanese men with asthma, after adjustment for potential confounders, including age, residential area, insurance premium, medication, hypertriglyceridemia, hypertension, DM, COPD, duration of hospitalization, and mortality. To the best of our knowledge, this is the first study to identify a relationship between asthma and prostate cancer risk in an Asian population.
The strengths of the present study should be emphasized. First, the selection criteria for enrolment of asthma patient were very rigorous. In addition to ICD-9 coding by experienced clinicians, we required at least 1 pulmonary function test as objective diagnostic evidence. Confirmation of prostate cancer and issuance of SDD certificates were done by independent experts in the NHI Bureau. These methods helped us to enroll “true” prostate cancer and asthma cases and diminished selection bias. Second, this nationwide study had a cohort size of >12,000 patients, who were studied over a long follow-up period. Third, we analyzed the relationship between the DDD of steroid for asthma and incidence of prostate cancer. In comparison to the previous studies, our results provided measurements that allow for dose-relative adjustment of confounding.
The relationship between asthma and overall cancer risk has been carefully investigated over several decades. Although numerous studies have attempted to address this complex relationship, the results remain contradictory. The National Health and Nutrition Examination Survey, a large-scale cohort study conducted from 1971 to 1975, enrolled a total of 6913 patients with various atopic disorders, one of which was asthma.26 After 10 years of follow-up, history of allergy was associated with an increase in the overall cancer risk (odds ratio [OR]: 1.4; 95% CI: 1.1–1.77); however, the association was not significant among asthma patients (OR: 1.02; 95% CI: 0.63–1.65). Another large population study of 34,198 patients also found no association between the history of asthma and cancer development.27 However, a positive relationship between asthma and various cancers was reported in a Finnish study: the risk of lung cancer was significantly elevated in both the sexes (standardized incidence ratio [SIR]: 1.32 and 1.68 among men and women, respectively).28 Subgroup analysis showed higher risks of rectal cancer in women (SIR, 1.42) and bladder cancer in men (SIR, 1.25), and lower risks of uterine cancer (SIR, 0.76) and multiple myeloma (SIR, 0.53) in women and laryngeal cancer (SIR, 0.63) in men. In a study using questionnaire-based interviews and self-reported asthma and medication history, El-Zein et al29 reported a borderline protective relationship between asthma and cancer risk in men (OR: 0.72; 95% CI: 0.5–1.1). These conflicting results may be due to methodological factors such as surveillance and selection bias, inconsistency in the definition of allergy, variation in the assessment tools used (eg, self-reported questionnaire, skin prick testing, and/or serum immunoglobulin E concentration), and reverse causality.30 In addition, several socioeconomic factors (eg, smoking, alcohol consumption, and/or occupation) that probably confound final outcomes are not recorded in large registry databases.
The prevalence of both asthma and the incidence of prostate cancer is increasing worldwide, and the possibility of an association has increased speculation and research interest.11,31 In the earlier mentioned Finnish study, prostate cancer incidence was not significantly higher in asthma patients (SIR: 1.1; 95% CI: 0.97–1.24).28 The recent Platz et al18 prospective questionnaire-based cohort study reported that men with a history of asthma had a 30% risk reduction of lethal prostate cancer. In contrast, a study in Busselton, Australia, found 86 of 1552 men with asthma had a prostate cancer diagnosis during follow-up.32 Patients with a history of asthma had a significantly higher risk of prostate cancer (HR: 1.89; 95% CI: 1.00–3.60). The results were very similar to those of a recent study in Melbourne, Australia. In the large prospective cohort analysis of 16,934 men with a detailed asthma medication history, Severi et al17 reported that prostate cancer risk was significantly higher in patients with asthma symptoms (HR: 1.25; 95% CI: 1.05–1.49). Moreover, the risk was further increased among those taking antiasthma medications such as inhaled glucocorticoids (HR: 1.39; 95% CI: 1.03–1.88), systemic glucocorticoids (HR: 1.71; 95% CI: 1.08–2.69), and bronchodilators (HR: 1.36; 95% CI: 1.05–1.76). In the present nationwide study, asthma was an independent factor for prostate cancer and was associated with a 136% increase in risk after careful adjustment for possible confounding factors. Notably, we did not find a direct association of prior use of glucocorticoids with a prostate cancer diagnosis. Glucocorticoids bound with mutated androgen receptors may have a role in the proliferation and progression of androgen-independent prostate cancer.33 However, there is insufficient evidence to support this theory in relation to prostate carcinogenesis.
In this study, we identified a cancer-susceptible population among asthma participants. In addition to age, a well-known risk factor for prostate cancer, we found that the risk of prostate cancer in hypertensive men was 2.75 times than that of normotensive men, after adjusting for confounders such as hypertriglyceridemia, DM, and COPD. Till date, the relationship between hypertension and prostate cancer remains uncertain. Martin et al34 found a 4% increase in prostate cancer risk per 1-SD increase in systolic blood pressure, and patients with higher blood pressures were associated with more advanced disease (HR: 1.16; 95% CI: 1.05–1.27). In the prospective Norwegian study that evaluated the link between metabolic syndrome and prostate cancer, several documented metabolic factors (ie, body mass index, waist circumference, waist–hip ratio, and hyperlipidemia) were not associated with prostate cancer.35 However, a 1-SD (12 mm Hg) increase in diastolic blood pressure was associated with an 8% increase in risk for prostate cancer. For researchers, the mechanism of hypertension-related prostate carcinogenesis remains a puzzle. Elevated blood pressure could enhance sympathetic tone, thereby stimulating androgen-mediated prostate cancer development.36 Some investigators have suggested that hypertension downregulates insulin growth factor-binding protein-1, thereby increasing the level of insulin growth factor-1 and prostate cancer risk.37 However, findings from a recent study of a cohort of Swedish construction workers argue against these hypotheses. In an analysis of 336,159 men during an average of 22.2 years of follow-up, systolic and diastolic blood pressures were inversely associated with prostate cancer risk.38 Men in the highest quintile of systolic blood pressure had a 16% lower risk of prostate cancer than those in the lowest quintile. In the present study, we carefully adjusted for confounding metabolic factors such as DM and hypertriglyceridemia and found that hypertension was an independent risk factor for prostate cancer. This finding indicates that clinicians must remain vigilant for such cancer-susceptible groups when developing screening protocols for prostate cancer.
This study has limitations. First, we were unable to collect laboratory data and the claims database included no explicit personal information, that is, on covariates such as family history of asthma, body mass index, tobacco and alcohol use, lifestyle, and environmental exposures. Second, the lack of detailed histopathologic information and definite tumor node metastasis staging of prostate cancer made subgroup analysis impossible. Third, because we used much more rigorous enrolment criteria and excluded many potential candidates who had only clinical diagnoses, the number of cases enrolled was smaller than that in similar previous studies.
In conclusion, we observed that asthma was an independent risk factor for prostate cancer in this retrospective case-cohort study of Asian men. Among patients with asthma, old age and hypertension were associated with increased risk of prostate cancer development. Therefore, active surveillance of prostate cancer should be incorporated into the treatment program for patients with asthma. Further research on the mechanisms underlying these associations is warranted.
Acknowledgments
This study was partially based on the data obtained from the National Health insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan, and managed by the National Health Research Institutes. The interpretations and conclusions contained herein did not represent those of the Bureau of National Health Insurance, Department of Health, or that of the National Health Research Institutes.
Footnotes
Abbreviations: CI = confidence interval, COPD = chronic obstructive pulmonary disease, DDD = defined daily dose, DM = diabetes mellitus, HR = hazard ratio, ICD-9 = International Classification of Diseases-Ninth Revision, IgE = immunoglobulin E, IGF-1 = insulin growth factor-1, IGFBP-1 = insulin growth factor-binding protein-1, IRB = Institutional Review Board, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, OR = odds ratio, SD = standard deviation, SDD = serious disabling disease, SIR = standard incidence ratio.
This research was supported by Kaohsiung Medical University Research Foundation. The funding authority had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Martinez FD, Vercelli D. Asthma. Lancet 2013; 382:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elmasri WM, Tran TH, Mulla ZD. A case-control study of asthma and ovarian cancer. Arch Environ Occup Health 2010; 65:101–105. [DOI] [PubMed] [Google Scholar]
- 3.Turner MC, Krewski D, Armstrong BK, et al. Allergy and brain tumors in the INTERPHONE study: pooled results from Australia, Canada, France, Israel, and New Zealand. Cancer Causes Control 2013; 24:949–960. [DOI] [PubMed] [Google Scholar]
- 4.Roncarolo F, Infante-Rivard C. Asthma and risk of brain cancer in children. Cancer Causes Control 2012; 23:617–623. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Xu T, Chen J, et al. Allergy and risk of glioma: a meta-analysis. Eur J Neuro 2010; 18:387–395. [DOI] [PubMed] [Google Scholar]
- 6.Santillan AA, Camargo CA, Colditz GA. A meta-analysis of asthma and risk of lung cancer (United States). Cancer Causes Control 2003; 14:327–334. [DOI] [PubMed] [Google Scholar]
- 7.Boffetta P, Ye W, Boman G, et al. Lung cancer risk in a population-based cohort of patients hospitalized for asthma in Sweden. Eur Respir J 2002; 19:127–133. [DOI] [PubMed] [Google Scholar]
- 8.Ji J, Shu X, Li X, et al. Cancer risk in hospitalised asthma patients. Br J Cancer 2009; 100:829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandini S, Lowenfels AB, Jaffee EM, et al. Allergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol Biomarkers Prev 2005; 14:1908–1916. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol 2003; 21:807–839. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 12.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013; 63:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakai Y, Nonomura N. Inflammation and prostate carcinogenesis. Int J Urol 2012; 20:150–160. [DOI] [PubMed] [Google Scholar]
- 14.Venkateswaran V, Klotz LH. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nat Rev Urol 2010; 7:442–453. [DOI] [PubMed] [Google Scholar]
- 15.Kazma R, Mefford JA, Cheng I, et al. Association of the innate immunity and inflammation pathway with advanced prostate cancer risk. PLoS One 2012; 7:e51680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toriola AT, Laukkanen JA, Kurl S, et al. Prediagnostic circulating markers of inflammation and risk of prostate cancer. Int J Cancer 2013; 133:2961–2967. [DOI] [PubMed] [Google Scholar]
- 17.Severi G, Baglietto L, Muller DC, et al. Asthma, asthma medications, and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2010; 19:2318–2324. [DOI] [PubMed] [Google Scholar]
- 18.Platz EA, Drake CG, Wilson KM, et al. Asthma and risk of lethal prostate cancer in the Health Professionals Follow-Up Study. Int J Cancer 2015; 137:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel T, Wambi CC, Berg W, et al. Prostate cancer disease characteristics for foreign-born South Asian men living in the United States. Indian J Cancer 2013; 50:159–163. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Yang B-X, Zhang H-T, et al. Prostate cancer: an emerging threat to the health of aging men in Asia. Asian J Androl 2011; 13:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jian Z-H, Lung C-C, Huang J-Y, et al. The coexistence of common pulmonary diseases on the histologic type of lung cancer in both genders in Taiwan. Medicine 2014; 93:e127–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo C-F, Luo S-F, See L-C, et al. Increased risk of cancer among gout patients: a nationwide population study. Joint Bone Spine 2012; 79:375–378. [DOI] [PubMed] [Google Scholar]
- 23.Hwang C-Y, Chen Y-J, Lin M-W, et al. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: a nationwide cohort study in Taiwan. Int J Cancer 2011; 130:1160–1167. [DOI] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 25.Gray R. cmprsk: subdistribution analysis of competing risks. R package version 2.2-1; 2010. http://CRAN.R-project.org/package=cmprsk Accessed date June 1, 2014. [Google Scholar]
- 26.McWhorter WP. Allergy and risk of cancer. A prospective study using NHANESI followup data. Cancer 1988; 62:451–455. [DOI] [PubMed] [Google Scholar]
- 27.Mills PK, Beeson WL, Fraser GE, et al. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am J Epidemiol 1992; 136:287–295. [DOI] [PubMed] [Google Scholar]
- 28.Vesterinen E, Pukkala E, Timonen T, et al. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol 1993; 22:976–982. [DOI] [PubMed] [Google Scholar]
- 29.El-Zein M, Parent ME, Kâ K, et al. History of asthma or eczema and cancer risk among men: a population-based case-control study in Montreal, Quebec, Canada. Ann Allergy Asthma Immunol 2010; 104:378–384. [DOI] [PubMed] [Google Scholar]
- 30.Josephs DH, Spicer JF, Corrigan CJ, et al. Epidemiological associations of allergy, IgE and cancer. Clin Exp Allergy 2013; 43:1110–1123. [DOI] [PubMed] [Google Scholar]
- 31.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355:2226–2235. [DOI] [PubMed] [Google Scholar]
- 32.Talbot-Smith A, Fritschi L, Divitini ML, et al. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol 2003; 157:606–612. [DOI] [PubMed] [Google Scholar]
- 33.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med 2000; 6:703–706. [DOI] [PubMed] [Google Scholar]
- 34.Martin RM, Vatten L, Gunnell D, et al. Blood pressure and risk of prostate cancer: cohort Norway (CONOR). Cancer Causes Control 2009; 21:463–472. [DOI] [PubMed] [Google Scholar]
- 35.Martin RM, Vatten L, Gunnell D, et al. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control 2009; 20:1181–1192. [DOI] [PubMed] [Google Scholar]
- 36.Gann PH, Daviglus ML, Dyer AR, et al. Heart rate and prostate cancer mortality: results of a prospective analysis. Cancer Epidemiol Biomarkers Prev 1995; 4:611–616. [PubMed] [Google Scholar]
- 37.McCarty MF. Up-regulation of IGF binding protein-1 as an anticarcinogenic strategy: relevance to caloric restriction, exercise, and insulin sensitivity. Med Hypotheses 1997; 48:297–308. [DOI] [PubMed] [Google Scholar]
- 38.Stocks T, Hergens M-P, Englund A, et al. Blood pressure, body size and prostate cancer risk in the Swedish Construction Workers cohort. Int J Cancer 2010; 127:1660–1668. [DOI] [PubMed] [Google Scholar]


