Abstract
In this nationwide population-based cohort study, we aimed to evaluate the effects of sleep-related breathing disorders (SBD) on migraine development.
Patients ages 20 years or more and diagnosed with SBD between 2000 and 2009 were evaluated as the SBD cohort (n = 3411), and compared with comparison cohort (n = 13,644). The adjusted hazard ratio (aHR) for developing migraine was calculated in both cohorts by multivariate Cox proportional hazards model.
The cumulative incidence of migraine was significantly higher in the SBD cohort than in the comparison cohort. In the SBD cohort, the overall aHR for developing migraine was 2.43 (95% confidence interval [CI] = 1.72–3.44). The risk of developing migraine was higher in men (aHR 2.71) than in women (aHR 2.29) with SBD. When stratifying by age, we observed increased incidence of migraine in patients ages 20 to 44 years and 45 to 64 years, with a higher aHR of 2.51 (95% CI = 1.47–4.30) and 2.68 (95% CI = 1.63–4.43), respectively. The risk of developing migraine in the patients with SBD with or without comorbidity exhibited nonsignificant differences. After stratifying by the use of hypnotics, the aHR for developing migraine was 2.39 in the patients with hypnotics use and 3.58 in the patients without hypnotics use.
Our findings indicate increased risk of developing migraine in adults, but not elderly ones, with SBD.
INTRODUCTION
Insomnia and headache are both common complaints that could affect the quality of our daily lives. In Asia, migraine is the most prevalent type of headache diagnosed at neurological clinics (66.6% of headache patients, ranging from 50.9% to 85.8% in various countries).1 Worldwide, the estimated annual prevalence of migraine is approximately 15% to 20% in women and 6% to 10% in men.2–4 On the other side, insomnia, especially the sleep-related breathing disorders (SBD) can temporarily or chronically happen in a person and would be highly associated with other medical disorders.5 Higher prevalence of SBD was usually observed in the elderly population,6,7 and was thought to threaten their lives with increasing the risk of various vascular diseases.6,7
Migraine is considered as a neurovascular disease and is associated with other vascular diseases, too.8,9 Previous studies have reported interacting relationships among migraine, various sleep disorders, anxiety, and depression.10–12 However, whether SBD can be considered a trigger or a predisposing factor for migraine development still remains unclear. Therefore, in this study, we used a Taiwanese nationwide population-based database to evaluate the risk of developing subsequent migraine in patients with SBD.
METHODS AND MATERIALS
Data Source
Taiwan launched a national health insurance (NHI) in 1995, operated by a single-buyer, the government. Medical reimbursement specialists and peer review should scrutinize all insurance claims. This retrospective population-based cohort study was conducted using the Longitudinal Health Insurance Database (LHID) of the National Health Insurance Research Database (NHIRD), established by the National Health Research Institutes, Department of Health, Taiwan. The NHIRD contains data from the NHI program, including registration files and claims data for reimbursement for approximately 99% of Taiwan's population. Data in the LHID are derived from the original files of the NHIRD. The LHID was established for research purposes and include claims data from 1996 to 2011 for 1 million people covered in the NHI program. The distributions of sex and age in patients in the LHID and all beneficiaries exhibited nonsignificant differences. The International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes were used to identify patients with specific diseases. Personal identification information was encrypted before the release of the research database to protect patient privacy and data security. This study was approved by the Institutional Review Board of China Medical University (CMU-REC-101-012).
Study Participants
Participants ages 20 years or more were evaluated for a causal correlation between SBD (ICD-9-CM Codes 780.51, 780.53, and 780.57) and subsequent migraine (ICD-9-CM Code 346). Patients with newly diagnosed SBD from 2000 to 2009 were identified as the SBD cohort (n = 3411) using the date of diagnosis as the index date. For every SBD patient, 4 individuals were randomly selected for comparison (comparison cohort, n = 13,644), frequency-matched according to age, sex, and index year. Meanwhile, SBD patients with a diagnosis of migraine before the index date were excluded.
Outcome Measures
The primary outcome variable was the development of migraine during the follow-up. All of the study participants were followed up from the index date until a diagnosis of migraine, withdrawal from the LHID database, or December 31, 2011. The diagnoses of SBD and migraine were based on the ICD-9-CM codes, which were judged and determined by related specialists and physicians according to the standard clinical criteria. If these hospitals or doctors made the wrong diagnoses or coding, they would be punished to pay a lot of penalty. Therefore, the diagnoses and codes for SBD and migraine used in this study should be correct and reliable.
The potential confounding risk factors for migraine, such as hypertension (ICD-9-CM Codes 401–405), hyperlipidemia (ICD-9-CM Code 272), diabetes (ICD-9-CM Code 250), stroke (ICD-9-CM Codes 430–438), chronic obstructive pulmonary disease (COPD; ICD-9-CM Codes 490–496), depression (ICD-9-CM Codes 296.2, 296.3, 296.82, 300.4, 309.0, 309.1, 309.28, and 311), and anxiety (ICD-9-CM Codes 300.0, 300.2, 300.3, 308.3, and 309.81), were identified before the study end date. History of hypnotics use (zolpidem, benzodiazepines [BZD], or both) was included in adjustments for drug use.
Statistical Analysis
Risk factors for migraine, including age, sex, comorbidity, and history of drug use, were compared between the SBD and comparison cohorts by using Student t test and Chi-squared test. The incidence rate of migraine (per 1000 person-year) was calculated in the 2 cohorts, according to demographic variables, comorbidity (yes/no), and drug use (yes/no). A Poisson regression model was used to estimate the incidence rate ratios and 95% confidence intervals (CIs) for migraine in the SBD and comparison cohorts according to the various variables. A multivariate Cox proportional hazards model was used to calculate the adjusted hazard ratio (aHR) and 95% CI for migraine in both cohorts, after controlling for potential confounding risk factors. The Cox model was also used to estimate the HR for migraine and SBD with various comorbidities.
In 2-tailed tests, P < 0.05 was considered statistically significant. Data management and analyses were performed using the SAS 9.3 statistical package (SAS Institute Inc, Cary, NC). R software (R Foundation for Statistical Computing, Vienna, Austria) was used to conduct a Kaplan–Meier analysis to measure the cumulative incidence of migraine, and to conduct a log-rank test to evaluate the differences between the cumulative incidence curves of the 2 cohorts.
RESULTS
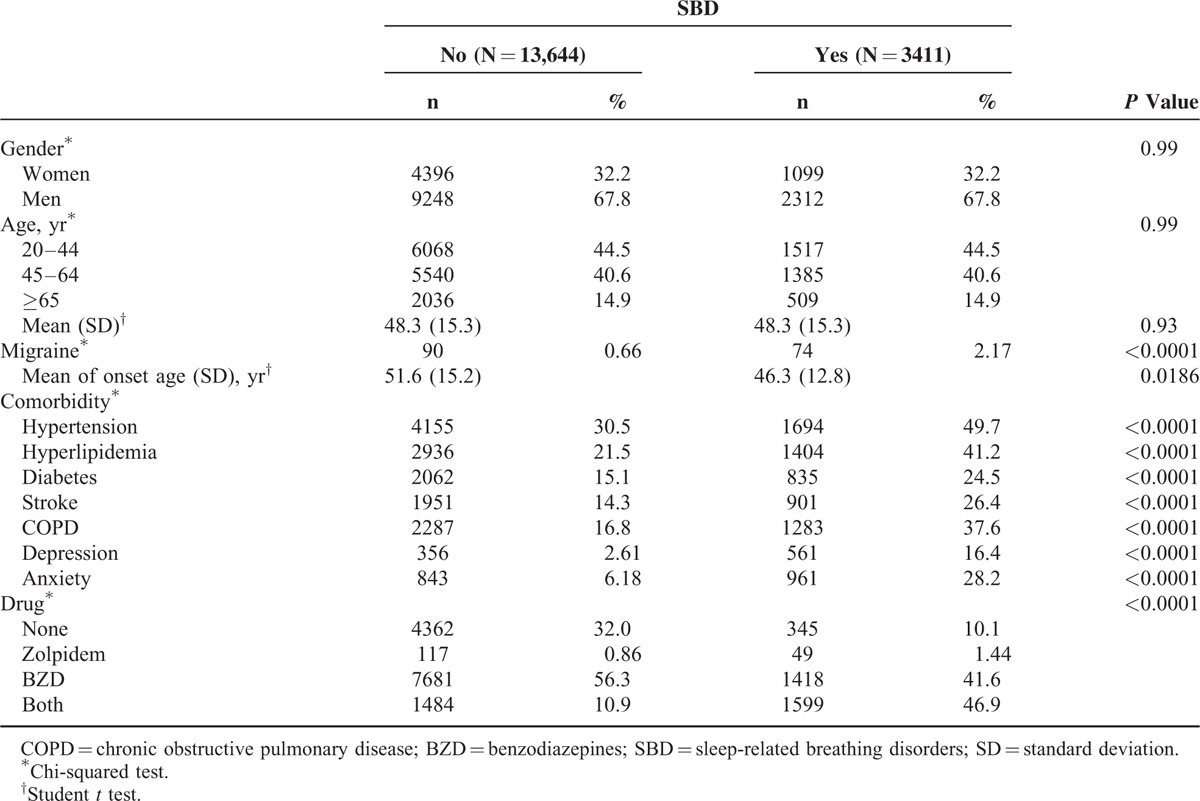
In this study, we evaluated 3411 patients with SBD diagnosed between 2000 and 2009 as the SBD cohort, and 13,644 comparison patients as the comparison cohort (Table 1). The distributions of sex and age were similar between the 2 cohorts. However, the majority of the patients were men (67.8%) and were ages <65 years (85.1%). The mean age (± standard deviation) of 2 cohorts was 48.3 ± 15.3 years. During the 12-year follow-up, the incidence of migraine development was 2.17% in the SBD cohort and 0.66% in the comparison cohort, with the mean migraine-onset age of 46.3 and 51.6 years, respectively. The proportion of patients with a comorbidity, such as hypertension (49.7% vs 30.5%), hyperlipidemia (41.2% vs 21.5%), diabetes (24.5% vs 15.1 %), stroke (26.4% vs 14.3%), COPD (37.6% vs 16.8%), depression (16.4% vs. 2.61%), and anxiety (28.2% vs. 6.18%), was substantially higher in the SBD cohort than in the comparison cohort. The proportion of patients using zolpidem, BZD, or both was higher in the SBD cohort than in the comparison cohort at the baseline (89.9% vs 68%). Surprisingly, most participants took BZD or both and only very few in the 2 cohorts used zolpidem alone (1.44% vs 0.86%; Table 1).
TABLE 1.
Comparison of Demographics, Comorbidity, and History of Drug Use Between SBD and Comparison Cohorts
As shown in Figure 1, the cumulative incidence of developing migraine during the follow-up was higher in the SBD cohort than in the comparison cohort (log-rank test, P < 0.0001). The overall aHR for developing migraine was 2.43 (95% CI = 1.72–3.44), and the incidence rate of migraine was higher in the SBD cohort than in the comparison cohort (3.83 vs 1.17 per 1000 person-year; Table 2). The risk of developing migraine was higher in men (aHR = 2.71, 95% CI = 1.64–4.47) than in women (aHR = 2.29, 95% CI = 1.41–3.72) with SBD. When stratifying by age, the patients ages between 45 and 64 years were associated with the highest risk of developing subsequent migraine, with an aHR of 2.68 (95% CI = 1.63–4.43). The aHR for developing migraine was 2.51 (95% CI = 1.47–4.30) in the patients ages 20 to 44 years and 1.09 in the patients ages ≥65 years. In both the SBD and comparison cohorts, the incidence of developing migraine was greater in patients with ≥1 type comorbidities. After stratifying the patients in the SBD and comparison cohorts by drug use, we identified an aHR of 2.39 (95% CI = 1.68–3.40) in the patients with drug use and 3.58 (95% CI = 0.42–30.7) in the patients without drug use.
FIGURE 1.
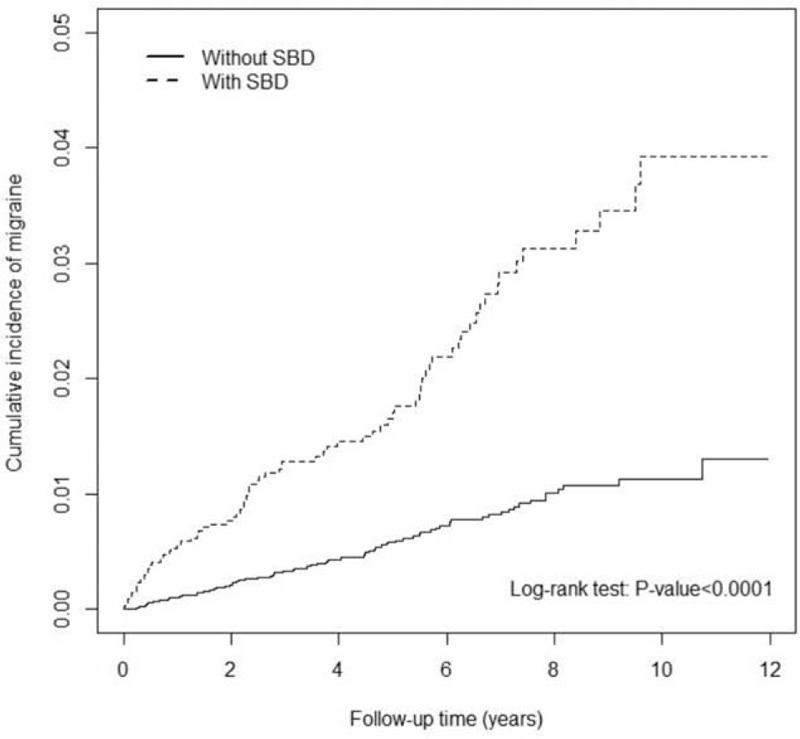
Comparison of cumulative incidence of developing migraine between participants with (dashed line) and without (solid line) sleep-related breathing disorders.
TABLE 2.
Incidence and Adjusted Hazard Ratio of Migraine Stratified by Sex, Age, Comorbidity (Yes/No) and Drug Use (Yes/No) Between SBD and Comparison Cohorts
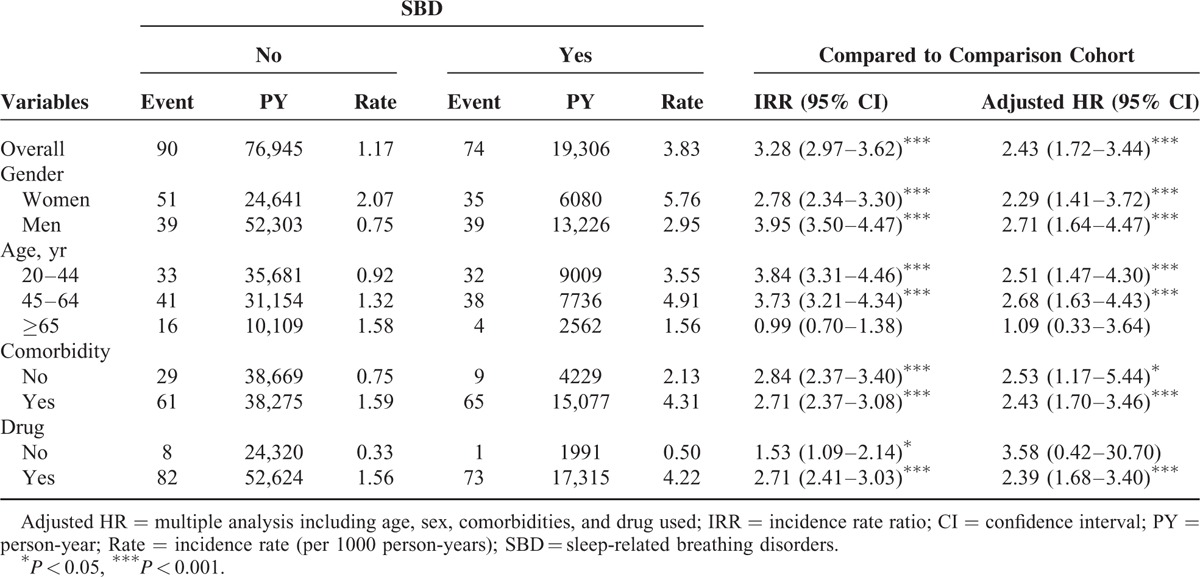
Table 3 shows the multiplicative risk of developing migraine in the patients with SBD and various comorbidities. The comorbidities having significant interaction with SBD for migraine development included hypertension (P = 0.0146), hyperlipidemia (P = 0.0227), diabetes (P = 0.0497), and stroke (P = 0.0367). But we also observed increased risk of developing migraine in the patients with SBD and COPD (aHR = 3.08, 95% CI = 1.96–4.83), depression (aHR = 4.44, 95% CI = 2.67–7.39), or anxiety (aHR = 5.12, 95% CI = 3.41–7.69).
TABLE 3.
Adjusted Hazard Ratios of Migraine Associated SBD Interaction With Comorbidity

DISCUSSION
Our results indicate significantly increased risk of developing subsequent migraine in patients with SBD ages between 20 and 64 years, but not in older patients. Migraine is a combining neuronal and vascular disorder, which associates with other vascular events.13–15 Murinova et al14 proposed that migraine would be considered a risk factor for other vascular diseases based on the vascular mechanism and that migraineurs might have different endothelial, structural, and genetic factors of vessels than regular people. Through the vascular mechanism, SBD can possibly result in repeated episodes of hypoxia and hypercapnia during sleep, and altered vascular endothelial function.16,17 Tissue hypoxia was also reportedly associated with the upregulated expression of transcription factors such as hypoxia inducible factor, nuclear factor kappa B, tumor necrosis factor (TNF), inducible nitric oxide synthase, and vascular endothelial growth factor.18 Therefore, migraine could be caused by the changed production of endothelium-derived hyperpolarizing factors, which dilates the meningeal vessels and presents a migraine headache. These changes might also lead to other vascular comorbidities, such as hypertension, atherosclerosis, ischemia, thrombosis, and stroke.19
Another study suggested that hypoxia might increase blood–brain barrier permeability, leading to brain edema, neurovascular uncoupling, and neuronal dysfunction and damage, which would be related to the neuronal or cortical mechanism of migraine.20 Such changes in brain cortices and neurons may lead to various neurological diseases, and a high degree of comorbidity has been observed among Alzheimer's disease, migraine, and epilepsy. Therefore, SBD might induce hypoxia and cause an unstable neuronal condition, resulting in the symptoms of migraine in patients. Furthermore, there is another possible mechanism causing from the comorbid condition of migraine and epilepsy. A sleep disorder itself can increase the occurrence of interictal spikes and epileptic seizures,21,22 and migraine like headache can be preceded to a subtle seizure before the epilepsy diagnosis being made. It is due to the well-known association between migraine and epilepsy in recent studies.23 In the future, we would like to study the prevalence of epilepsy development in patients with SBD and migraine to investigate the detail association of these diseases.
Migraine is known to be more prevalent in young adults than in elderly people, and is usually more prevalent in women than in men.2–4 Due to we intended to enroll the subjects with SBD in this retrospective study, overall 55.5% of the study subjects were 45 years old or more. It resulted the mean age of migraine onset being 46.3 years in the SBD cohort. The data should not be compared with the migraine-onset age in general population because of the difference in study groups. We also found that the risk of developing migraine was higher in men than in women with SBD. It implies that men are more vulnerable to develop migraine than women if they already have SBD. A recent study reported that interleukin (IL)-1B, IL-6, and TNF considerably increase the expression on the trigeminal ganglion neurons in male mice compared with female mice.24 The sex differences of proinflammatory cytokines expressions are thought to be associated with the risk difference of migraine development.25,26
As shown in Figure 1, we observed that the risk of developing subsequent migraine increased in the SBD cohort over time. We analyzed the interactions between various comorbidities and SBD, and considered the major confounding factors for migraine development. Vascular-related comorbidities, such as hypertension, hyperlipidemia, diabetes, and stroke, were significantly associated with SBD and migraine development (Table 3). However, nonvascular-related comorbidities, such as COPD, depression, and anxiety, were nonsignificantly associated with SBD and migraine development. As shown in Table 2, comorbidity and drug use exerted nonsignificant effects on the HRs for developing migraine. However, because of limited numbers of SBD patients who had no comorbidity or without drug use, these results could not be considered reliable to us in the interpretation.
The strengths of this study are its nationwide population-based design and representativeness of the 2 cohorts. However, this study also has some limitations. First, information on migraine frequency, presence or absence of aura, smoking habits, alcohol consumption, body mass index or weight, socioeconomic status, and family history were not available in the NHIRD, all of which might represent confounding factors for developing migraine. Second, evidence deriving from a cohort study is subject to several biases related to adjustment for confounders. Although our study design included adequate control for confounding factors, bias could have remained in the presence of unmeasured or unknown confounders. Finally, the diagnoses in the NHI claims data are primarily used for administrative affairs and do not undergo verification for scientific purposes due to the anonymity of patient identification numbers. We were unable to approach the patients directly to confirm the details of them and the accuracy of their diagnoses. Also, we could not obtain the characteristics of their migraine (with or without aura, episodic or chronic) and the medication use to categorize subgroups for strengthening the data analysis. However, some previous studies reported the high accuracy and validity of diagnoses from ICD-9-CM codes in the NHIRD research,27,28 and those convinced us that some valuable evidences had be shown in this study about the causal correlation between SBD and subsequent migraine development.
CONCLUSION
The findings from this population-based cohort study indicate increased risk of developing subsequent migraine in adults, but not elderly ones, with SBD, which could be thought as a trigger or a predisposing factor for migraine development. Additional large unbiased population-based studies with further categorization and analysis are required to confirm these findings.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, BZD = benzodiazepines, CI = confidence interval, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IL = interleukin, LHID = the Longitudinal Health Insurance Database, NHI = national health insurance, NHIRD = the National Health Insurance Research Database, SBD = sleep-related breathing disorder, TNF = tumor necrosis factor.
Author contributions: Conception/design: TH, C-HK; provision of study materials and patients: C-HK; collection and assembly of data: all authors; data analysis and interpretation: all authors; manuscript preparation: all authors; final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wang SJ, Chung CS, Chankrachang S, et al. Migraine disability awareness campaign in Asia: migraine assessment for prophylaxis. Headache 2008; 48:1356–1365. [DOI] [PubMed] [Google Scholar]
- 2.Cheung RT. Prevalence of migraine, tension-type headache, and other headaches in Hong Kong. Headache 2000; 40:473–479. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001; 41:646–657. [DOI] [PubMed] [Google Scholar]
- 4.Stovner LJ, Zwart JA, Hagen K, et al. Epidemiology of headache in Europe. Eur J Neurol 2006; 13:333–345. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM. Observation of the natural evolution of insomnia in the American general population cohort. Sleep Med Clin 2009; 4:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panossian L, Daley J. Sleep-disordered breathing. Continuum (Minneap Minn) 2013; 19:86–103. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurth T, Gaziano JM, Cook NR, et al. Migraine and risk of cardiovascular disease in women. JAMA 2006; 296:283–291. [DOI] [PubMed] [Google Scholar]
- 9.Kurth T, Gaziano JM, Cook NR, et al. Migraine and risk of cardiovascular disease in men. Arch Intern Med 2007; 167:795–801. [DOI] [PubMed] [Google Scholar]
- 10.Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol 2008; 7:354–361. [DOI] [PubMed] [Google Scholar]
- 11.Pompili M, Cosimo DD, Innamorati M, et al. Psychiatric comorbidity in patients with chronic daily headache and migraine: a selective overview including personality traits and suicidal risk. J Headache Pain 2009; 10:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache 2005; 45:904–910. [DOI] [PubMed] [Google Scholar]
- 13.Metso TM, Tatlisumak T, Debette S, et al. Migraine in cervical artery dissection and ischemic stroke patients. Neurology 2012; 78:1221–1228. [DOI] [PubMed] [Google Scholar]
- 14.Murinova N, Krashin DL, Lucas S. Vascular risk in migraineurs: interaction of endothelial and cortical excitability factors. Headache 2014; 54:583–590. [DOI] [PubMed] [Google Scholar]
- 15.Scher AI, Terwindt GM, Picavet HSJ, et al. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology 2005; 64:614–620. [DOI] [PubMed] [Google Scholar]
- 16.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010; 7:677–685. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Li AY, Guo QH, et al. Effects of cyclic intermittent hypoxia on ET-1 responsiveness and endothelial dysfunction of pulmonary arteries in rats. PLoS ONE 2013; 8:e58078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Rosa DP, Forgiarini LF, Baronio D, et al. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm 2012; 2012:879419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev 2013; 66:102–192. [DOI] [PubMed] [Google Scholar]
- 20.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab 2012; 32:1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain SV, Kothare SV. Sleep and epilepsy. Semin Pediatr Neurol 2015; 22:86–92. [DOI] [PubMed] [Google Scholar]
- 22.Khachatryan SG, Prosperetti C, Rossinelli A, et al. Sleep-onset central apneas as triggers of severe nocturnal seizures. Sleep Med 2015; 16:1017–1019.doi: 10.1016/j.sleep.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Toldo I, Perissinotto E, Menegazzo F, et al. Comorbidity between headache and epilepsy in a pediatric headache center. J Headache Pain 2010; 11:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzawińska O, Lis K, Cudna A, et al. Gender differences in the neurochemical response of trigeminal ganglion neurons to peripheral inflammation in mice. Acta Neurobiol Exp 2014; 74:227–232. [DOI] [PubMed] [Google Scholar]
- 25.Sarchielli P, Alberti A, Baldi A, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006; 46:200–207. [DOI] [PubMed] [Google Scholar]
- 26.Capuano A, De Corato A, Lisi L, et al. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain 2009; 5:43.doi: 10.1186/1744-8069-5-43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 28.Yu YB, Gau JP, Liu CY, et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost 2012; 108:225–235. [DOI] [PubMed] [Google Scholar]


