Abstract
This study investigates parental attitudes and factors associated with varicella vaccination among preschool and schoolchildren prior to introduction of the vaccine into Hong Kong's universal Childhood Immunization Program.
Fourteen kindergartens and 5 primary schools in Hong Kong were randomly selected in 2013. Parents of the students were invited to answer the self-administered questionnaires. Acquired information included demographic characteristics and socioeconomic statuses of families, children's history of chickenpox infection and vaccination, and reasons for getting children vaccinated. Logistic regression was applied to examine the factors associated with vaccination.
From the 3484 completed questionnaires, the calculated rates of varicella infection and vaccination were 20.7% and 69.0%, respectively. Barriers to vaccination included parental uncertainties about vaccine effectiveness, lack of recommendation from the government, and concerns on adverse effects. Overall, 71.8%, 69.0%, and 45.7% of the parents rated family doctors, specialists, and the government, respectively, as very important motivators of vaccination. Higher parental educational level and family income, better perceived knowledge of varicella and chance of infection, discussion with a family doctor, and positive health belief towards vaccination were associated with vaccination (all P < 0.05).
The rate of vaccination in Hong Kong was higher than that of some other countries that also did not include the vaccine in their routine immunization programs. More positive parental attitudes, higher socioeconomic status, and discussion with a family doctor are associated with greater vaccination rates. The important roles that health professionals and the government play in promoting varicella vaccination were emphasized.
INTRODUCTION
Chickenpox (varicella) is a highly contagious disease recognized by a characteristic and often pathognomonic maculopapular vesicular rash. Varicella may lead to serious complications, including secondary bacterial skin and soft-tissue infections, cerebellitis, encephalitis, pneumonia, and coagulopathy.1 Neonates, adolescents, and immunocompromised individuals are more susceptible to complications.2–4 Reported rates of complications from varicella range from 40.7% to 83.3% among hospitalized children.5–9 Mortality rates of 2 to 4 for every 100,000 infected people were noted.1 More than 95% of the non-vaccinated populations were infected with varicella, and most of them are younger than 20 years.10 Children younger than 10 years of age, particularly those between 3 and 6 years, exhibit a high incidence of infection when they do not receive varicella vaccine as part of a universal vaccination program.11
In 1998, the World Health Organization (WHO) recommended routine childhood varicella vaccination in countries where chickenpox significantly affects public health.12 Since the introduction of universal vaccination, a number of countries have witnessed declines in varicella incidence, hospitalization, and/or mortality, including the United States of America,13,14 Australia,15 Italy,16 Germany,17 and Taiwan.18 Varicella vaccination provides long-term effectiveness in preventing varicella and may reduce the risk of herpes zoster.19 Furthermore, varicella vaccination is cost-effective in the Asian regions. In Singapore, the benefit–cost ratio of vaccination is 2.25, which indicates that every US$1.00 invested in vaccination would save US$2.25.20 Similar findings were reported in 2 studies in Taiwan, which reported a benefit–cost ratio of 2.06 21 and 0.9,22 respectively.
Although varicella is a global health issue, disease burden varies by geographical region, climate, population density, and socioeconomic development.23–25 For example, the onset of varicella infection may be delayed in tropical countries because of virus inactivation by high ambient temperature and humidity.26 In Hong Kong, a city with subtropical climate, the average annual varicella notification and hospital attendance rates in children younger than 18 years are 981 and 285 per 100,000 individuals, respectively.27 Moreover, complication rate is 47% among hospitalized cases.28 Even though the varicella complication rate in Hong Kong is comparable with international data,5–9 the varicella vaccine was only introduced into Hong Kong's universal Childhood Immunization Program (CIP) as a free but optional vaccine in July 2014 for children born on or after January 1, 2013.29
Before the implementation of universal varicella vaccination, children in Hong Kong can only be vaccinated in a private sector. A survey conducted by Hong Kong's Department of Health in 2009 reported that only 32.4% of local-born children had received varicella vaccine at the time.30 A 2012 survey of kindergartens revealed the varicella infection rates was 19.5% and the uptake of varicella vaccination rate was 57.6%.31 Although varicella vaccine coverage has increased in Hong Kong, it remains below that of the United States (79.9%–92.0%).32 Previously noted barriers to vaccination in Hong Kong include uncertainties about the effectiveness and side effects of the vaccine and the lack of recommendations from doctors or the government.31 Cross-sectional surveys conducted in the United States and Turkey showed that positive parental health beliefs on the effect of varicella vaccine may affect parental decisions to vaccinate their children.33,34 However, both studies did not correlate parental health beliefs and vaccination status.
In the present study, we investigate the varicella vaccination rates among preschool (kindergarten) and primary schoolchildren before the vaccine's incorporation into Hong Kong's universal CIP; the association between the rates and health beliefs and demographic characteristics of the parents of these children; and the motivators and barriers of having the vaccination.
METHODS
A cross-sectional questionnaire survey was conducted between September and November 2013. A complete list of kindergarten and primary schools was downloaded from Hong Kong's Education Bureau (www.edb.gov.hk), which comprise 948 kindergartens and 615 local primary schools. Fourteen kindergarten (1.5%) and 5 primary schools (0.8%) were randomly selected and their school principals were invited to participate. Sample size determination is summarized in the following section.
Although no data are available on the age-specific varicella cumulative incidence in Hong Kong, several overseas studies reported varicella seroprevalence rates in children.35–38 Various countries such as England,35 Italy,36 Australia,37 and Korea 38 have varicella seroprevalence rates ranging from 58% to 75% by the age of 5 years, and 83% to 93% by the age of 12 years. From these figures, the sample size was estimated to be 1400 kindergarten students (350 cases for each age group within 2–5 years of age), and 1610 primary school students (230 cases for each age group within 6–12 years of age), which sum up to a total of 3010 students. This estimation gave a precision of 5% at a confidence interval of 95% for age-specific varicella cumulative incidence in our schoolchildren population.39 According to the education statistics (www.edb.gov.hk) and by assuming that the number of students in each kindergarten and primary school in Hong Kong are around 100 and 400, respectively, we determined that 14 kindergartens and 5 primary schools can satisfy the sample size.
After obtaining consent from the principals, questionnaire packages that contain information, consent forms, and self-administered questionnaires were distributed to the students. The students were then asked to bring home the packages for filling out by their respective parent or guardian. The questionnaire consisted of 4 parts: demographic information of the children and the respondents, children's history of varicella infection and vaccination, 10 parental health belief statements regarding the varicella vaccine,33 knowledge on varicella transmission and therapy,40 and reasons/barriers for their having/not having varicella vaccination.31
Most questions in the survey were in multiple-choice format, except for personal health beliefs and motivators/barriers for vaccination, in which participants were requested to answer with numbers in pre-specified ranges. Each of the 10 parental health belief statements were scored from 1 (completely disagree) to 6 (completely agree). The motivators/barriers of vaccination were graded using a 6-point Likert scale from 0 (completely disagree) to 5 (completely agree). Only the parents of vaccinated children were asked to answer on the motivators of vaccination, whereas only the parents of unvaccinated children were asked to answer on the barriers. Perceived knowledge and chance of infection were assessed by categorical responses ranging from “very inadequate/very unlikely” to “very adequate/very likely.”
Completed questionnaires were collected, and data checking and entry into a pre-developed SPSS database were performed by student assistants. Parents were contacted when necessary to clarify incomplete information. Frequencies and percentages were computed for demographic variables. Median and interquartile ranges were calculated by vaccination status for the variables that used the Likert scale and subsequently compared by employing the Mann–Whitney U test. Crude odds ratios (ORs) and their 95% confidence intervals were computed for each variable by vaccination status. ORs with P values <0.1 were entered to the multivariate logistic regression, and a stepwise procedure was used to select the final model. All analyses were conducted using SPSS version 20 (SPSS Inc, Chicago, IL). As individual schools may affect vaccination status, a school variable was added as a random factor in the regression using the approach of generalized linear mixed-effects model.41 The SAS function PROC GLIMMIX was used for the estimation.42
Ethical approval was obtained from the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Committee (Ref: CRE-2012.393).
RESULTS
A total of 5617 questionnaires were distributed, and 3484 completed questionnaires were returned, giving a response rate of 62.0%. Previous history of varicella infection was noted in 20.7% (721) of the children. After excluding those with history of varicella infection and 36 with missing infection information, 2727 children were included in the succeeding analyses. Among these 2727 children, 69.0% (1881) were reported to have been vaccinated against varicella, 53.2% (1452) were boys, and 91.8% (2503) were born in Hong Kong. Furthermore, 56.2% attended kindergarten (mean age: 4.14 ± 0.96 years), 24.4% attended junior primary schools (mean age: 7.28 ± 0.92 years), and 16.7% attended senior primary schools (mean age: 10.36 ± 0.90 years). Of the questionnaires, 77.9% (2124) were completed by mothers and 18.0% (492) by fathers (Table 1 ). Moreover, 94.5% of respondents indicated that they would allow their children to be vaccinated if the vaccine was included in the government's universal CIP. Significant differences in vaccination rates were noted in relation to the child's place of birth and school grade, respondent's educational level and occupation, family income, and housing type (Table 1 ). Significant differences were also noted in perceived knowledge, perceived chance of infection, discussion with a family doctor, and willingness to pay (Table 1 ).
TABLE 1.
Demographic Characteristics of Children and Respondents (N = 2727)
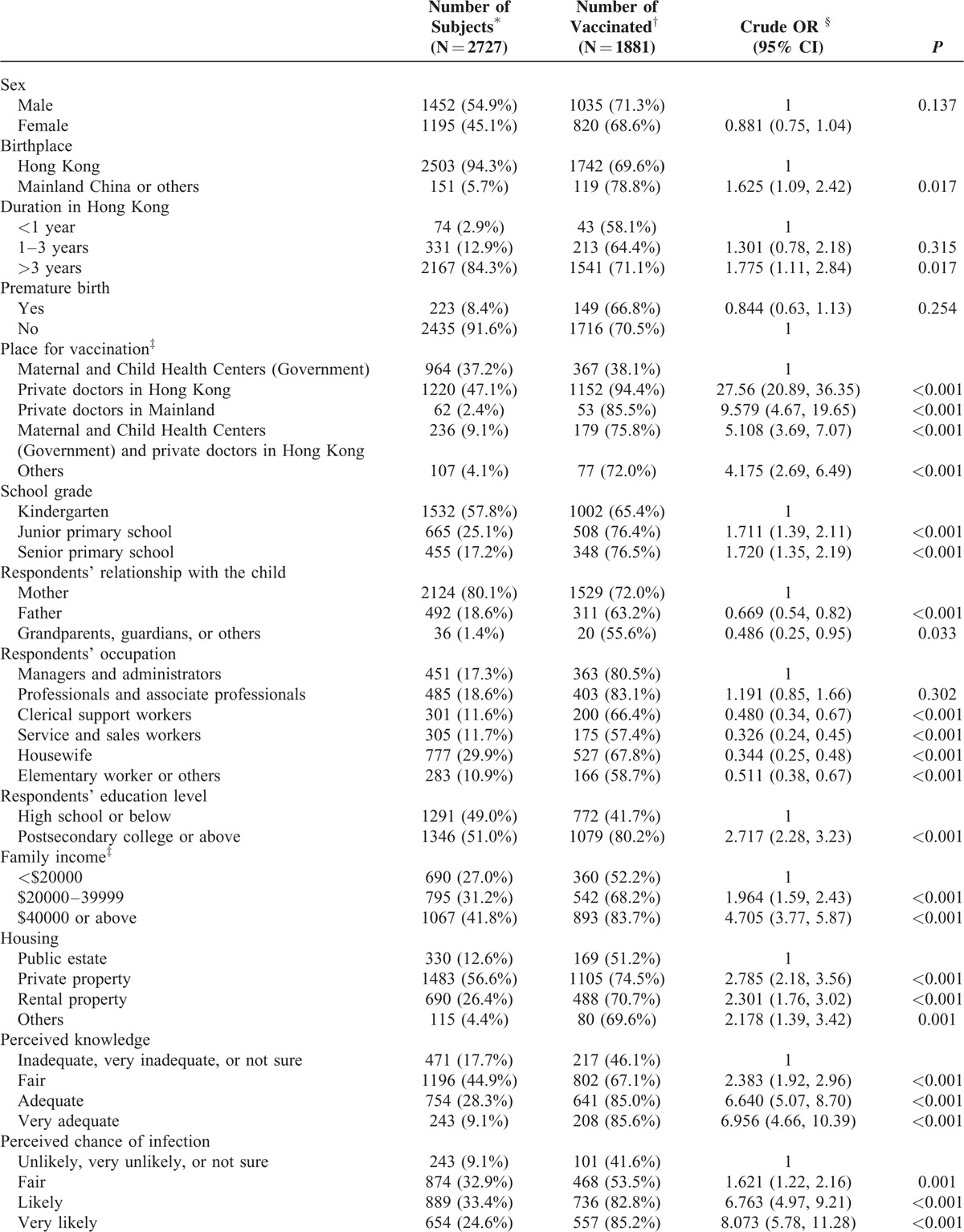
Results of the scoring of the 10 parental health belief statements by vaccination status are presented below. Among the respondents with vaccinated children, 6 statements obtained a median score of 5, which implies that >50% of respondents strongly agreed with these statements (Table 2). For respondents with unvaccinated children, only 3 statements obtained median scores of 5. All but one statement showed significant differences between the vaccinated and nonvaccinated groups (Table 2).
TABLE 1 (Continued).
Demographic Characteristics of Children and Respondents (N = 2727)
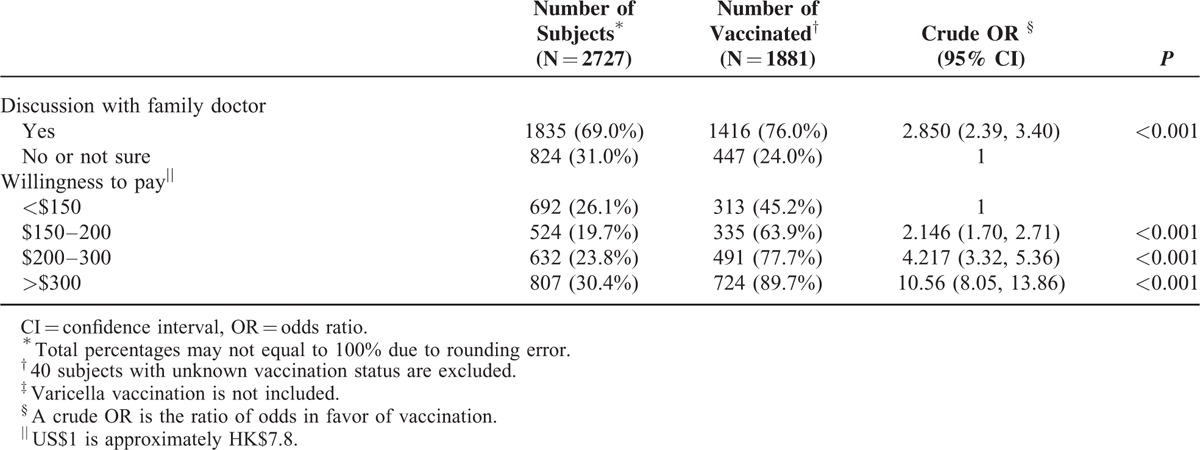
Adjusted ORs and their 95% confidence intervals from the logistic regression were calculated based on the final model selected using the stepwise procedure (Table 3). Children who were studying in senior primary school, respondents with educational attainments higher than the secondary level, family income of ≥$40,000, very adequate perceived knowledge on varicella, very high perceived chance of children being infected by varicella, discussion with a family doctor, and willingness to pay >$300 for the vaccine were associated with higher vaccination rates. Except for “my child may not need the chickenpox vaccine because chickenpox is only a minor illness,” 4 other health beliefs were significantly associated with high vaccination rates. These health beliefs are as follows: “the chickenpox vaccine is very effective in preventing chickenpox,” “my child is very likely to contract chickenpox if he/she does not receive the vaccine,” “the chickenpox vaccine is worthwhile even if it does not provide lifelong immunity to chickenpox for my child,” and “I am uncomfortable with the number of shots my child is receiving.” The results from the application of the generalized linear mixed model with school as random effect are also presented in Table 3. The ORs virtually matched those computed from the logistic regression.
TABLE 2.
Parental Health Beliefs on Having Their Children Vaccinated Against Varicella Infection
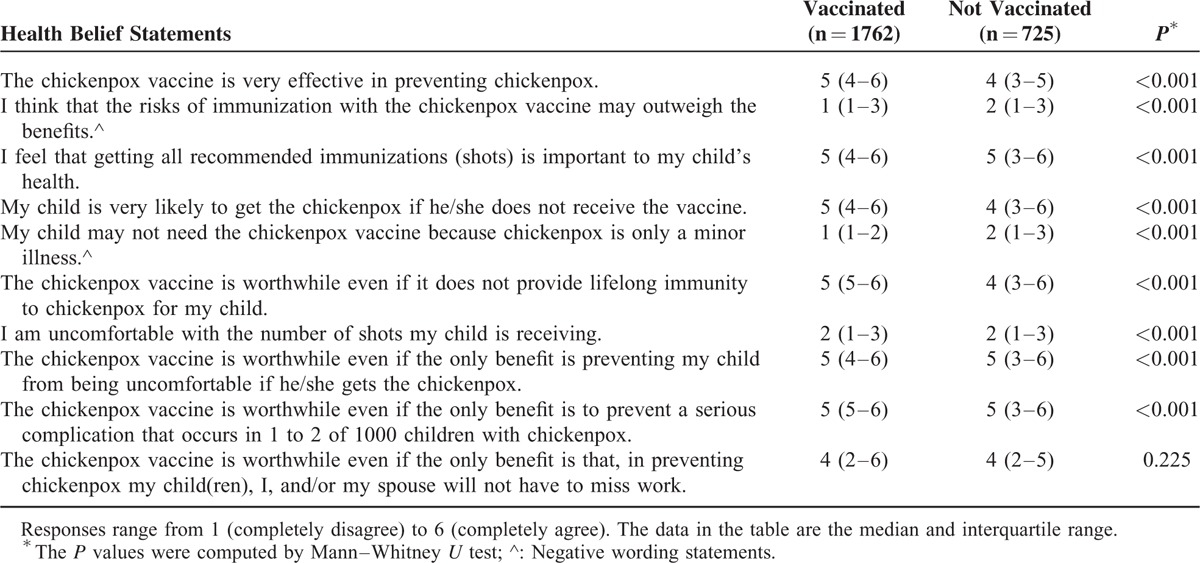
Reasons for varicella vaccination were scored from 0 (not important) to 5 (very important). The median scores for “recommendation by family doctors” and “recommendation by specialists” were both 5, which implies that >50% of respondents thought that these recommendations were very important (Table 4). “Recommendation on TV/newspaper/magazine” obtained the lowest median score (3) among the 6 recommendations. Of the parents with vaccinated children, 71.8%, 69.0%, and 45.7% rated family doctors, specialists, and the government, respectively, as very important motivators of vaccination.
TABLE 3.
Multivariate Analysis of the Associations Between Demographic Characteristics and the Rate of Varicella Vaccination
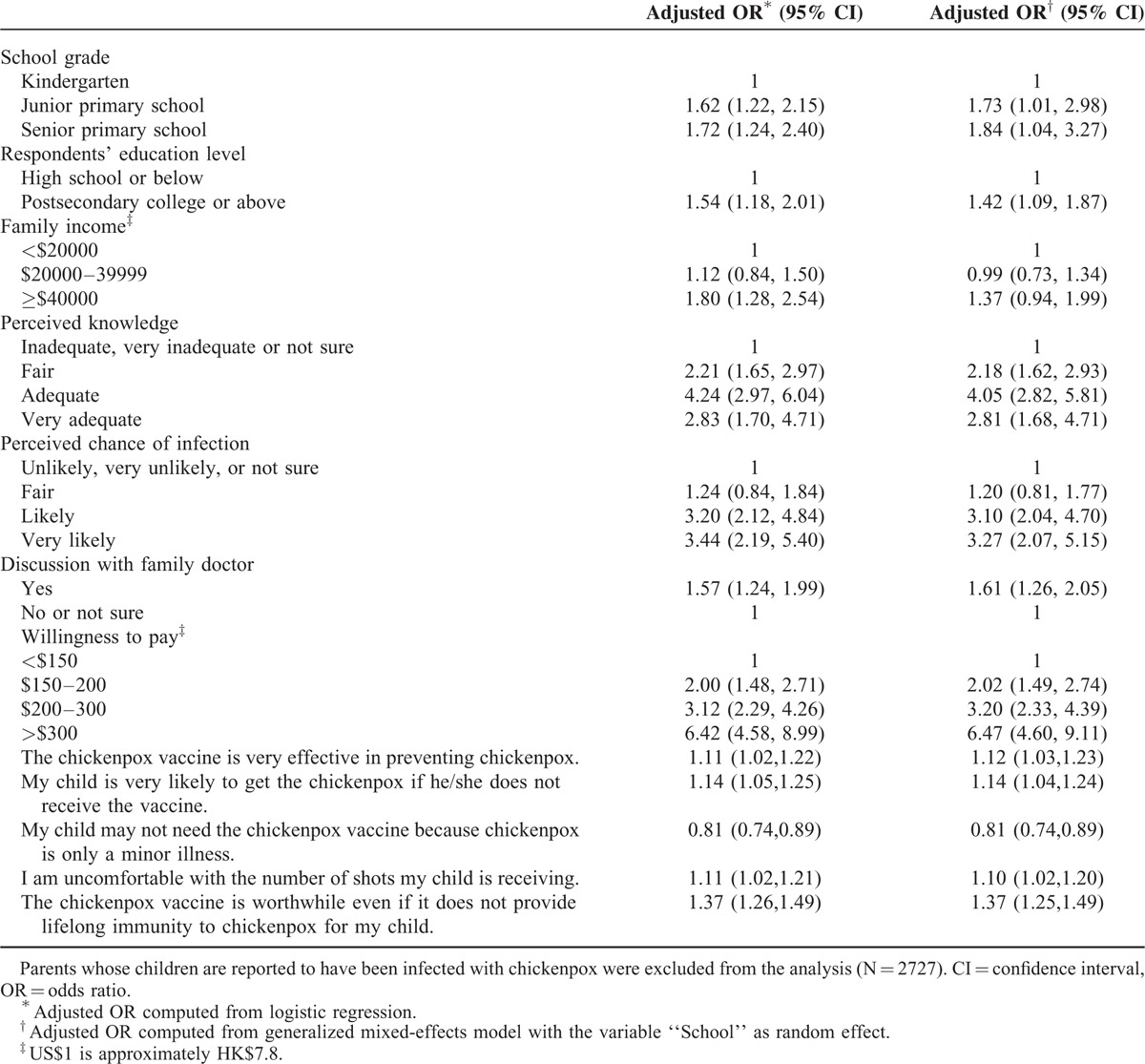
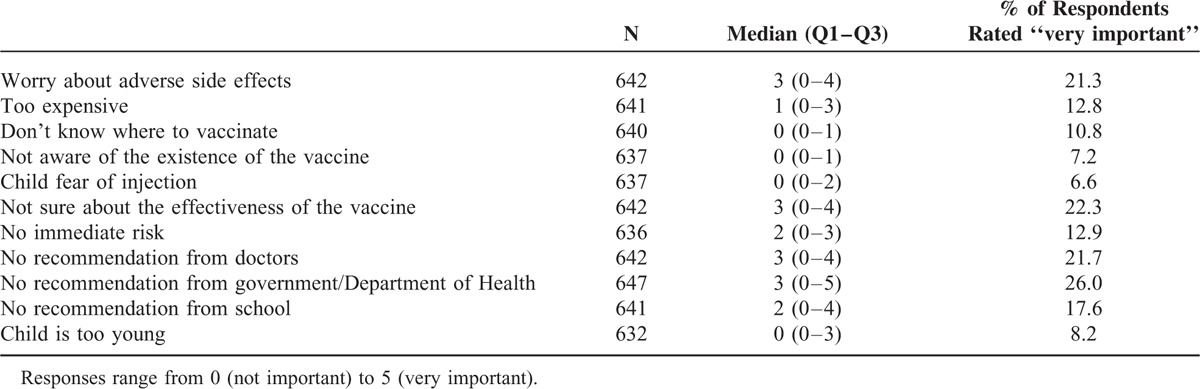
Four statements on the barriers to varicella vaccination (“worry about adverse side effects,” “not sure about the effectiveness of the vaccine,” “no recommendation from doctors,” and “no recommendation from the government/Department of Health”) obtained the highest median scores (3). By contrast, 4 other statements (“don’t know where to vaccinate,” “not aware of the existence of the vaccine,” “child fear of injection,” and “child is too young”) obtained the lowest median scores (0), which shows that >50% respondents thought that these factors were unimportant barriers (Table 5). Of the parents of unvaccinated children, 21.3%, 22.3%, 21.7%, and 26.0% rated adverse effects, uncertain effectiveness, no recommendation from doctors, and no recommendation from the government, respectively, as very important barriers to vaccination.
TABLE 4.
Reported Motivators for Vaccination Among Parents of Vaccinated Students
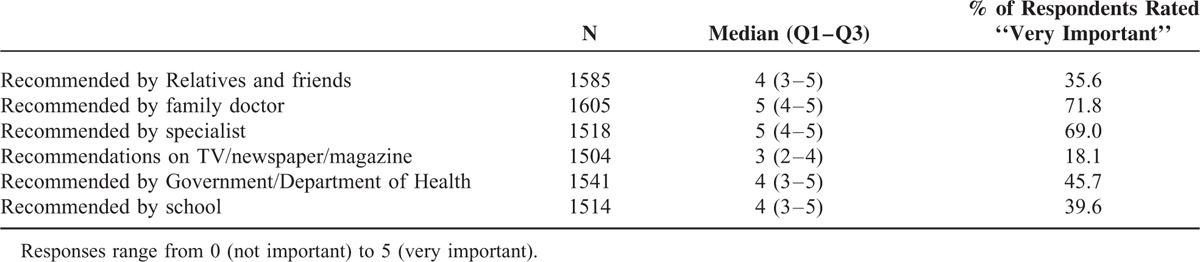
TABLE 5.
Reported Barriers for Vaccination Among Parents of Unvaccinated Children
DISCUSSION
Varicella can pose serious threats of disease to fetuses, neonates, and non-immune or immunocompromised children and adults. The present study found that 69.0% of Hong Kong preschool and school children were reported to have received vaccination against varicella, although the vaccine was not yet included in Hong Kong's CIP at the time. This rate is higher than that in a similar study in Hong Kong reported a few years ago.31 The rate increase may be due to the educational campaigns conducted by the government and the discussions on including the vaccine in the CIP over the recent years. The vaccine has been included as a free optional vaccine in the CIP program since 2014.
Hong Kong's rate of varicella vaccination appears slightly higher than those of several other countries that also do not include the vaccine in their routine immunization programs. The corresponding vaccination rates for these countries were 61.1% in Athens, Greece,43 50% in Japan,44,45 and 62.0% in Shandong Province, China.45 The higher rate of vaccination in Hong Kong may indicate the greater awareness or value placed on varicella prevention by Hong Kong parents.
This study showed that parents with higher educational attainments, greater family income, greater perceived knowledge of varicella, and higher perceived chance of infection were significantly associated with positive vaccination. In the United States, higher maternal educational levels positively correlated with varicella vaccination.46 Similarly, families with better parental educational attainments and higher family income showed greater vaccination rates in Turkey and Taiwan.34,47 Promoting parental knowledge on vaccination is a key factor in improving immunization rates, especially in families with lower educational backgrounds and from cities with low socioeconomic ranking, as observed in Israel.48
Discussion with a family doctor is significantly associated with a greater propensity for vaccination, which indicates that an expert's opinion importantly influences the perspective of the parents. “Recommendation from family doctors” and “recommendation from specialists” were the major reasons that the parents allowed their children to receive the vaccine. By contrast, other parents did not seek vaccination for their children mainly because of their lack of knowledge about the vaccine and lack of recommendation from the experts or government officials. A previous study conducted in Sweden 49 also showed the great importance of the role of well-trained healthcare staff in providing advice and discussing measles, mumps, and rubella vaccination with concerned parents.
Our study evaluated the effect of parental health beliefs on varicella vaccination rate. The results may be explained by the relationship between health beliefs and other parental attitudes. “The chickenpox vaccine is very effective in preventing chickenpox” and “my child may not need the chickenpox vaccine because chickenpox is only a minor illness” may be related to the perceived knowledge of parents on vaccination. Parents are more willing to vaccinate their children when with adequate awareness on the effectiveness of vaccine and potential complications of varicella. “My child is very likely to contract chickenpox if he/she does not receive the vaccine” may be associated with the perceived chance of children being infected. Parents were more likely to allow vaccination of their children if they perceive that their children are otherwise at risk. “The chickenpox vaccine is worthwhile even if it does not provide lifelong immunity to chickenpox for my child” was also associated with parental knowledge. When promoting varicella vaccination, clarifying the period of vaccine effectiveness is important. Although children cannot be immunized throughout their lives, long-term health benefits still encourage vaccination. Surprisingly, “I am uncomfortable with the number of shots my child is receiving” positively correlated with vaccination. Greater awareness on the risk of infection and more knowledge on vaccination may have outweighed the discomfort parents feel while their children are being vaccinated. This explanation, however, should be justified in future studies.
Despite its effectiveness in preventing varicella in the United States,14 the varicella vaccine has not been included in the immunization program of many countries, including Japan and mainland China.44,45 Furthermore, our survey was completed only before varicella vaccination was added to Hong Kong's CIP. This immunization program is funded by the Hong Kong government and includes vaccines that are free of cost. The government announced in December 2012 the possible addition of the varicella vaccine to the CIP, which was then launched to fruition in July 2014.29 The present study indicated that 94.5% of the respondents would allow their children to be vaccinated if the vaccine was included in the CIP. Furthermore, high vaccination rates (95%) have been reported for all other vaccines included in CIP.50 Hence, high varicella vaccination rates are anticipated in the near future.
The strength of this study lies in the large sample of >2700 participants recruited from 14 kindergartens and 5 primary schools randomly selected across Hong Kong. However, an important limitation of this study is the potential for recall bias; the vaccination status of the children reported by parents was not validated by official records. Another limitation is that the number of children without varicella infection is likely less than the figure reported because of possible previous unrecognized infection. Furthermore, the total of 19 schools recruited only accounted for a small proportion of all the schools in Hong Kong, which might limit the generalizability despite the large sample size. We did not acquire the status of other vaccinations already included in the CIP. Children who received more vaccines are highly likely to receive varicella vaccination. This suggestion could explain the greater likelihood of varicella vaccination among parents who felt uncomfortable for their children receiving more shots of vaccines, as revealed in present study. This hypothesis can be examined in future studies. Despite the aforementioned limitations, this survey has provided valuable information on the association between parental attitudes and varicella vaccination in the period before the inclusion of the vaccine to Hong Kong's CIP. Our study suggested that to increase vaccination rates, government officials should organize more promotional activities to raise parents’ awareness on the inclusion of the vaccine in the CIP as a free optional vaccine.
Once varicella vaccine is implemented as part of Hong Kong's CIP for a period of time and anticipated high vaccination rates are achieved, conducting a follow-up study would be valuable to demonstrate the effect of the policy change on parental attitudes and practices regarding varicella vaccination. If possible, evidence in terms of morbidity and complications could be gathered by reviewing hospital admission data to shed greater light on the general impact of the CIP inclusion. In countries with universal immunization programs that still exclude the varicella vaccine, parents should be informed of the risks of varicella infection and its potential complications. These countries do not include United States, Australia, Italy, Germany, and Taiwan, which have already been providing universal varicella vaccination. We hope to witness a future increase in the number of countries that would follow the WHO recommendation on including the vaccine in their routine immunization programs.
Among parents of Hong Kong preschool and schoolchildren, parental attitudes and demographic characteristics were associated with rates of varicella vaccination. As emphasized in the study, health professionals and the government play important roles in promoting varicella vaccination to the public.
Acknowledgments
The authors would like to thank the schools, families, and students for their cooperation and participation in their study. They also extend their gratitude to Professor Hesham Rashwan and Dr. Taylor for allowing the use of the questionnaires.
Footnotes
Abbreviations: CI = confidence interval, CIP = Childhood Immunization Program, OR = odds ratio, WHO = World Health Organization.
WWST, Part of the work was conducted when the author worked in the JC School of Public Health and Primary Care, the Chinese University of Hong Kong.
The study was supported by the Health and Medical Research Fund (Ref No: 12110552). The funding source held no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
The authors have no conflicts of interest to disclose regarding the present study.
REFERENCES
- 1.Heininger U, Seward JF. Varicella. Lancet 2006; 368:1365–1376. [DOI] [PubMed] [Google Scholar]
- 2.Boelle PY, Hanslik T. Varicella in non-immune persons: incidence, hospitalization and mortality rates. Epidemiol Infect 2002; 129:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchetto S, de Benedictis FM, de Martino M, et al. Epidemiology of hospital admissions for chickenpox in children: an Italian multicentre study in the pre-vaccine era. Acta paediatrica 2007; 96:1490–1493. [DOI] [PubMed] [Google Scholar]
- 4.Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56:1–40. [PubMed] [Google Scholar]
- 5.Galil K, Brown C, Lin F, et al. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J 2002; 21:931–935. [DOI] [PubMed] [Google Scholar]
- 6.Lin F, Hadler JL. Epidemiology of primary varicella and herpes zoster hospitalizations: the pre-varicella vaccine era. J Infect Dis 2000; 181:1897–1905. [DOI] [PubMed] [Google Scholar]
- 7.Carapetis JR, Russell DM, Curtis N. The burden and cost of hospitalised varicella and zoster in Australian children. Vaccine 2004; 23:755–761. [DOI] [PubMed] [Google Scholar]
- 8.Dubos F, Grandbastien B, Hue V. Hospital Network for Evaluating Management of Common Childhood D, Martinot A. Epidemiology of hospital admissions for paediatric varicella infections: a one-year prospective survey in the pre-vaccine era. Epidemiol Infect 2007; 135:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandelcwajg A, Quinet B, Castello B, et al. [Causes of hospitalization of patients with ongoing varicella in a French children hospital: evolution between 1990 and 2001]. Arch Pediatr 2006; 13:429–435. [DOI] [PubMed] [Google Scholar]
- 10.Banz K, Wagenpfeil S, Neiss A, et al. The cost-effectiveness of routine childhood varicella vaccination in Germany. Vaccine 2003; 21:1256–1267. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics 2007; 120:221. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Varicella vaccines WHO position paper. Wkly Epidemiol Rec 1998; 73:241–248. [PubMed] [Google Scholar]
- 13.Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics 2011; 128:214–220. [DOI] [PubMed] [Google Scholar]
- 14.Baxter R, Tran TN, Ray P, et al. Impact of vaccination on the epidemiology of varicella: 1995-2009. Pediatrics 2014; 134:24–30. [DOI] [PubMed] [Google Scholar]
- 15.Marshall HS, McIntyre P, Richmond P, et al. Changes in patterns of hospitalized children with varicella and of associated varicella genotypes after introduction of varicella vaccine in Australia. Pediatr Infect Dis J 2013; 32:530–537. [DOI] [PubMed] [Google Scholar]
- 16.Giammanco G, Ciriminna S, Barberi I, et al. Universal varicella vaccination in the Sicilian paediatric population: rapid uptake of the vaccination programme and morbidity trends over five years. Euro Surveill 2009. 14. [PubMed] [Google Scholar]
- 17.Spackova M, Muehlen M, Siedler A. Complications of varicella after implementation of routine childhood varicella vaccination in Germany. Pediatr Infect Dis J 2010; 29:884–886. [DOI] [PubMed] [Google Scholar]
- 18.Tseng HF, Tan HF, Chang CK. Use of National Health Insurance database to evaluate the impact of public varicella vaccination program on burden of varicella in Taiwan. Vaccine 2006; 24:5341–5348. [DOI] [PubMed] [Google Scholar]
- 19.Baxter R, Ray P, Tran TN, et al. Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics 2013; 131:e1389–e1396. [DOI] [PubMed] [Google Scholar]
- 20.Jean-Jasmin LM, Lynette SP, Stefan M, et al. Economic burden of varicella in Singapore-a cost benefit estimate of implementation of a routine varicella vaccination. Southeast Asian J Trop Med Public Health 2004; 35:693–696. [PubMed] [Google Scholar]
- 21.Hsu HC, Lin RS, Tung TH, et al. Cost-benefit analysis of routine childhood vaccination against chickenpox in Taiwan: decision from different perspectives. Vaccine 2003; 21:3982–3987. [DOI] [PubMed] [Google Scholar]
- 22.Tseng HF, Tan HF, Chang CK. Varicella epidemiology and cost-effectiveness analysis of universal varicella vaccination program in Taiwan. Southeast Asian J Trop Med Public Health 2005; 36:1450–1458. [PubMed] [Google Scholar]
- 23.Fairley CK, Miller E. Varicella-zoster virus epidemiology-a changing scene? J Infect Dis 1996; 174 Suppl 3:S314–S319. [DOI] [PubMed] [Google Scholar]
- 24.Gershon AA, Takahashi M, CJ W. Plotkin SA, WA O. Varicella vaccine. Vaccines. Philadephia: Saunders; 1999. 475–507. [Google Scholar]
- 25.Yawn BP, Yawn RA, Lydick E. Community impact of childhood varicella infections. J Pediatr 1997; 130:759–765. [DOI] [PubMed] [Google Scholar]
- 26.Lokeshwar MR, Agrawal A, Subbarao SD, et al. Age related seroprevalence of antibodies to varicella in India. Indian Pediatr 2000; 37:714–719. [PubMed] [Google Scholar]
- 27.Chan YC, Kwan YW, Chow CB, CW L. Hospitalisations for varicella among children and adolescents in a tertiary referral hospital in Hong Kong, 2004 to 2008. Hong Kong J Paediatr 2010; 15:116–125. [Google Scholar]
- 28.Chan JY, Tian L, Kwan Y, et al. Hospitalizations for varicella in children and adolescents in a referral hospital in Hong Kong, 2004 to 2008: a time series study. BMC Public Health 2011; 11:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Department.of.Health. Chickenpox vaccine to incorporate into DH Childhood Immunisation Programme on July 2 2014 (Press release, 24 June 2014). In: Family Health Services Department of Health, HKSAR Government., ed2014. [Google Scholar]
- 30.Desmond C, CS K, WSC K, Allen C, et al. Immunisation coverage among hildren aged two to five: findings of the 2009 immunisation survey. Public Health Epidemiolo Bull 2010; 19:11. [Google Scholar]
- 31.Chan JY, Leung KM, Tam WW, et al. Varicella vaccine uptake and associated factors in children in Hong Kong. Epidemiol Infect 2014; 142:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez AS, Cardemil C, Pabst LJ, et al. Two-dose varicella vaccination coverage among children aged 7 years-six sentinel sites, United States, 2006-2012. MMWR 2014; 63:174–177. [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor JA, Newman RD. Parental attitudes toward varicella vaccination. The Puget Sound Pediatric Research Network. Arch Pediatr Adolesc Med 2000; 154:302–306. [DOI] [PubMed] [Google Scholar]
- 34.Gundogdu Z, Gundogdu O. Parental attitudes and varicella vaccine in Kocaeli. Turkey Prev Med 2011; 52:278–280. [DOI] [PubMed] [Google Scholar]
- 35.Vyse AJ, Gay NJ, Hesketh LM, et al. Seroprevalence of antibody to varicella zoster virus in England and Wales in children and young adults. Epidemiol Infect 2004; 132:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabutti G, Rota MC, Guido M, et al. The epidemiology of Varicella Zoster Virus infection in Italy. BMC Public Health 2008; 8:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gidding HF, MacIntyre CR, Burgess MA, et al. The seroepidemiology and transmission dynamics of varicella in Australia. Epidemiol Infect 2003; 131:1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi WS, Noh JY, Huh JY, et al. Seroprevalence of Varicella-Zoster Virus in Korea. J Med Virol 2010; 82:2123–2126. [DOI] [PubMed] [Google Scholar]
- 39.Cengage Learning, Scheaffer R, Mendenhall W, III, Ott R, et al. Elementary survey sampling. 2011. [Google Scholar]
- 40.Hesham R, Cheong JY, Mohd Hasni J. Knowledge, Attitude and Vaccination Status of Varicella Among Students of Universiti Kebangsaan Malaysia (UKM). Med J Malaysia 2009; 64:118–123. [PubMed] [Google Scholar]
- 41.John Wiley & Sons Limited, McCulloch CE JMN. Generalized, linear, and mixed models. 2001. [Google Scholar]
- 42.SAS institute, Littell RC, Stroup WW, Milliken GA, et al. SAS for mixed models. 2006. [Google Scholar]
- 43.Pavlopoulou ID, Michail KA, Samoli E, et al. Immunization coverage and predictive factors for complete and age-appropriate vaccination among preschoolers in Athens, Greece: a cross-sectional study. BMC Public Health 2013; 13:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozaki T. Varicella vaccination in Japan: necessity of implementing a routine vaccination program. J Infect Chemother 2013; 19:188–195. [DOI] [PubMed] [Google Scholar]
- 45.Xu A, Xu Q, Fang X, et al. Varicella vaccine uptake in Shandong Province China. Hum Vaccin Immunother 2012; 8:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawai K, O’Brien MA, Conway JH, et al. BJK. Factors associated with receipt of two doses of varicella vaccine among adolescents in the United States. Pediatr Infect Dis J 2013; 32:538–542. [DOI] [PubMed] [Google Scholar]
- 47.Liao SL, Huang T, Huang YC.DDJ. Survey of the status of self-paid varicella vaccination among children one to six years of age in Taiwan. J Microbiol Immunol Infect 2007; 40:112–115. [PubMed] [Google Scholar]
- 48.Adler A, Herring E, Babilsky H, et al. Parent-dependent barriers to varicella immunization in Israel: the importance of adequate information. Acta Paediatr 2007; 96:428–431. [DOI] [PubMed] [Google Scholar]
- 49.Dannetun E, Tegnell A, Hermansson G, et al. Parents’ reported reasons for avoiding MMR vaccination. A telephone survey. Scand J Prim Health Care 2005; 23:149–153. [DOI] [PubMed] [Google Scholar]
- 50.Department of Health. Hong Kong Reference Framework for Preventive Care for Children in Primary Care Settings: Module on Immunisation. In: Primary Care Office, ed2013. [Google Scholar]


