Abstract
Enhanced Recovery or Fast Track Recovery after Surgery protocols (ERAS) have significantly changed perioperative care following colorectal surgery and are promoted as reducing the stress response to surgery.
The present systematic review aimed to examine the impact on the magnitude of the systemic inflammatory response (SIR) for each ERAS component following colorectal surgery using objective markers such as C-reactive protein (CRP) and interleukin-6 (IL-6).
A literature search was performed of the US National Library of Medicine (MEDLINE), EMBASE, PubMed, and the Cochrane Database of Systematic Reviews using appropriate keywords and subject headings to February 2015.
Included studies had to assess the impact of the selected ERAS component on the SIR using either CRP or IL-6.
Nineteen studies, including 1898 patients, were included. Fourteen studies (1246 patients) examined the impact of laparoscopic surgery on the postoperative markers of SIR. Ten of these studies (1040 patients) reported that laparoscopic surgery reduced postoperative CRP. One study (53 patients) reported reduced postoperative CRP using opioid-minimising analgesia. One study (142 patients) reported no change in postoperative CRP following preoperative carbohydrate loading. Two studies (108 patients) reported conflicting results with respect to the impact of goal-directed fluid therapy on postoperative IL-6. No studies examined the effect of other ERAS components, including mechanical bowel preparation, antibiotic prophylaxis, thromboprophylaxis, and avoidance of nasogastric tubes and peritoneal drains on markers of the postoperative SIR following colorectal surgery.
The present systematic review shows that, with the exception of laparoscopic surgery, objective evidence of the effect of individual components of ERAS protocols in reducing the stress response following colorectal surgery is limited.
INTRODUCTION
Surgery for colorectal disease is associated with variable short-term outcomes. Recent advances in perioperative care methods have attempted to improve these outcomes. The development and widespread application of enhanced recovery or fast track surgical protocols (ERAS), in combination with laparoscopic surgery, represent a paradigm shift in perioperative care.1 ERAS involves multimodal, protocol-driven peri-operative care which proponents of have stated reduces the stress response to surgery.2
The trauma of surgery leads to well-understood metabolic, neuroendocrine, and immune responses, the aims of which are to promote physiological stability and wound healing.3 The cellular response to surgical injury is to activate neutrophils and macrophages of the innate immune system by the production of proinflammatory cytokines such as tumor necrosis factor (TNF) alpha, and the interleukins (ILs), for example, IL-1 and IL-6.4,5 Proinflammatory cytokines alter the levels of circulating acute phase proteins, for example, C-reactive protein (CRP), albumin, ferritin, transferrin, and fibrinogen, through their action on hepatocytes.6 Indeed, it has been reported that concentrations of circulating acute phase proteins and cytokines are associated with the magnitude of the stress response, that is, the systemic inflammatory response (SIR) to surgery.7 Furthermore, CRP and IL-6 have been reported to have the strongest association with the magnitude of the surgical injury, although CRP is perhaps the most clinically useful of these.7 Moreover, this knowledge forms the basis of an objective examination of the evidence for the impact of ERAS protocols and their components.
Although it is recognized that laparoscopic surgery generates a reduced postoperative SIR following colorectal surgery, the impact of individual components of ERAS protocols, in terms of SIR, has not been examined in a systematic manner. The aim of the present review was to examine the evidence in relation to ERAS protocols and their components having an effect on objective markers of the postoperative SIR.
METHODS
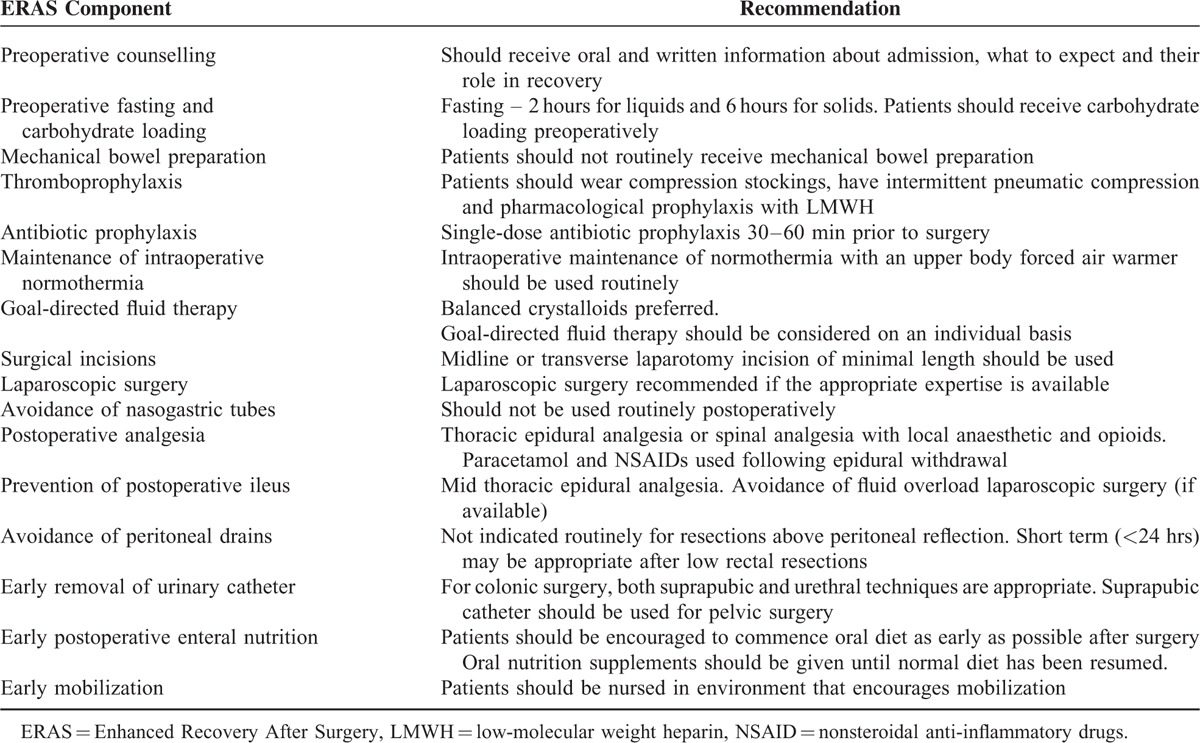
Recent separate guidelines on ERAS recommendations following elective colonic and elective rectal/pelvic surgery have been published.8,9 The present systematic review focuses on the components reported in the ERAS Group consensus review in colorectal surgery.1 These recommendations for patients undergoing colorectal surgery are summarized in Table 1.
TABLE 1.
Components of Enhanced Recovery After Surgery—ERAS Group Recommendations
A systematic literature search of the US National Library of Medicine (MEDLINE), the Excerpta Medica Database (EMBASE), PubMed, and the Cochrane Database of Systemic Reviews (CDSR) was made using the following search criteria: “ERAS component” AND (systemic inflammation OR SIR OR stress response OR C-reactive protein OR CRP OR IL-6) AND surgery. This was performed independently by the 2 lead authors and any conflicts that were encountered were discussed with the senior authors. Included data were from the inception of the searched databases until February 2015. From this search, abstracts of articles were analyzed for relevance and the bibliographies of relevant studies as well as the bibliography of the consensus review of perioperative care following colorectal surgery1 were hand-searched for any additional studies. Included studies had to assess the impact of the selected ERAS component on the SIR using either CRP or interleukin-6 (IL-6). Both prospective clinical trials and observational trials were included.
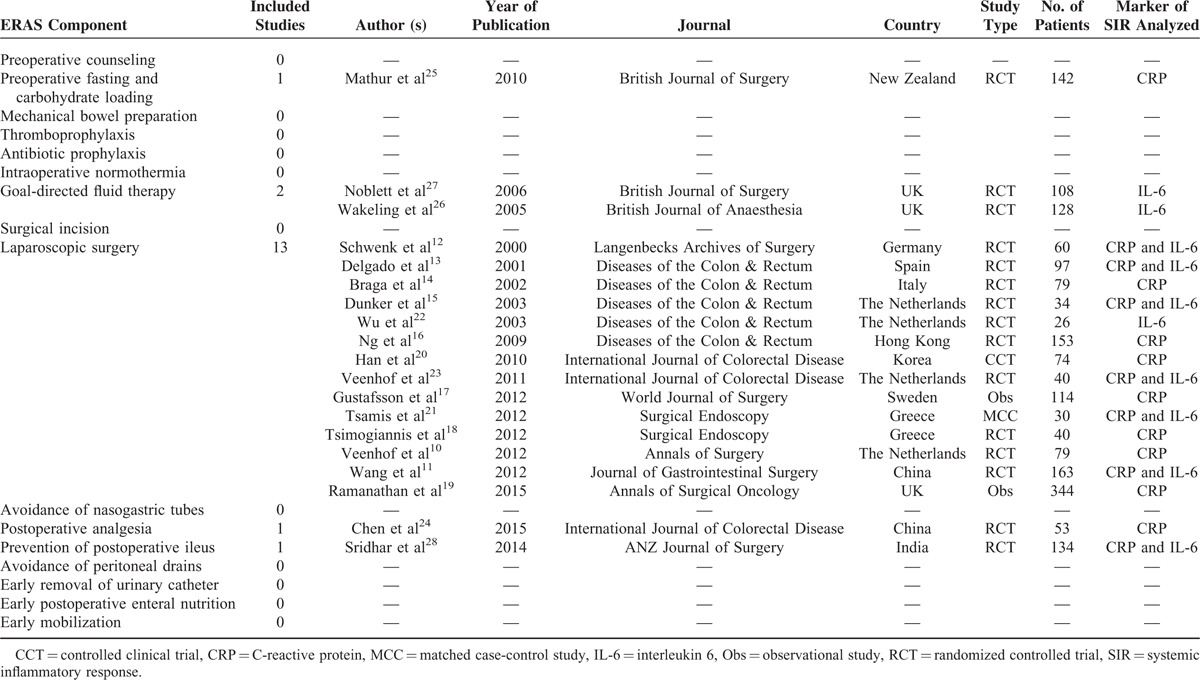
The selection process is summarized in Figure 1. Using the aforementioned search strategy, relevant abstracts were obtained for each ERAS component. Articles were excluded if they were animal studies, not in the English language, were review articles, were not related to colorectal surgery or did not use either CRP or IL-6 as the marker of the SIR. Table 2 summarizes the included studies for each ERAS component and the marker of the SIR analyzed.10–28 Evidence relating to other outcomes of ERAS programmes such as length of stay or postoperative complications were obtained from the most recent Cochrane Reviews or meta-analyses on the specific topic. Meta-analysis of included studies was not performed because of significant heterogeneity among study methodology, populations, and outcomes measured.
FIGURE 1.
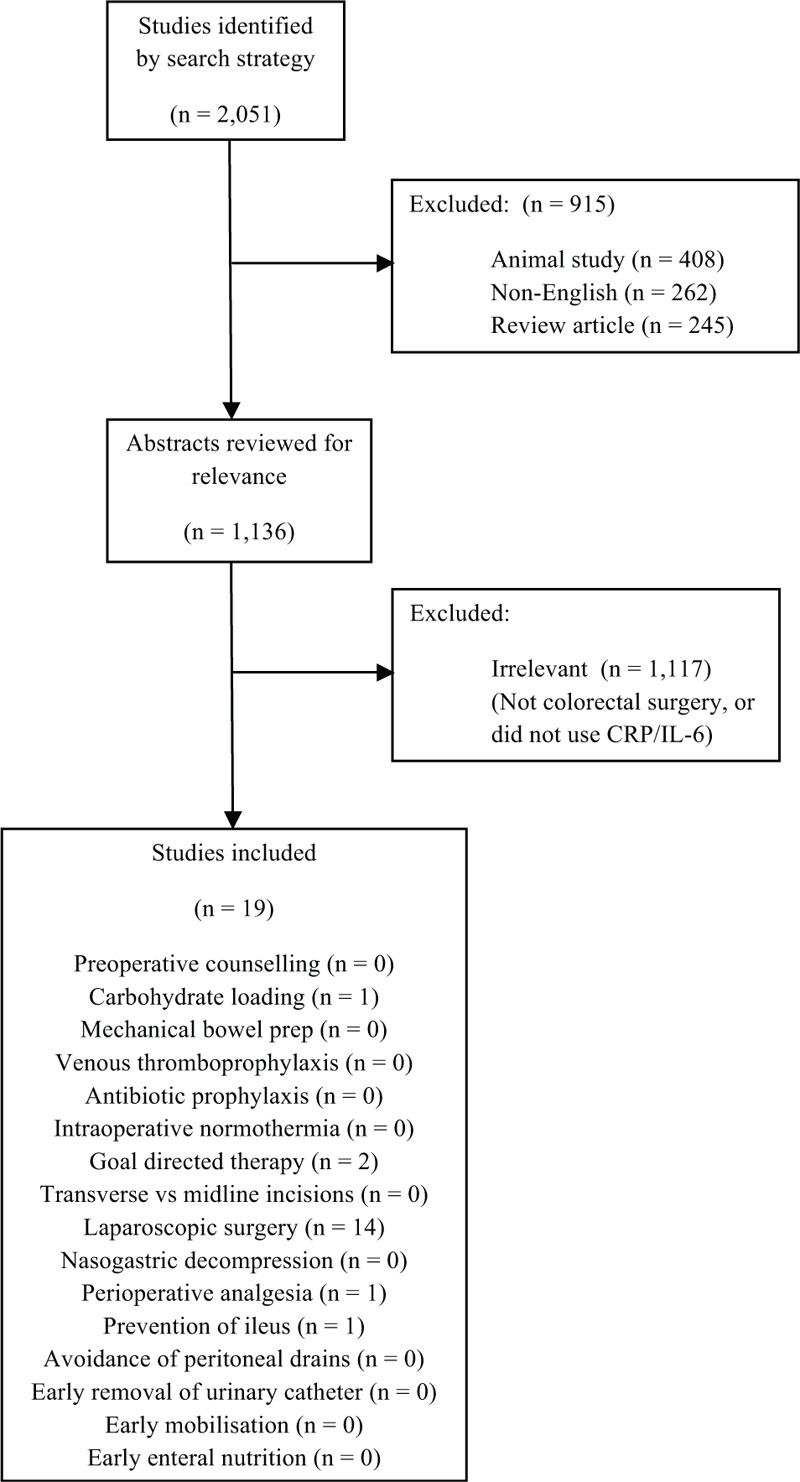
PRISMA flowchart demonstrating study selection.
TABLE 2.
Summary of included studies for each ERAS component and the marker of the systemic inflammatory response analysed
Subjective assessment of study validity was carried out by two authors independently (DW and SM) using the Cochrane Collaboration tool provided by Review Manager version 5.3 (RevMan 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Any uncertainties were resolved by consensus following discussion with the senior authors (PH and DM). Both prospective clinical trials and observational trials were included.
Ethical approval was not required for the present study as this was a systematic review of published data.
RESULTS
Assessment of Included Study Validity
The validity of included studies is summarized in Figure 2. The present systematic review included 15 randomized controlled trials (RCTs), 1 controlled clinical trial (CCT), and 3 observational studies. The included studies were of varying methodological quality. In particular, those RCTs investigating the impact of laparoscopic versus open surgery on postoperative IL-6 or CRP had issues relating to blinding to both patients and clinicians. All included studies examined the impact of ERAS components on patients undergoing elective colorectal surgery. No studies included emergency surgery or presentation.
FIGURE 2.
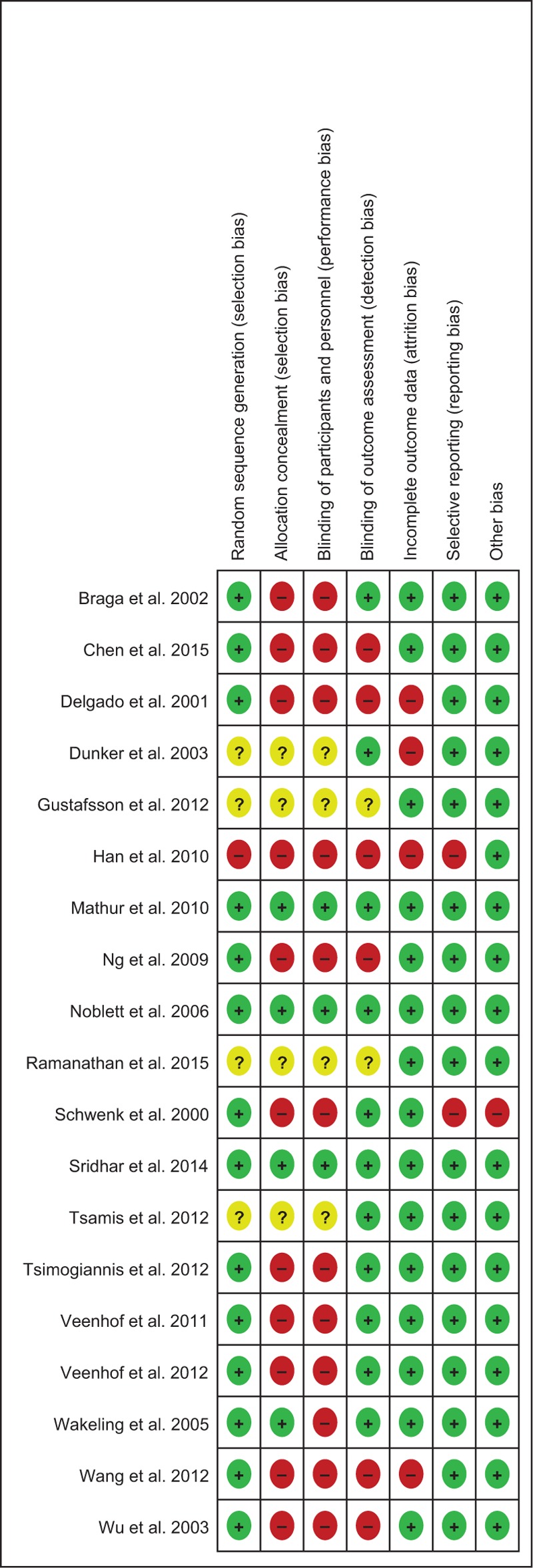
Cochrane risk of bias summary.
ERAS Protocols
Among the published literature surrounding ERAS, there is considerable variation in the number and nature of the ERAS components applied and also in the outcomes measured.29–31 Indeed, a recent meta-analysis reported that using fewer ERAS components was associated with a greater reduction in mortality and complications compared with those with a greater number of components.31 Some evidence suggests that only the provision of laparoscopic surgery, early enteral nutrition, and early mobilization shortens length of stay following colorectal surgery within the ERAS framework.32 Two studies including 249 patients examined the impact of ERAS protocols when compared with standard perioperative care and laparoscopic surgery when compared with open procedures, on postoperative CRP and IL-6.10,11 Wang et al reported that, in 170 patients, CRP and IL-6 on postoperative days 1 and 3 were lower in those cared for using an ERAS protocol, independent of the mode of surgery. Veenhof et al, in their study of 79 patients, reported that no observed difference in postoperative CRP or IL-6 could be attributed solely to the use of an ERAS protocol. No studies examined the impact of ERAS protocols as a whole versus standard perioperative care on the postoperative SIR in colorectal surgery without the inclusion of a laparoscopic surgery versus open surgery arm, making the interpretation of the impact of ERAS protocols alone difficult.
Laparoscopic Surgery
Laparoscopic colorectal surgery has been reported to shorten length of stay and reduce postoperative pain when compared with open surgery.33 Furthermore, it has been reported to produce equivalent oncological outcomes when compared with open surgery.34,35 Ten studies including a total of 1040 patients have demonstrated lower postoperative CRP following laparoscopic colorectal surgery when compared with open procedures.10,11,12–19 Furthermore, a recent review has also reported similar findings in both benign and malignant colorectal disease.7 Four studies, including 206 patients, reported no difference in postoperative serum CRP when those undergoing laparoscopic colorectal surgery were compare with those undergoing open procedures.20–23 Therefore, laparoscopic surgery appears to be associated with a reduction in the postoperative SIR, as evidenced by circulating concentrations of CRP, following colorectal surgery, and is likely to reduce the surgical stress response as part of an ERAS protocol.
Postoperative Analgesia
Systemic opioids provide effective analgesia but are associated with side effects, including nausea, vomiting, gut dysfunction, respiratory depression, and drowsiness, which are likely to prolong hospital stay.36,37 Methods of analgesia, which minimize the amount of opioids used are therefore key components of ERAS programmes.
Epidural analgesia is an effective method of analgesia that can be used with local anaesthetic and low-dose opioids. However, they are associated with a failure rate of approximately 30%, epidural hematoma and hypotension.38 In a recent meta-analysis comparing epidural analgesia (EA) versus opioid analgesia in patients undergoing colorectal surgery, the use of EA decreased the duration of postoperative ileus, allowed more intensive postoperative physiotherapy and mobilization and resulted in a reduction in pain scores without a significant reduction in length of stay.37 EA has been reported to result in a decrease in CRP levels following colorectal surgery.24
Local anesthesia techniques such as the transversus abdominis plane block (TAP) have been reported to be successful in multiple surgical specialties and operations.39 Subsequent meta-analysis of the use of TAP blocks showed a reduction in morphine use 24 hours postoperatively and reduced postoperative nausea and vomiting.39 A continuous infusion of local anesthetic (LA) delivered directly into the surgical wound via a catheter allows longer benefit from the local anesthetic, is easy to insert, and associated with few complications and a low failure rate.40 When compared with EA, wound catheters were reported to be of equal efficacy in terms of pain scores at 48 hours postoperatively with a lower rate of urinary retention.41 However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery.
Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used to good effect in the postoperative period. Some studies have suggested that combining NSAIDs and opioid medications results in decreased opioid consumption over a 24-hour period.42 If an EA has been used, the combination of paracetamol and an NSAID provides good analgesia during the period around EA removal. However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. In summary, there is some evidence that epidural anesthesia but not local anesthesia or NSAIDs can reduce the surgical stress response as part of an ERAS protocol.
Preoperative Fasting and Carbohydrate Loading
Surgery performed in a fasted state is thought to worsen the catabolic state and delay patient recovery. A recent Cochrane review reported that preoperative carbohydrate loading was only associated with a small reduction in length of stay and had no effect on complication rates.43 One study reported no effect on systemic inflammation, as evidenced by CRP and IL-6, in patients undergoing major abdominal surgery.25 Therefore, there is no evidence that carbohydrate loading can reduce the surgical stress response as part of an ERAS protocol.
Mechanical Bowel Preparation
A recent Cochrane review has reported that there is no statistically significant evidence that the use of mechanical bowel preparation (MBP) prevents postoperative complications such as anastomotic leak in patients undergoing colorectal surgery.44
The benefits of MBP in rectal surgery remain unclear with some studies reporting no difference in anastomotic leak rates45 and others reporting that MBP reduced infective complication rates.46 No studies analyzed whether MBP had an impact on the SIR. Therefore, although avoiding MBP has no adverse effect on postoperative complication rates, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that MBP can reduce the surgical stress response as part of an ERAS protocol.
Goal-Directed Fluid Therapy
Goal-directed therapy is the use of intravenous fluids and vasoactive drugs to meet defined targets for blood flow to achieve optimal oxygen delivery.47 A recent Cochrane Review of goal-directed therapy, including 5291 patients from 31 studies across several surgical specialties, demonstrated a modest reduction in postoperative complications and hospital stay when compared with conventional fluid regimens.48 Two studies have demonstrated a reduction in postoperative complications following the use of goal-directed therapy in colorectal surgery.26,27 One study demonstrated a significant reduction in postoperative serum interleukin-6 when goal directed therapy was compared to conventional fluid management in colorectal surgery.27 however this has not been reproduced in other studies.26 No study has specifically examined the impact of goal directed therapy on CRP following colorectal surgery. Therefore, there is no evidence that goal-directed fluid therapy can reduce the surgical stress response as part of an ERAS protocol.
Prevention of Postoperative Ileus
Postoperative ileus is a common problem following colorectal surgery and often results in the patient feeling nauseous and bloated and can delay discharge from hospital.49 The exact cause of an ileus is unknown, but it is thought to be multifactorial.50 The use of thoracic epidural analgesia instead of opioids has been shown to improve gut motility and reduce the length of postoperative ileus.37,51,52 Avoidance of gut edema due to fluid overloading during surgery cn improve gut function postoperatively53, as can the use of laparoscopic surgery.54
Other strategies that have been reported to reduce postoperative ileus include the use of chewing gum and the use of intravenous lignocaine intraoperatively. Some studies report that the use of chewing gum in the postoperative period reduced postoperative ileus and inpatient stay,49,55 whereas others reported only a mild reduction in time to flatus with no difference in length of stay or complication rate.56 In one study analyzing the effect of intravenous lignocaine given intraoperatively, they reported a reduction in postoperative analgesic requirements as well as improved time to flatus and reduced postoperative nausea and vomiting.28 As previously stated, there is little reported evidence on the effects of different analgesic methods on the systemic inflammatory response following colorectal surgery. One study reported that those receiving intravenous lignocaine had lower postoperative levels of both CRP and IL-6 following major abdominal surgery.28 Therefore, there is some evidence that strategies to reduce postoperative ileus such as intravenous lidocaine and epidural anesthesia but not chewing gum can reduce the surgical stress response as part of an ERAS protocol.
Early Postoperative Enteral Nutrition
A Cochrane Review of 14 trials including 1224 patients undergoing colorectal surgery57 reported a no significant trend toward fewer complications, in particular infections, in patients allowed enteral nutrition within 24 hours of surgery. However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that early postoperative enteral nutrition can reduce the surgical stress response as part of an ERAS protocol.
Avoidance of Nasogastric Tubes
A Cochrane Review of nasogastric tube decompression, including 5240 patients from 33 studies undergoing abdominal surgery, reported earlier return of bowel function and fewer pulmonary complications in those without routine nasogastric tube.58 There was no significant increase in other complications. A recent meta-analysis, including 1416 patients from 7 trials, in patients undergoing elective colorectal surgery reported similar results.59 However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that avoidance of nasogastric tubes can reduce the surgical stress response as part of an ERAS protocol.
Avoidance of Peritoneal Drains
There is no evidence to support the routine use of peritoneal drains following colorectal surgery to reduce neither the incidence or severity of anastomotic leaks nor the development of intra-abdominal collections.9,60–62 Moreover, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that avoidance of peritoneal drains can reduce the surgical stress response as part of an ERAS protocol.
Early Removal of Urinary Catheter
A meta-analysis of patients undergoing abdominal surgery reported reduced rates of bacteriuria and discomfort when suprapubic catheters were compared with transurethral catheters.63 A systematic review of urinary catheter management following colorectal surgery suggests removal of urinary catheters on the first postoperative day in colonic resections and from postoperative day 3 to 6 in rectal surgeries.64 This is associated with a lower incidence of urosepsis and a nonsignificant increase in urinary retention and recatheterization; however, this is based on a single small RCT and several observational studies.65–68 However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that early removal of urinary catheter can reduce the surgical stress response as part of an ERAS protocol.
Surgical Incisions
A Cochrane Review, including 3464 patients from 19 trials comparing transverse and vertical midline abdominal incisions across several surgical specialties, reported reduced rates of incisional hernia, analgesia requirement, and improved pulmonary function when transverse incisions were employed.69 Similar results were reported in a more recent meta-analysis of 24 trials, which also included paramedian incisions; however, neither study reported a significant reduction in pulmonary complications or recovery time.70 Two small RCTs included in the above review articles focused on colorectal surgery, describe conflicting results with regards to the impact of transverse or midline incisions on postoperative pulmonary function and pain.71,72 However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that different surgical incisions can reduce the surgical stress response as part of an ERAS protocol.
Early Mobilization
Within an enhanced recovery programme following colorectal surgery, mobilization on postoperative days 1 to 3 was associated with a reduced length of hospital stay.32,73 There is no specific evidence for early mobilization following colorectal surgery out with the context of enhanced recovery programmes. However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that early mobilization can reduce the surgical stress response as part of an ERAS protocol.
Thromboprophylaxis
Patients with cancer have a 4- to 6-fold higher risk than the general population of developing venous thromboembolism.74 Pharmacological methods (low-molecular-weight heparin [LMWH] or unfractionated heparin) and mechanical methods (intermittent pneumatic compression; graduated compression stockings) are used either solely75,76 or in combination with each other.77 No studies have analyzed thromboprophylaxis in an ERAS setting, although conclusions regarding the benefits can be drawn from non-ERAS patient groups. Furthermore, no studies reported whether the use of thromboprophylaxis had any impact on the SIR. Therefore, although the use of LMWH is effective in reducing VTE rates, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that thromboprophylaxis can reduce the surgical stress response as part of an ERAS protocol.
Antibiotic Prophylaxis
A recent Cochrane Review78 of antimicrobial prophylaxis in colorectal surgery, including 43,451 patients from 260 trials, has demonstrated a significant reduction in postoperative wound infections when prophylactic antibiotics are compared with placebo (relative risk 0.34, 95% confidence interval 0.28–0.41). Furthermore, additional benefit in terms of wound infection reduction was reported when combination oral and intravenous antibiotics were used and when antibiotics with anaerobic cover were used.78 Although it is understood that an exaggerated postoperative SIR, determined by measuring serum CRP, is associated with the development of infective complications,79,80 there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that antibiotic prophylaxis can reduce the surgical stress response as part of an ERAS protocol.
Maintenance of Intraoperative Normothermia
The development of hypothermia in the perioperative period is multifactorial, and can be attributed to general anesthesia, surgical technique, and the theatre environment.81 In the context of colorectal surgery, warming with forced air blankets and intravenous fluids to maintain normothermia has been shown to reduce wound infection rates,82 blood transfusion requirements, and complication rates.83 However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that maintenance of intraoperative normothermia can reduce the surgical stress response as part of an ERAS protocol.
Preoperative Counseling
Preoperative patient counseling has been reported to reduce a patient's anxiety84 and allow quicker recovery and discharge1,85 following surgery. If patients are aware of what is likely to happen during their hospital stay and are given targets postoperatively, then their recovery and hospital discharge are perceived to be quicker.86 However, there would appear to be no literature examining the impact of this strategy on the SIR following colorectal surgery. Therefore, there is no evidence that preoperative counseling can reduce the surgical stress response as part of an ERAS protocol.
DISCUSSION
The results of the present review shows that although there is evidence of the benefits of ERAS protocols in terms of reducing length of hospital stay and the reduction of postoperative complications following colorectal surgery, evidence of a effect on the stress response is surprisingly limited. Moreover, with the exception of laparoscopic surgery, evidence of an effect of individual components of ERAS protocols on the surgical stress response is also limited. Without such information, establishment of an optimal ERAS protocol will be based on subjective evidence rather than evidence of a beneficial effect on the surgical stress response.
In the present review, only laparoscopic surgery was shown to have substantial evidence demonstrating its beneficial effect on the reduction of markers of the postoperative SIR. It is of interest therefore that it has recently been reported that of the recommended ERAS components, only laparoscopic surgery, early oral intake, and early mobilization were identified as independent determinants of early recovery.32 It could be considered from a clinical point of view that ERAS protocols were developed to reduce pain and hospital stay and to reduce the time to return to work. Implicit in this is the assumption that the surgical stress response would also be reduced. From our review it is clear, with respect to laparoscopic surgery, there is evidence that these aims have been achieved. However, it would appear that there is no evidence that other components of ERAS protocols fulfil the above criteria. Future studies could utilize an objective marker of the postoperative SIR, such as CRP, which has recently been reported to be reflective of the magnitude of surgical trauma7 to assess which individual components of ERAS programmes modulated the SIR. These elements could then form future ERAS protocols, which would have the proven ability to reduce the surgical stress response. On the basis of the present review, these new protocols would likely be more streamlined and would perhaps allow easier implementation of the ERAS approach in clinical practice.
Initial studies into enhanced recovery were mainly performed in patients undergoing colorectal surgery, but ERAS is now used in many different surgical specialties and procedures. Indeed there are now consensus guidelines from the ERAS society for patients undergoing upper gastrointestinal surgery,87 urological surgery,88 colorectal surgery,1,8,9 and hepatobiliary and pancreatic surgery.89 This guidance has resulted in the widespread acceptance of the ERAS principles and adoption of these programmes in surgical units across the UK and Europe. However, no standard protocol exists for such programmes and as such the number of components used varies between units, making comparison of different ERAS studies problematic.30
Furthermore, it has previously been presumed that the greater the number of elements in an ERAS programme the better the outcomes. However, often studies make no distinction between the numbers of elements intended to be included versus the number that were actually successfully implemented.90 Indeed, it has recently been reported that increasing compliance with ERAS protocols improves outcomes in patients undergoing elective colorectal cancer resection.91 It has also been reported that studies with fewer ERAS components were associated with greater reduction in mortality and complications.12 This finding raises the possibility that some of the components of ERAS programmes offer little additional benefit to the overall outcomes.
The recently reported LAFA-study was designed to identify whether laparoscopic or open surgery, in combination with ERAS or standard care, was the optimal approach for colorectal surgery.92 Interestingly, the authors reported that length of stay was shortest in the laparoscopic/ERAS group (5 days), followed by the laparoscopic/standard group (6 days) and then by both open groups (7 days). These findings would appear to imply that although ERAS and laparoscopic surgery work synergistically, perhaps the majority of the benefit seen in these programmes is due to the use of laparoscopic surgery.90 Additional evidence for this hypothesis, in the context of the post-operative SIR, is provided by the finding that the two groups undergoing laparoscopic surgery had a lower postoperative CRP than either open surgery group regardless of perioperative care methods.10
Despite laparoscopic surgery appearing to be the key component in ERAS protocols, the majority of patients undergoing resection for colorectal cancer are still likely to receive an open procedure. Recent data from the National Bowel Cancer Audit 2014 has reported that of the 19 4533 patients who underwent resection of colorectal cancer, less than half (45%) had laparoscopic surgery.93 Therefore, rather than increasing efforts to deliver ERAS protocols it may be more appropriate to increase the number of laparoscopic procedures carried out.
In the present study, a potential confounding factor would be the duration of operation since this may vary with surgical approach. However, few of the included studies that examined the impact of laparoscopic versus open colorectal surgery on the postoperative SIR reported operation duration. In a recent audit of our center, there was no association between operation duration and the postoperative SIR following either laparoscopic or open surgery for colorectal cancer (McSorley et al 2015, submitted to press).
In conclusion, the present systematic review shows that, with the exception of laparoscopic surgery, objective evidence of the effect of individual components of ERAS protocols in reducing the stress response following colorectal surgery is limited. This review, examining the literature pertaining to a known indicator of surgical trauma and predictor of post-operative complication severity, may be the first step in stimulating further research in this area.
Acknowledgments
The authors declare no conflict of interest and no sources of funding.
Footnotes
Abbreviations: CRP = C-reactive protein, EA = epidural analgesia, ERAS = Enhanced Recovery After Surgery, IL-6 = interleukin-6, LA = local anaesthetic, LMWH = low molecular weight heparin, MBP = mechanical bowel preparation, NSAID = non-steroidal anti-inflammatory drug, SIR = systemic inflammatory response, TAP = transversus abdominis plane, TNF = tumor necrosis factor.
DGW and STM are joint first authors.
No sources of financial support or funding were obtained for this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144:961–969. [DOI] [PubMed] [Google Scholar]
- 2.Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001; 322:473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbertson DP. Second annual Jonathan E. Rhoads Lecture. The metabolic response to injury and its nutritional implications: retrospect and prospect. JPEN J Parenter Enteral Nutr 1979; 3:108–129. [DOI] [PubMed] [Google Scholar]
- 4.Baigrie RJ, Lamont PM, Kwiatkowski D, et al. Systemic cytokine response after major surgery. Br J Surg 1992; 79:757–760. [DOI] [PubMed] [Google Scholar]
- 5.Marik PE, Flemmer M. The immune response to surgery and trauma: implications for treatment. J Trauma Acute Care Surg 2012; 73:801–808. [DOI] [PubMed] [Google Scholar]
- 6.Gabay C, Kushner I. Mechanisms of disease: acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340:448–454. [DOI] [PubMed] [Google Scholar]
- 7.Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 2015; 157:362–380. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012; 31:783–800. [DOI] [PubMed] [Google Scholar]
- 9.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012; 31:801–816. [DOI] [PubMed] [Google Scholar]
- 10.Veenhof AA, Vlug MS, van der Pas MH, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg 2012; 255:216–221. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Jiang Z, Zhao K, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg 2012; 16:1379–1388. [DOI] [PubMed] [Google Scholar]
- 12.Schwenk W, Jacobi C, Mansmann U, et al. Inflammatory response after laparoscopic and conventional colorectal resections - results of a prospective randomized trial. Langenbecks Arch Surg 2000; 385:2–9. [DOI] [PubMed] [Google Scholar]
- 13.Delgado S, Lacy AM, Filella X, et al. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum 2001; 44:638–646. [DOI] [PubMed] [Google Scholar]
- 14.Braga M, Vignali A, Zuliani W, et al. Metabolic and functional results after laparoscopic colorectal surgery: a randomized, controlled trial. Dis Colon Rectum 2002; 45:1070–1077. [DOI] [PubMed] [Google Scholar]
- 15.Dunker MS, Ten Hove T, Bemelman WA, et al. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral blood mononuclear cells in patients after laparoscopic vs. conventional bowel resection: a randomized study. Dis Colon Rectum 2003; 46:1238–1244. [DOI] [PubMed] [Google Scholar]
- 16.Ng SS, Leung KL, Lee JF, et al. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum 2009; 52:558–566. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson UO, Tiefenthal M, Thorell A, et al. Laparoscopic-assisted and open high anterior resection within an ERAS protocol. World J Surg 2012; 36:1154–1161. [DOI] [PubMed] [Google Scholar]
- 18.Tsimogiannis KE, Tellis CC, Tselepis AD, et al. Toll-like receptors in the inflammatory response during open and laparoscopic colectomy for colorectal cancer. Surg Endosc 2012; 26:330–336. [DOI] [PubMed] [Google Scholar]
- 19.Ramanathan ML, MacKay G, Platt J, et al. The impact of open versus laparoscopic resection for colon cancer on C-reactive protein concentrations as a predictor of postoperative infective complications. Ann Surg Oncol 2015; 22:938–943. [DOI] [PubMed] [Google Scholar]
- 20.Han SA, Lee WY, Park CM, et al. Comparison of immunologic outcomes of laparoscopic vs open approaches in clinical stage III colorectal cancer. Int J Colorectal Dis 2010; 25:631–638. [DOI] [PubMed] [Google Scholar]
- 21.Tsamis D, Theodoropoulos G, Stamopoulos P, et al. Systemic inflammatory response after laparoscopic and conventional colectomy for cancer: a matched case-control study. Surg Endosc 2012; 26:1436–1443. [DOI] [PubMed] [Google Scholar]
- 22.Wu FP, Sietses C, von Blomberg BM, et al. Systemic and peritoneal inflammatory response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum 2003; 46:147–155. [DOI] [PubMed] [Google Scholar]
- 23.Veenhof AA, Sietses C, von Blomberg BM, et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis 2011; 26:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WK, Ren L, Wei Y, et al. General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. Int J Colorectal Dis 2015; 30:475–481. [DOI] [PubMed] [Google Scholar]
- 25.Mathur S, Plank LD, McCall JL, et al. Randomized controlled trial of preoperative oral carbohydrate treatment in major abdominal surgery. Br J Surg 2010; 97:485–494. [DOI] [PubMed] [Google Scholar]
- 26.Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95:634–642. [DOI] [PubMed] [Google Scholar]
- 27.Noblett SE, Snowden CP, Shenton BK, et al. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 2006; 93:1069–1076. [DOI] [PubMed] [Google Scholar]
- 28.Sridhar P, Sistla SC, Ali SM, et al. Effect of intravenous lignocaine on perioperative stress response and post-surgical ileus in elective open abdominal surgeries: a double-blind randomized controlled trial. ANZ J Surg 2014. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed J, Khan S, Lim M, et al. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Colorectal Dis 2012; 14:1045–1051. [DOI] [PubMed] [Google Scholar]
- 30.Neville A, Lee L, Antonescu I, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg 2014; 101:159–170. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014; 101:172–188. [DOI] [PubMed] [Google Scholar]
- 32.Vlug MS, Bartels SA, Wind J, et al. Which fast track elements predict early recovery after colon cancer surgery? Colorectal Dis 2012; 14:1001–1008. [DOI] [PubMed] [Google Scholar]
- 33.Schwenk W, Haase O, Neudecker J, et al. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005; CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhry E, Schwenk W, Gaupset R, et al. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev 2008; 34:498–504. [DOI] [PubMed] [Google Scholar]
- 35.Vennix S, Pelzers L, Bouvy N, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2014; 4:CD005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kehlet H. Postoperative opioid sparing to hasten recovery: what are the issues? Anesthesiology 2005; 102:1083–1085. [DOI] [PubMed] [Google Scholar]
- 37.Marret E, Remy C, Bonnet F. Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg 2007; 94:665–673. [DOI] [PubMed] [Google Scholar]
- 38.Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth 2012; 109:144–154. [DOI] [PubMed] [Google Scholar]
- 39.Johns N, O’Neill S, Ventham NT, et al. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorectal Dis 2012; 14:E635–E642. [DOI] [PubMed] [Google Scholar]
- 40.Thornton PC, Buggy DJ. Local anaesthetic wound infusion for acute postoperative pain: a viable option? Br J Anaesth 2011; 107:656–658. [DOI] [PubMed] [Google Scholar]
- 41.Ventham NT, Hughes M, O’Neill S, et al. Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg 2013; 100:1280–1289. [DOI] [PubMed] [Google Scholar]
- 42.Maund E, McDaid C, Rice S, et al. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth 2011; 106:292–297. [DOI] [PubMed] [Google Scholar]
- 43.Smith MD, McCall J, Plank L, et al. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev 2014; 8:CD009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guenaga KF, Matos D, Wille-Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011; CD001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van’t Sant HP, Weidema WF, Hop WCJ, et al. The influence of mechanical bowel preparation in elective lower colorectal surgery. Ann Surg 2010; 251:59–63. [DOI] [PubMed] [Google Scholar]
- 46.Bretagnol F, Panis Y, Rullier E, et al. Rectal cancer surgery with or without bowel preparation the French Greccar III multicenter single-blinded randomized trial. Ann Surg 2010; 252:863–867. [DOI] [PubMed] [Google Scholar]
- 47.Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia 2008; 63:44–51. [DOI] [PubMed] [Google Scholar]
- 48.Grocott MP, Dushianthan A, Hamilton MA, et al. Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane Systematic Review. Br J Anaesth 2013; 111:535–548. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald JEF, Ahmed I. Systematic review and meta-analysis of chewing-gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J Surg 2009; 33:2557–2566. [DOI] [PubMed] [Google Scholar]
- 50.Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch of Surg 2003; 138:206–214. [DOI] [PubMed] [Google Scholar]
- 51.Jorgensen H, Wetterslev J, Moiniche S, et al. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev 2000; CD001893. [DOI] [PubMed] [Google Scholar]
- 52.Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol 2003; 4:365–372. [DOI] [PubMed] [Google Scholar]
- 53.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002; 359:1812–1818. [DOI] [PubMed] [Google Scholar]
- 54.Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorectal Dis 2006; 8:375–388. [DOI] [PubMed] [Google Scholar]
- 55.Li S, Liu Y, Peng Q, et al. Chewing gum reduces postoperative ileus following abdominal surgery: a meta-analysis of 17 randomized controlled trials. J Gastroenterol Hepatol 2013; 28:1122–1132. [DOI] [PubMed] [Google Scholar]
- 56.Su’a BU, Pollock TT, Lemanu DP, et al. Chewing gum and postoperative ileus in adults: a systematic literature review and meta-analysis. Int J Surg 2015; 14C:49–55. [DOI] [PubMed] [Google Scholar]
- 57.Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev 2006; CD004080. [DOI] [PubMed] [Google Scholar]
- 58.Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2007; CD004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao W, Zhang X, Zhang J, et al. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis 2011; 26:423–429. [DOI] [PubMed] [Google Scholar]
- 60.Jesus EC, Karliczek A, Matos D, et al. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev 2004; CD002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karliczek A, Jesus EC, Matos D, et al. Drainage or nondrainage in elective colorectal anastomosis: a systematic review and meta-analysis. Colorectal Dis 2006; 8:259–265. [DOI] [PubMed] [Google Scholar]
- 62.Puleo FJ, Mishra N, Hall JF. Use of intra-abdominal drains. Clin Colon Rectal Surg 2013; 26:174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McPhail MJ, Abu-Hilal M, Johnson CD. A meta-analysis comparing suprapubic and transurethral catheterization for bladder drainage after abdominal surgery. Br J Surg 2006; 93:1038–1044. [DOI] [PubMed] [Google Scholar]
- 64.Hendren S. Urinary catheter management. Clin Colon Rectal Surg 2013; 26:178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benoist S, Panis Y, Denet C, et al. Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery 1999; 125:135–141. [PubMed] [Google Scholar]
- 66.Basse L, Hjort Jakobsen D, Billesbolle P, et al. A clinical pathway to accelerate recovery after colonic resection. Ann Surg 2000; 232:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahokehr A, Sammour T, Zargar-Shoshtari K, et al. Recovery after open and laparoscopic right hemicolectomy: a comparison. J Surg Res 2010; 162:11–16. [DOI] [PubMed] [Google Scholar]
- 68.Zmora O, Madbouly K, Tulchinsky H, et al. Urinary bladder catheter drainage following pelvic surgery-is it necessary for that long? Dis Colon Rectum 2010; 53:321–326. [DOI] [PubMed] [Google Scholar]
- 69.Brown SR, Goodfellow PB. Transverse verses midline incisions for abdominal surgery. Cochrane Database Syst Rev 2005; CD005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bickenbach KA, Karanicolas PJ, Ammori JB, et al. Up and down or side to side? A systematic review and meta-analysis examining the impact of incision on outcomes after abdominal surgery. Am J Surg 2013; 206:400–409. [DOI] [PubMed] [Google Scholar]
- 71.Lindgren PG, Nordgren SR, Oresland T, et al. Midline or transverse abdominal incision for right-sided colon cancer-a randomized trial. Colorectal Dis 2001; 3:46–50. [DOI] [PubMed] [Google Scholar]
- 72.Brown SR, Goodfellow PJ, Adam IJ, et al. A randomised controlled trial of transverse skin crease vs. vertical midline incision for right hemicolectomy. Tech Coloproctol 2004; 8:15–18. [DOI] [PubMed] [Google Scholar]
- 73.Maessen J, Dejong CH, Hausel J, et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg 2007; 94:224–231. [DOI] [PubMed] [Google Scholar]
- 74.Imberti D, Agnelli G, Ageno W, et al. Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the MASTER Registry. Haematologica 2008; 93:273–278. [DOI] [PubMed] [Google Scholar]
- 75.Borly L, Wille-Jorgensen P, Rasmussen MS. Systematic review of thromboprophylaxis in colorectal surgery - an update. Colorectal Dis 2005; 7:122–127. [DOI] [PubMed] [Google Scholar]
- 76.Levine MN, Raskob G, Landefeld S, et al. Hemorrhagic complications of anticoagulant treatment. Chest 2001; 119 (1 Suppl):108S–121S. [DOI] [PubMed] [Google Scholar]
- 77.Wille-Jorgensen P. Prophylaxis of postoperative thromboembolism with combined methods. Semin Thromb Hemost 1991; 17 Suppl 3:272–279. [PubMed] [Google Scholar]
- 78.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014; 5:CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Platt JJ, Ramanathan ML, Crosbie RA, et al. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 2012; 19:4168–4177. [DOI] [PubMed] [Google Scholar]
- 80.Singh PP, Zeng IS, Srinivasa S, et al. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 2014; 101:339–346. [DOI] [PubMed] [Google Scholar]
- 81.Sessler DI. Perioperative heat balance. Anesthesiology 2000; 92:578–596. [DOI] [PubMed] [Google Scholar]
- 82.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996; 334:1209–1215. [DOI] [PubMed] [Google Scholar]
- 83.Wong PF, Kumar S, Bohra A, et al. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg 2007; 94:421–426. [DOI] [PubMed] [Google Scholar]
- 84.Kiyohara LY, Kayano LK, Oliveira LM, et al. Surgery information reduces anxiety in the pre-operative period. Rev Hosp Clin Fac Med Sao Paulo 2004; 59:51–56. [DOI] [PubMed] [Google Scholar]
- 85.Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med 2004; 32 (4 Suppl):S76–S86. [DOI] [PubMed] [Google Scholar]
- 86.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005; 24:466–477. [DOI] [PubMed] [Google Scholar]
- 87.Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br J Surg 2014; 101:1209–1229. [DOI] [PubMed] [Google Scholar]
- 88.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS((R))) society recommendations. Clin Nutr 2013; 32:879–887. [DOI] [PubMed] [Google Scholar]
- 89.Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012; 31:817–830. [DOI] [PubMed] [Google Scholar]
- 90.Vlug MS, Bemelman WA. Reply to Letter: “The LAFA Study”. Ann Surg 2015; 261:e31–e32. [DOI] [PubMed] [Google Scholar]
- 91.Group EC. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 2015; 261:1153–1159. [DOI] [PubMed] [Google Scholar]
- 92.Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011; 254:868–875. [DOI] [PubMed] [Google Scholar]
- 93.HQUIP. National Bowel Cancer Audit Report 2014. Available at: http://www.hscic.gov.uk/catalogue/PUB16021/nati-clin-audi-supp-prog-bowe-canc-2014-rep1.pdf Accessed 16/03/2015. [Google Scholar]


