Abstract
Rituximab causes hepatitis B virus (HBV) reactivation in HBV surface antigen (HBsAg)-seronegative patients with CD20-positive B-cell non-Hodgkin lymphoma (CD20+ NHL), especially for those seropositive to the antibody of core antigen (anti-HBc). Clinical hepatitis usually develops after reverse seroconversion of HBsAg (HBV-RS), indicated by the reappearance of HBsAg in serum. Because of the relatively high prevalence of anti-HBc seropositivity in unvaccinated HBsAg-seronegative adults in an HBV hyperendemic area, we aimed to investigate additional factors influencing the development of rituximab-associated HBV-RS.
Between January 2000 and December 2010, unvaccinated HBsAg-seronegative adults with CD20+ NHL who had received rituximab-containing therapy but not anti-HBV agents were enrolled. Patients with and without HBV-RS were compared in terms of clinical factors and treatments including the number of cycles of rituximab therapy, and transplantation. Competing risk regression was used to identify the factors associated with HBV-RS.
For the 482 patients enrolled, the serological status of anti-HBc was available in 75.9%, with a seropositivity rate of 86.6%. At the last follow-up, a total of 33 (6.85%) patients had HBV-RS, with 95.8% anti-HBc seropositive, 78.9% anti-HBs seropositive, and none anti-HCV seropositive. HBV-RS patients have received more cycles (≥6) and prolonged durations of rituximab therapy, and hematopoietic stem cell transplantation. The overall survival was not different between patients with and those without HBV-RS. At the time of HBV-RS, a total of 25 (78.1%) patients had hepatitis flare, especially when HBV-RS appeared during/after induction therapy (100%, 10 of 10). Three (9.1%) patients had fulminant hepatitis, resulting in death in 1 (3%) patient. A higher rituximab cycle intensity was associated with a higher rate of hepatitis flare at the time of HBV-RS. When death in the absence of HBV-RS was considered as the competing risk, the univariate and multivariate regression analyses showed that several factors were independently associated with the development of HBV-RS, including anti-HCV seronegativity, histological subtype of posttransplant lymphoproliferative disorders, ≥6 cycles of rituximab therapy, and succeeding hematopoietic stem cell transplantation.
The findings of our study identify additional factors influencing the development of rituximab-associated HBV-RS in HBsAg-seronegative adults with CD20+ NHL.
INTRODUCTION
The anti-CD20 monoclonal antibody rituximab is the backbone of therapy for CD20-positive B-cell non-Hodgkin lymphoma (CD20+ NHL), and causes hepatitis B virus (HBV) reactivation in HBV surface antigen (HBsAg)-seronegative patients.1–15 The incidence of HBV reactivation varied from 2% to 5% to 23.8% according to the definition based on the appearance of HBsAg (ie, reverse seroconversion of HBsAg, HBV-RS) or HBV DNA.14 In these events, hepatitis flare usually develop after HBV-RS.14 Efforts to avoid such events included identification of risk factors, close monitoring, and preemptive and even prophylactic use of anti-HBV agents.13,14,16
In terms of risk factors of rituximab-associated HBV reactivation in HBsAg-seronegative CD20+ NHL patients, the patients’ HBV serological status before rituximab therapy was especially highlighted. The anti-HBc seropositivity and anti-HBs seronegativity were associated with a significantly increased risk of HBV reactivation.9,14,16,17 However, these serological factors may not be enough, especially for HBV-hyperendemic areas where there is a high prevalence of anti-HBc seropositivity in CD20+ NHL patients.9,14–17 Although Taiwan was the first to initiate an HBV mass vaccination in 1984,18 most of the CD20+ NHL patients were unvaccinated, and >70% of them were seropositive to anti-HBc and anti-HBs at the diagnosis of lymphoma.10,13,14,17 Second, most of the reports on rituximab-associated HBV-RS usually focus on the risk during/after induction therapy,9 but did not consider those patients with rituximab therapy extended into so-called maintenance or salvage therapy, or those who further received high-dose therapy and hematopoietic stem cell transplantation (HSCT). Finally, the calculation of HBV-RS risks in previous reports did not consider the so-called competing risk—“death in the absence of HBV reactivation”—and might have overestimated the incidence.19
To reduce or resolve the hazard of rituximab-associated HBV-RS in HBV hyperendemic areas, the cost-effective approach may be to identify patients at a higher risk. In the current study, we enrolled all rituximab-treated, unvaccinated HBsAg-seronegative patients with CD20+ NHL from a single institute in Taiwan, one of the HBV-hyperendemic areas since 2000, and used HBV-RS as the indicator of HBV reactivation to identify additional risk factors of rituximab-associated HBV reactivation.
PATIENTS AND METHODS
Patients and Data Collection
Patients with histologically proven CD20+ NHL, who received rituximab alone or in combination with chemotherapy at Taipei Veterans General Hospital between January 2000 and December 2010, were retrospectively reviewed. Patients who fulfilled all of the following inclusion criteria at diagnosis were eligible for inclusion in the study: 18 years of age or older, HBV unvaccinated, seronegative to HBsAg and human immunodeficiency virus, not using prophylactic anti-HBV agents, and with ≥1 cycle(s) of rituximab administered at the last follow-up.
The collected data included demographics, histological subtypes, treatments (chemotherapy, number of cycles of rituximab, and HSCT), blood biochemistry tests including liver function tests, viral serology tests of hepatitis virus (HBsAg, antibody to HBsAg [anti-HBs], anti-HBc, and antibody to HCV [anti-HCV]), virological data (HBV DNA), and outcome. The event of HBV reactivation, determined by the conversion from being HBsAg seronegative to being seropositive, was identified, with additional data collected on liver function, viral serology, and virological tests. This study was approved by the Institutional Regulatory Board of Taipei Veterans General Hospital.
Definition of HBV Reactivation and Hepatitis Flare
Here, HBV-RS was used to represent HBV reactivation and defined as the reappearance of HBsAg in serum. Elevated serum alanine aminotransferase (ALT) level that exceeds the upper limit of normal (ULN, ie, 40 IU/L) was attributed to HBV reactivation if it was preceded or accompanied by HBV-RS and/or an HBV viral load of >2000 IU/mL, if applicable.13 Hepatitis flare was defined as a >3-fold increase of serum ALT level that exceeds 100 IU/L.13 Hepatitis was designated as severe when a hepatitis flare with an ALT increase to >10-fold the ULN or bilirubin increase to >1.5-fold ULN was observed,14 and as fulminant when hepatic encephalopathy and irregular blood coagulation (prothrombin time prolonged for >10 s) were noted.20
Biochemistry, Viral Serology, and Virological Tests
Biochemical liver function tests including ALT, aspartate aminotransferase, alkaline phosphate, gamma-glutamyl transferase, and total bilirubin (TB) were checked at baseline, at the start of every new cycle of rituximab-containing therapy, and during the follow-up period after the completion of therapy. Serological tests of HIV, HBsAg, and anti-HCV were routinely done at diagnosis, and were performed if clinically indicated at the discretion of the attending physician. Tests for serum hepatitis B e antigen (HBeAg) and hepatitis B e antibody (anti-HBe) were usually done when HBsAg was seropositive. Anti-HBs and anti-HBc are usually tested for HSCT recipients; however, they were not universally tested for patients with CD20+ NHL until the late 1990s.
Biochemical liver function tests, viral serology, and virological tests were done as previously described.13,20 The normal ranges were <40 IU/L for ALT and <1.6 mg/dL for TB. Qualitative analysis of HBsAg and anti-HBc was done by using the microparticle enzyme immunoassay. HBeAg and anti-HBe were tested by using a radioimmunoassay kit (Abbott Laboratories, North Chicago, IL). Anti-HCV was measured by using a second-generation enzyme immunoassay kit (Abbott Laboratories). Detection of HBV DNA in serum was performed with an in-house assay in the early 2000s and with a commercial kit in the late 2000s, by using a Cobas Amplicor HBV monitor (Roche Molecular Systems, Pleasanton, CA) to determine the HBV viral load (detection limit of 12 IU/mL). The data of HBV DNA from the in-house assay were appropriately converted into International Units (IU).
Lymphoma-Related Therapy
The use of rituximab-containing therapy for CD20+ NHL patients was generally based on the guidelines recommended by the National Comprehensive Cancer Network, and, in addition, its coverage by the National Health Insurance of Taiwan. For indolent CD20+ NHL, rituximab was given as salvage therapy since April 2002 and as induction and maintenance therapy of follicular lymphoma (FL) since March 2006 and February 2008, respectively. For diffuse large B-cell lymphoma (DLBCL), the addition of rituximab in induction therapy of the elderly (>60 years) began in January 2004, and then for the remaining patients since March 2006.
Rituximab is used alone or in combination with chemotherapy, with a dose of 375 mg/m2 every 3 to 4 weeks in induction and salvage therapy, and every 2 to 3 months in maintenance therapy.21 In combination with chemotherapy, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) or a CHOP-like regimen was usually given as induction therapy for patients with aggressive CD20+ NHL for 6 to 8 cycles if treatment was tolerated. Rituximab was given alone as maintenance after induction therapy for 8 to 12 cycles if treatment was tolerated, or in combination with salvage chemotherapy of relapsed or refractory diseases for 3 to 6 cycles. The regimens of salvage chemotherapy included ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin), EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin), and ICE (ifosfamide, carboplatin, and etoposide).
High-dose therapy followed by autologous HSCT was usually given after induction therapy in patients with poor prognostic factors at diagnosis, or salvage therapy for those with relapse or refractory diseases. Allogeneic HSCT was given only to patients with multiple relapses or refractory diseases. The preparative regimens of autologous HSCT were BEAM (BCNU, etoposide, cytarabine, and melphalan) and BEAC (BCNU, etoposide, cytarabine, and cyclophosphamide). The nonmyeloablative regimen containing fludarabine, busulfan, and ATG-Fresenius was used for allogeneic HSCT of CD20+ NHL, especially for indolent CD20+ NHL patients.22 The regimen for prophylaxis of acute graft-versus-host diseases included a short course of methotrexate and cyclosporine A. The dose of cyclosporine A was decreased by 5% weekly until 6 months after transplantation. Methotrexate was not used in patients receiving a nonmyeloablative regimen.22 After HSCT, rituximab and an anti-HBV agent was not routinely used to prevent posttransplant lymphoproliferative disorders (PTLD) and HBV reactivation, respectively.
The application of rituximab in maintenance and salvage therapy, and the enforcement of HSCT were determined according to the treatment response at the discretion of the attending physician. Then, modification of chemotherapy dosage and the use of component therapy or granulocyte colony-stimulating factor were performed according to the patients’ adverse events.
Statistical Analysis
The first end point was to characterize the incidence, clinical and laboratory features, and rituximab-containing treatments of patients with HBV-RS. First, all patients with and without HBV-RS were compared, and factors with a statistical difference were considered as potential risk factors of rituximab-associated HBV-RS, which will be further evaluated in the regression analysis. Second, patients with HBV-RS were further categorized into 3 groups according to the phase of rituximab therapy administered and whether they have received transplantation, and were compared. Group 1 included HBV-RS that developed during/after induction therapy, and group 2 indicated HBV-RS that did not appear until during/after salvage/maintenance therapy. Group 3 included HBV-RS that developed after transplantation, including 8 patients who had received HSCT before HBV-RS and 2 who received rituximab therapy for PTLD. The number of cycles of rituximab, chemotherapy, and HSCT administered were coded until the development of HBV-RS or the last follow-up if no HBV-RS developed. The overall survival (OS) was calculated from rituximab administration until the last follow-up. Finally, to explore the possible relationship between the dose intensity of rituximab and the severity of hepatitis following HBV-RS, the “rituximab cycle intensity” was defined here as the total number of cycles administered according to the duration of rituximab therapy (weeks). The association of rituximab cycle intensity and variable severity of hepatitis at the time of HBV-RS was analyzed. The chi-square test (or Fisher exact test when applicable), Student t-test (or 1-way ANOVA when applicable), and Mann-Whitney U-test (or Kruskal-Wallis H-test when applicable) were applied for categorical, parametrically continuous, and nonparametrically continuous variables between groups, respectively. Log-rank test was used to assess differences in OS between patients with and without HBV-RS.
The second end point was to estimate the risk factors of HBV-RS after rituximab-containing therapy. The Fine and Gray model was used to assess the impact of different variables on the cumulative incidence of HBV-RS, with death in the absence of HBV-RS considered as a competing event.23 The cumulative incidence of reactivation in the presence of competing risks was calculated. Subhazard ratios with the relative 95% confidence interval were reported for the Fine and Gray analysis, respectively. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 17.0 (Statistical Package for the Social Sciences; SSPS Inc, Chicago, IL), Stata version 11.0 (Stata Corp, College Station, TX), and XLSTAT (Addinsoft SARL, Paris, France).
RESULTS
Patient Characteristics
As shown in Table 1, 482 unvaccinated HBsAg-seronegative patients with CD20+ NHL were enrolled, with a median age of 69.3 years and 298 (61.8%) being male. The major histological subtypes included DLBCL and FL in 290 (60.2%) and 77 (16%) patients, respectively (also see in Table 2). In addition, PTLD was noted in 5 patients, including 4 and 1 who received renal transplantation and HSCT, respectively. The serological status of anti-HCV, anti-HBs, and anti-HBc at diagnosis was available in 97.5%, 79%, and 75.9% of patients, respectively, with seropositivity rates of 7%, 78%, and 86.6%, respectively. Rituximab therapy started at 4.1 weeks after the diagnosis, and consisted of a mean of 6.55 cycles during a period of 20.9 weeks. About 60% of patients had received ≥6 cycles of rituximab therapy. At the time of HBV-RS or at the last follow-up, a total of 58 (12%) patients had also received HSCT.
TABLE 1.
Characteristics of 482 Unvaccinated HBsAg-Seronegative Patients With CD20+ NHL
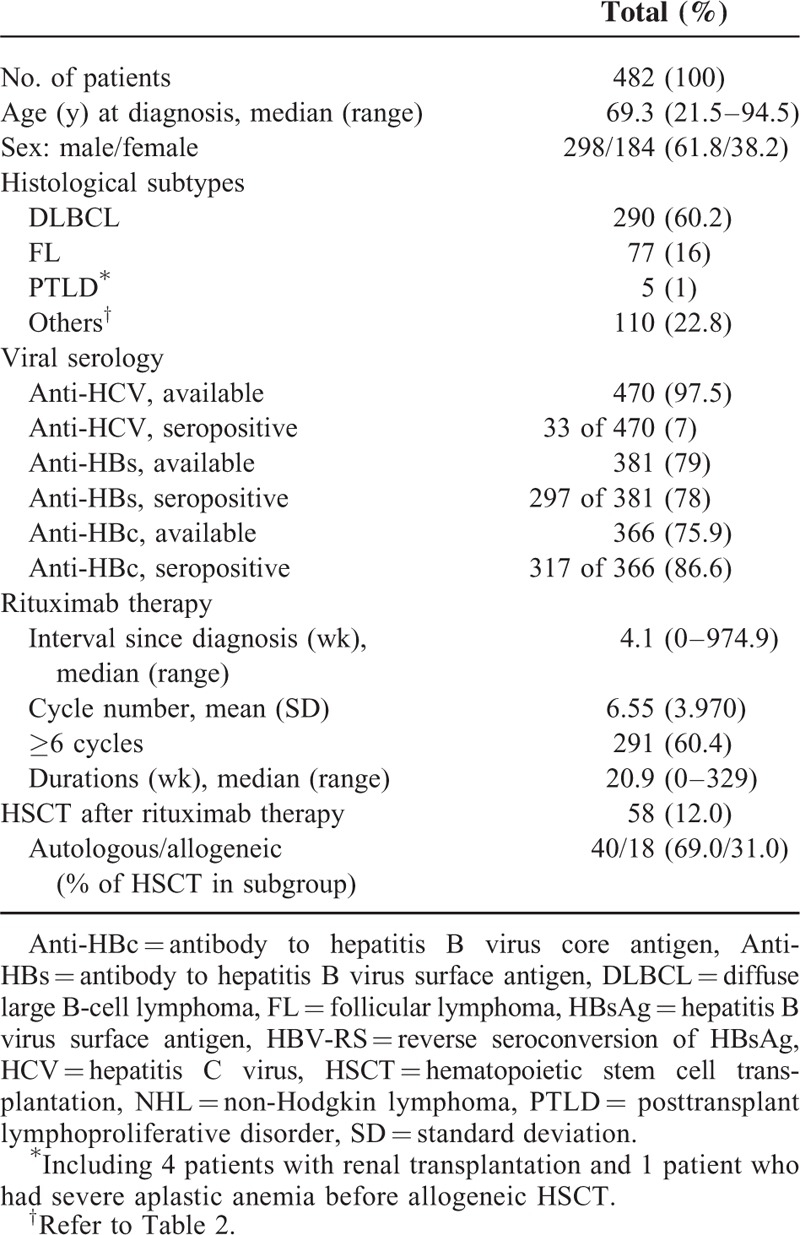
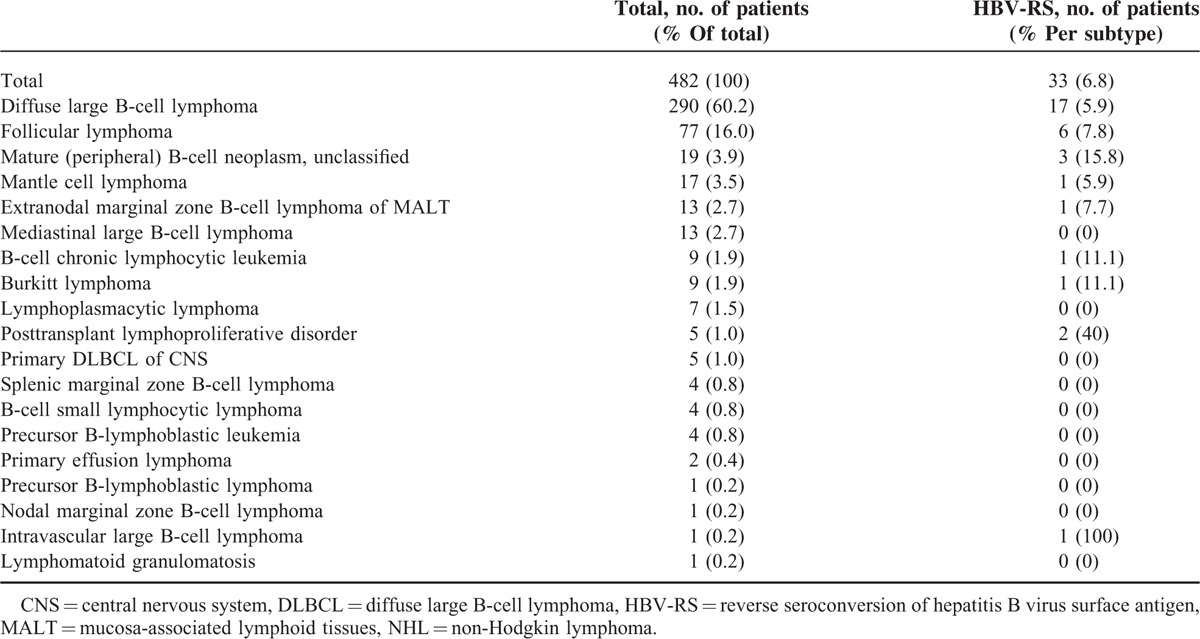
TABLE 2.
Reverse Seroconversion of Hepatitis B According to the Histological Subtypes of CD20+ NHL
Comparison of Patients With and Without HBV-RS
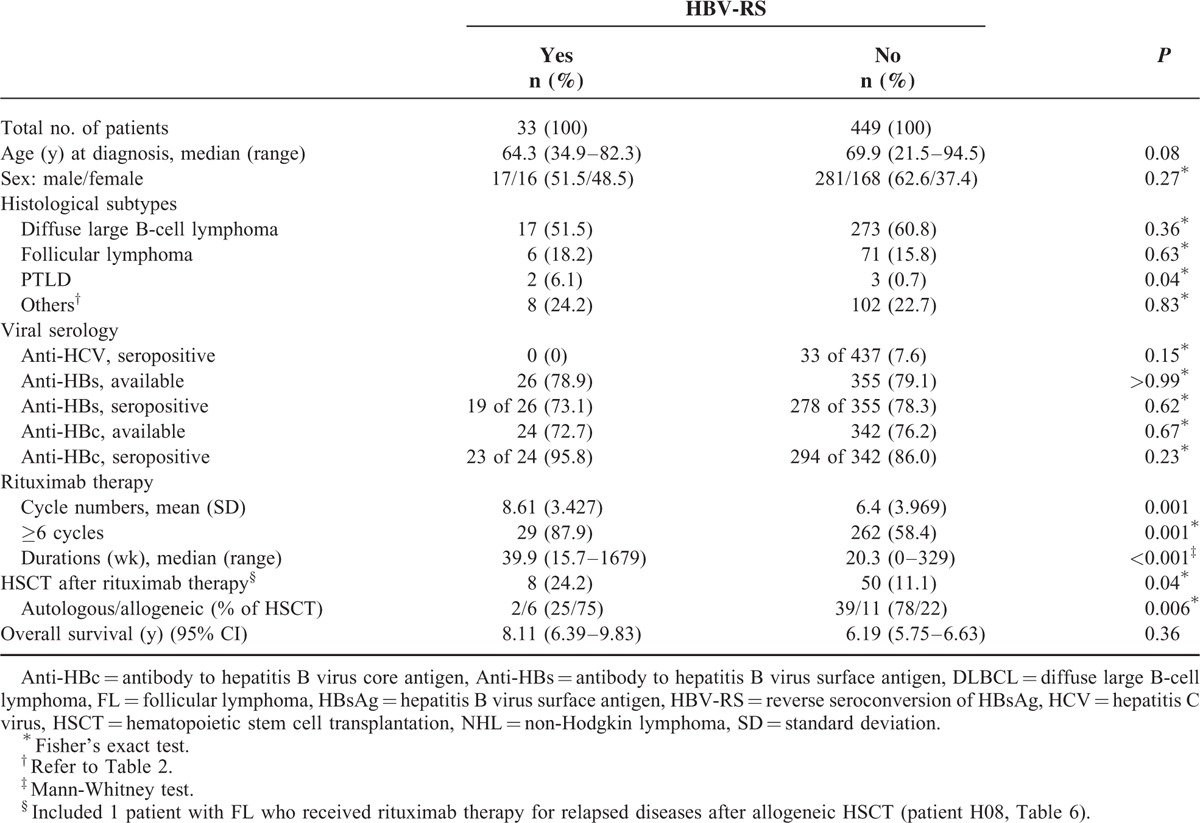
A total of 33 (6.85%) patients had HBV-RS, with 95.8% of them being anti-HBc seropositive, 78.9% anti-HBs seropositive, and none seropositive to anti-HCV (in Table 3). Compared with those without HBV-RS, patients with HBV-RS had a higher proportion of the histological subtype PTLD (also see in Table 2), received more cycles and longer durations of rituximab therapy, and more frequently had also received HSCT. There was no significant difference in OS between patients with and those without HBV-RS.
TABLE 3.
Comparison of 482 Unvaccinated HBsAg-Seronegative Patients With CD20+ NHL According to the Presence of Rituximab-Associated HBV-RS
Clinical and Laboratory Features of Patients With HBV-RS
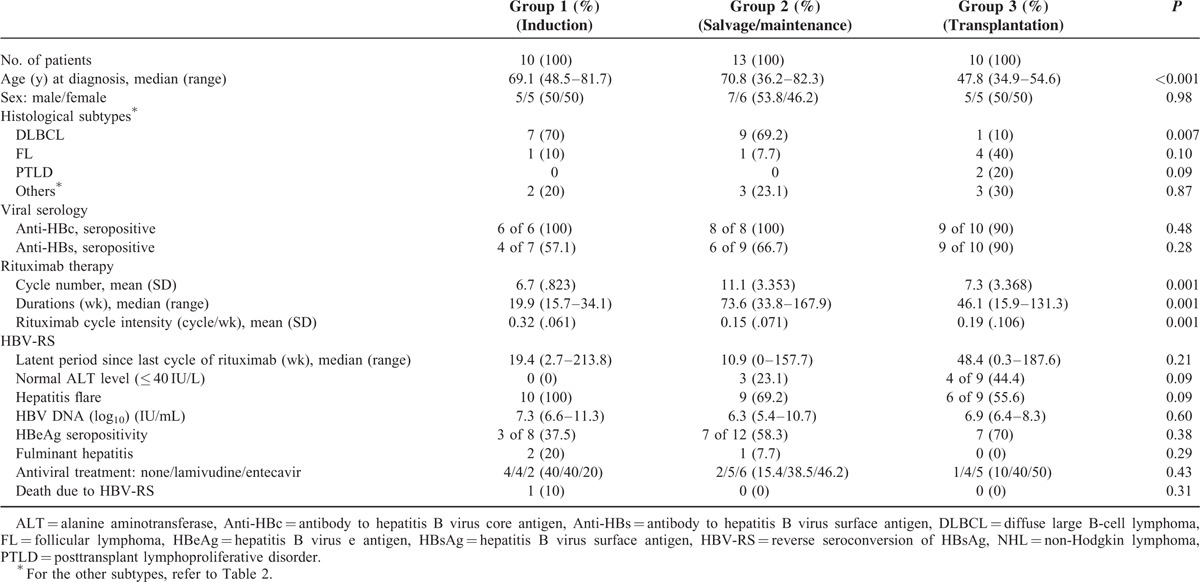
As described above, 33 patients with HBV-RS were categorized into 3 groups: group 1—HBV-RS occurred during/after induction therapy (n = 10) (in Table 4), group 2—HBV-RS occurred during/after salvage (n = 4)/maintenance (n = 9) therapy (in Table 5), and group 3—transplantation was done before HBV-RS (n = 10) (in Table 6). When the 3 groups were compared (in Table 7), the patients of group 1 had a higher proportion of the histological subtype DLBCL and a higher “rituximab cycle intensity.” Group 2 had a lower “rituximab cycle intensity” than group 1, although a higher number of rituximab cycles was administered. Group 3 consisted of 7 patients who had received rituximab therapy before HSCT, 2 with PTLD after allogeneic HSCT and renal transplantation (patients P01 and P02, in Table 6), and the 1 with FL who received rituximab therapy for relapsed diseases after allogeneic HSCT (patient H08, in Table 6). Four of these 8 non-PTLD patients (50% of 8) had only received <6 cycles of rituximab therapy before HBV-RS (in Table 6). At the time of HBV-RS, 25 (78.1%) patients had hepatitis flare, especially in group 1 (100%, 10 of 10); 3 (9.1%) patients had fulminant hepatitis, resulting in death in 1 (3%) patient (in Table 7).
TABLE 4.
Characteristics of HBV-RS During/After Rituximab-Containing Induction Therapy of 10 Patients With CD20+ NHL (Group 1)
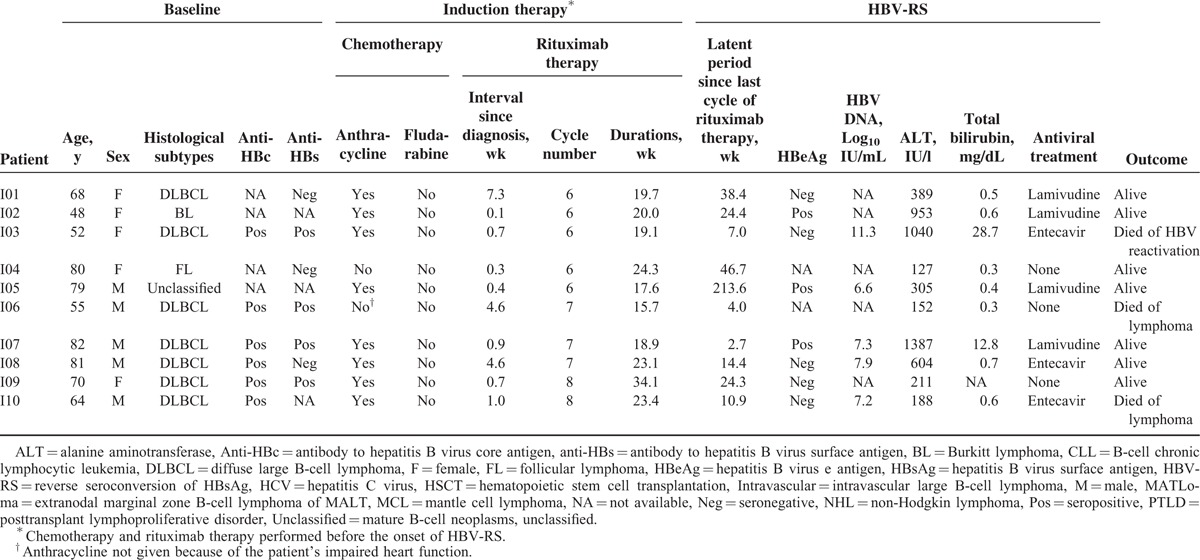
TABLE 5.
Characteristics of HBV-RS During/After Rituximab-Containing Salvage or Maintenance Therapy of 13 Patients With CD20+ NHL (Group 2)
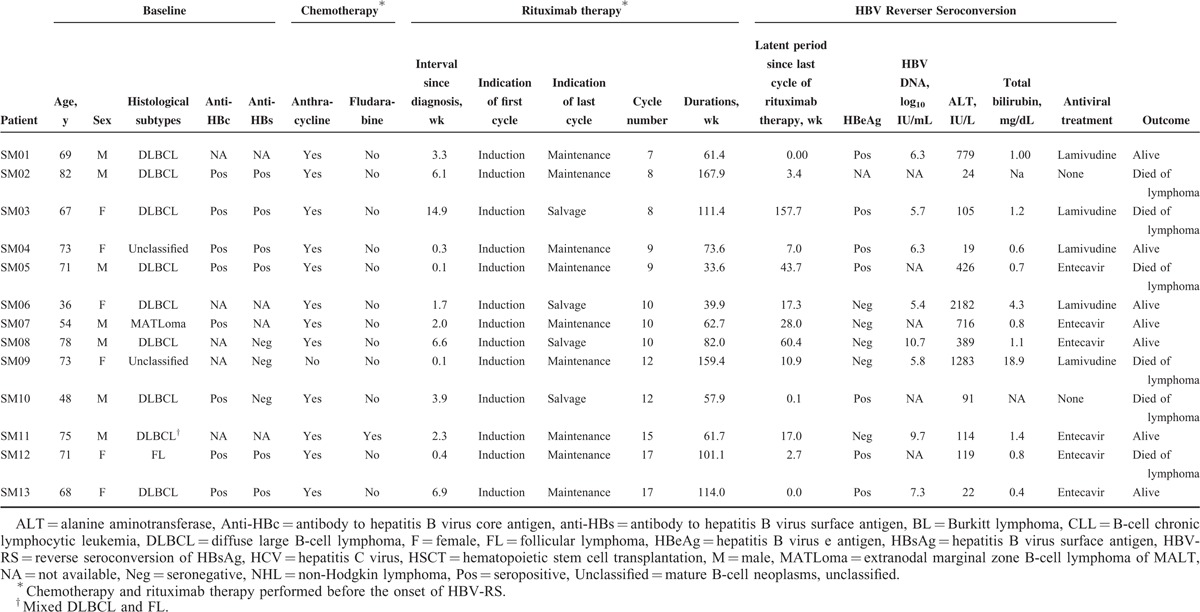
TABLE 6.
Characteristics of HBV-RS After Rituximab-Containing Therapy and Transplantation of 10 Patients With CD20+ NHL (Group 3)
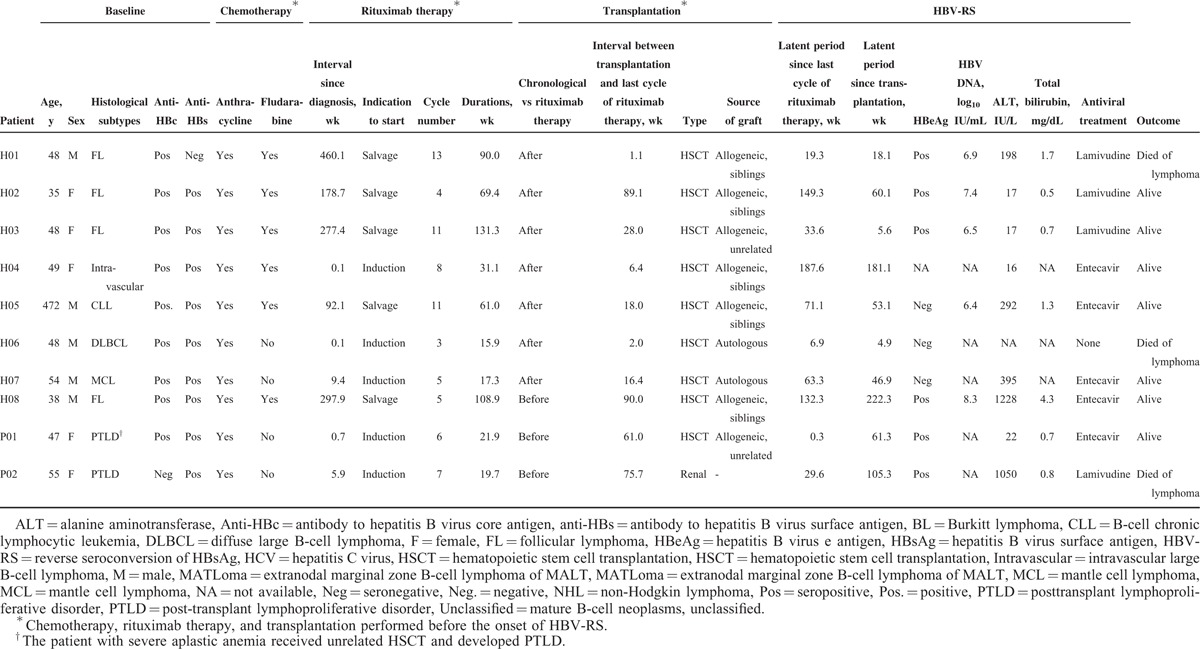
TABLE 7.
HBV-RS in 33 Unvaccinated HBsAg-Seronegative Patients With CD20+ NHL According to the Phase of Rituximab Therapy
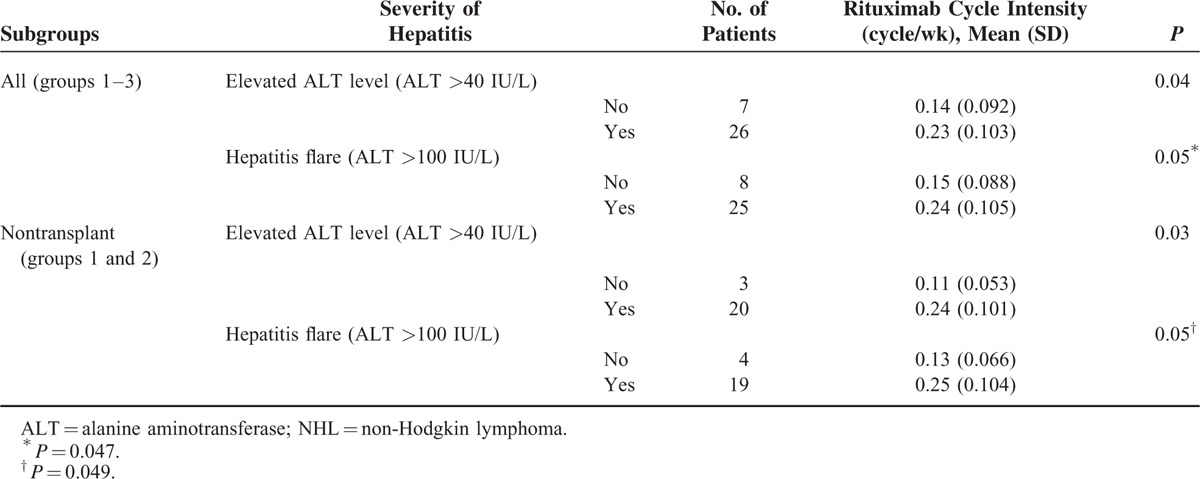
The association of the rituximab cycle intensity and the severity of hepatitis at the time of HBV-RS was assessed. As shown in in Table 8, a higher rituximab cycle intensity was associated with a higher frequency of “elevated ALT level” or “hepatitis flare” at the time of HBV-RS in all patients with HBV-RS, or those without transplantation (ie, group 1 and group 2).
TABLE 8.
Correlation of Rituximab Cycle Intensity and Hepatitis Flare in 33 CD20+ NHL With Reverse Seroconversion of Hepatitis B Virus Surface Antigen
Factors Associated With HBV-RS
When death in the absence of HBV-RS was considered as the competing risk, the cumulative incidence of HBV-RS estimated was 0.9%, 2%, and 4.8% at 6, 12, and 24 months after the initiation of rituximab therapy, respectively (Figure 1). The univariate and multivariate regression analyses showed that several factors independently influenced the development of HBV-RS (in Table 9), including anti-HCV seropositivity (Figure 2A), the PTLD histological subtype (Figure 2B), administration of ≥6 cycles of rituximab therapy (Figure 2C), and HSCT after rituximab therapy (Figure 2D).
FIGURE 1.
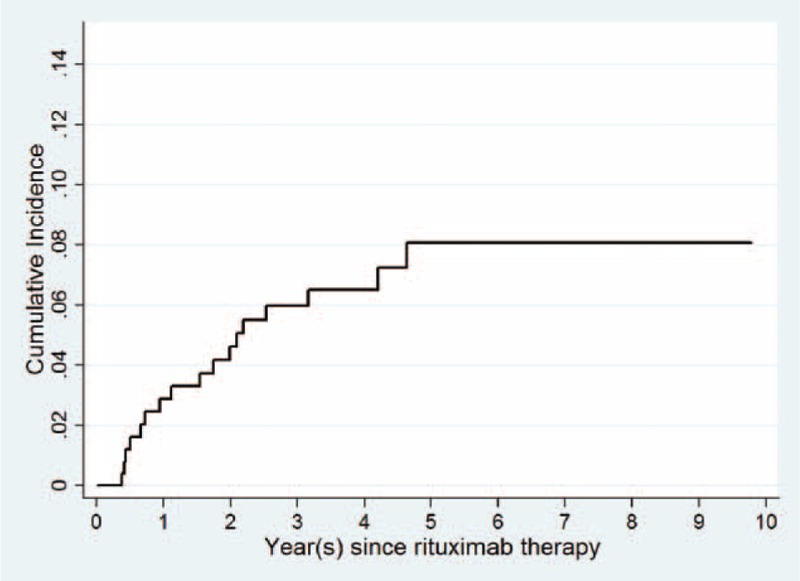
Cumulative incidence of rituximab-associated reverse seroconversion of HBsAg in 482 unvaccinated patients with CD20+ NHL, with death in the absence of reverse seroconversion of HBsAg as the competing risk.
TABLE 9.
Competing Risk Regression for Factors Influencing Rituximab-Associated HBV-RS, With the Outcome “Death in the Absence of HBV-RS” as the Competing Risk
FIGURE 2.
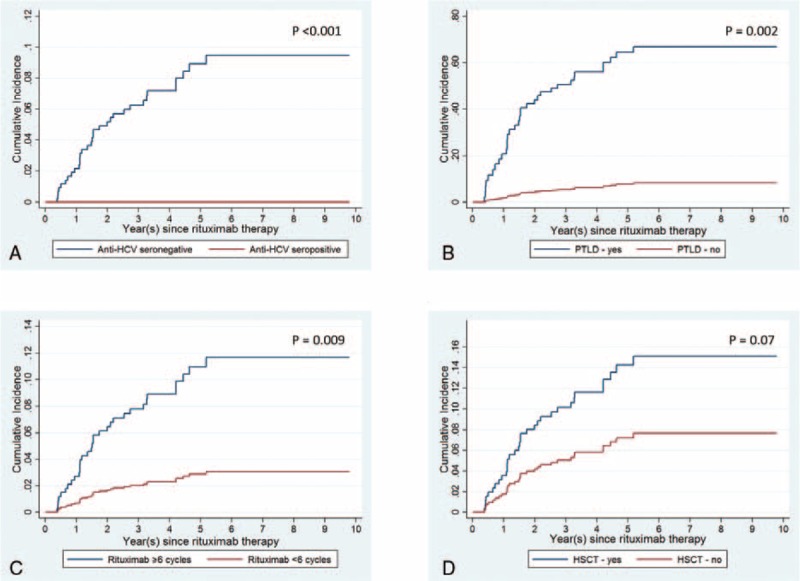
Cumulative incidence of rituximab-associated reverse seroconversion of HBsAg in 482 unvaccinated patients with CD20+ NHL, with death in the absence of reverse seroconversion of HBsAg as the competing risk, according to (A) anti-HCV seropositivity, (B) PTLD histological subtype, (C) rituximab therapy ≥6 cycles, and (D) additional hematopoietic stem cell transplantation (HSCT) after rituximab therapy.
DISCUSSION
The study identified additional factors influencing the development and severity of rituximab-associated HBV reactivation, which would provide clues for a cost-effective approach in reducing or preventing the resulting morbidity and mortality, especially for unvaccinated patients with CD20+ NHL. The impact of these factors can be elucidated according to the clinical course of rituximab-treated CD20+ NHL patients. At diagnosis, a preexisting HCV infection in CD20+ NHL patients would prevent the development of rituximab-associated HBV-RS (in Tables 3 and 9, Figure 2A). In a Japanese study of HCV-infected DLBCL patients treated with rituximab-containing chemotherapy, Ennishi et al24 showed that HCV infection was associated with severe hepatic toxicity; however, at the time of hepatic dysfunction, none of HBV-DNA and HBsAg was seropositive, and in all patients, severe hepatic toxicity improved with no anti-HBV treatment. Similarly, in our CD20+ NHL patients with HCV infection, hepatitis flare was common during rituximab-containing therapy; however, additional serological tests of HBV infection did not show HBV-RS. It was reported that HBV replication was suppressed in patients with HBV and HCV coinfections.25,26 Kao et al27 suggested that occult HBV infection does not have clinical significance in chronic hepatitis C patients residing in areas where HBV infection is endemic. On the contrary, patients with CD20+ PTLD might have the highest risk of rituximab-associated HBV-RS (Tables 2, 3 and 9, and Figure 2B), as seen in our 2 (40% of 5) patients (patients P01 and P02, Table 6). Similarly, Mikulska et al28 recently reported that rituximab therapy of PTLD after allogeneic HSCT caused HBV reactivation in anti-HBc seropositive patients. It is possible that preexisting immunosuppression before rituximab therapy might further increase the risk. Similarly, there was 1 patient with FL who received rituximab therapy for relapsed diseases after allogeneic HSCT, and finally developed HBV-RS (patient H08, Table 6). Because of a relatively small number of PTLD patients in our study, this finding deserves more patients to validate in the future.
Second, during rituximab-containing therapy, the effect of the number of cycles on the development of rituximab-associated HBV-RS was highlighted, especially whenever rituximab was administered at ≥6 cycles, and was continuously given as maintenance and/or salvage therapy. In a recent report on DLBCL patients,15 a higher number (>8) of rituximab cycles was shown to be associated with an increased risk of HBV reactivation, as defined according to HBV-RS and/or HBV DNA elevation. The number of cycles of rituximab therapy required to induce HBV-RS may vary according to the difference in histologic subtypes, presence and intensity of concomitant chemotherapy, and statistical method used. Except for the case reported by Koo et al,29 the risk of HBV-RS during the period of rituximab maintenance therapy was not understood, and an additional 9 cases are shown here (Table 5). In contrast, the severity of hepatitis flare at the time of HBV-RS was possibly related to the “rituximab cycle density” rather than the number of cycles of rituximab therapy (Tables 7 and 8). Especially notably, HBV-RS during/after rituximab-containing induction therapy (group 1) tended to have a higher severity of hepatitis flare (Table 7). Although an increased cycle number of rituximab would theoretically lead to a more prolonged B-cell depletion, the concept of “rituximab cycle intensity” might provide a more reasonable answer. A higher “rituximab cycle intensity” in induction therapy may cause a more rapid and profound B-cell depletion and resulting reduction of anti-HBs, thus rapidly depleting its protective effect on HBV-RS. In contrast, for patients who did not develop HBV-RS during/after rituximab-containing induction therapy, they would have a relatively slow and lower degree of B-cell depletion whenever rituximab is given as maintenance, or would have a period of remission or stable disease to allow at least partial recovery of B cells before rituximab-containing salvage therapy is given for the relapse.
Third, similar to the impact of PTLD, a succeeding HSCT would further precipitate the risk of HBV-RS in rituximab-treated CD20+ NHL patients, although <6 cycles of rituximab therapy were administered. Because HBsAg-seronegative/anti-HBc-seropositive patients with other hematological malignancies (other than CD20+ NHL) were shown to have a high risk of HBV-RS after allogeneic HSCT,30 the development of HBV-RS after allogeneic HSCT in our patients with CD20+ NHL might be simply caused by the immunosuppression after allogeneic HSCT itself, rather than by rituximab therapy. However, the findings—2 patients with autologous HSCT (patients H06 and H07, Table 6) and 4 patients with rituximab therapy of <6 cycles—might provide physicians with additional cautionary examples of the occurrence of HBV-RS after HSCT.
This study has several limitations. First, because of a relatively high prevalence of anti-HBc seropositivity and a low incidence of rituximab-associated HBV-RS, we enrolled all rituximab-treated CD20+ NHL patients who were unvaccinated, including those with unknown serological status of anti-HBc, to have a larger number of patients for analysis. This approach might limit our examination of the impact of anti-HBc seropositivity on rituximab-associated HBV-RS; however, 23 of our 24 HBV-RS patients with known anti-HBc status were seropositive. The only anti-HBc-seronegative patient had PTLD after renal transplantation and developed HBV-RS after rituximab therapy. Several patients with seronegative anti-HBc developed HBV-RS after a different anticancer therapy,31,32 and the possible causes included a lower titer of anti-HBc and core gene mutation in occult HBV. Second, similar to the findings of our previous reports,13,15 we did not demonstrate an increased risk of anti-HBs seronegativity in this study, although several recent studies showed such an increase.9,14,16,17 Third, other factors including the International Prognostic Index and chemotherapeutic agents used were not analyzed in the present study because of heterogeneities of histological subtypes and treatment modalities in our patients. At present, there is no evidence to support the relation of these factors with rituximab-associated HBV-RS. Finally, the retrospective design of this study might underestimate the incidence of HBV-RS.
In conclusion, we analyzed a large number of unvaccinated HBsAg-seronegative adults with CD20+ NHL in Taiwan—an HBV hyperendemic area—and highlighted several additional factors influencing the development of rituximab-associated HBV-RS, including preexisting HCV infection, the histological type PTLD, the number of rituximab cycles, and succeeding HSCT. In addition, HBV-RS after rituximab-containing induction therapy tended to have a higher severity of hepatitis, and the cycle number-dependent risk should be considered with caution when rituximab is continuously given as maintenance and/or salvage therapy.
Acknowledgments
The authors thank research assistants Hsiao-Ling Liu and Wen-Chun Chang for their help with experimental analysis.
Footnotes
Abbreviations: ALT = alanine aminotransferase, anti-HBc = antibody to core antigen, anti-HBs = antibody to HBsAg, CD20+ NHL = CD20-positive B-cell non-Hodgkin lymphoma, DLBCL = diffuse large B-cell lymphoma, FL = follicular lymphoma, HBsAg = HBV surface antigen, HBV = hepatitis B virus, HBV-RS = reverse seroconversion of hepatitis B virus surface antigen, HCV = hepatitis C virus, HR = hazard ratio, HSCT = hematopoietic stem cell transplantation, PTLD = posttransplant lymphoproliferative disorders, TB = total bilirubin, ULN = upper limit of normal.
This work was supported by grants from the Taiwan Clinical Oncology Research Foundation, Taipei Veterans General Hospital (V99C1–191, V100C-111, and V103E2–002 to L-T), National Science Council (NSC 99-2314-B-075-017-MY2, 101-2325-B-075-008, 102-2325-B-075-005 to L-T), and Ministry of Science and Technology (MOST 103-2325-B-075-004 to L-T).
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med 2001; 344:68–69. [DOI] [PubMed] [Google Scholar]
- 2.Ng HJ, Lim LC. Fulminant hepatitis B virus reactivation with concomitant listeriosis after fludarabine and rituximab therapy: case report. Ann Hematol 2001; 80:549–552. [DOI] [PubMed] [Google Scholar]
- 3.Hamaki T, Kami M, Kusumi E, et al. Prophylaxis of hepatitis B reactivation using lamivudine in a patient receiving rituximab. Am J Hematol 2001; 68:292–294. [DOI] [PubMed] [Google Scholar]
- 4.Skrabs C, Muller C, Agis H, et al. Treatment of HBV-carrying lymphoma patients with Rituximab and CHOP: a diagnostic and therapeutic challenge. Leukemia 2002; 16:1884–1886. [DOI] [PubMed] [Google Scholar]
- 5.Westhoff TH, Jochimsen F, Schmittel A, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 2003; 102:1930. [DOI] [PubMed] [Google Scholar]
- 6.Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother 2005; 11:189–191. [DOI] [PubMed] [Google Scholar]
- 7.Hui CK, Cheung WW, Zhang HY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006; 131:59–68. [DOI] [PubMed] [Google Scholar]
- 8.Khaled Y, Hanbali A. Hepatitis B virus reactivation in a case of Waldenstrom's macroglobulinemia treated with chemotherapy and rituximab despite adefovir prophylaxis. Am J Hematol 2007; 82:688. [DOI] [PubMed] [Google Scholar]
- 9.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009; 27:605–611. [DOI] [PubMed] [Google Scholar]
- 10.Pei SN, Chen CH, Lee CM, et al. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol 2010; 89:255–262. [DOI] [PubMed] [Google Scholar]
- 11.Evens AM, Jovanovic BD, Su YC, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol 2011; 22:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung C, Tsoi E, Burns G, et al. An argument for the universal prophylaxis of hepatitis B infection in patients receiving rituximab: a 7-year institutional experience of hepatitis screening. Oncologist 2011; 16:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013; 31:2765–2772. [DOI] [PubMed] [Google Scholar]
- 14.Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology 2014; 59:2092–2100. [DOI] [PubMed] [Google Scholar]
- 15.Wu CY, Hsiao LT, Chiou TJ, et al. Lymphocyte/monocyte ratio and cycles of rituximab-containing therapy are risk factors for hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma and resolved hepatitis B. Leuk Lymphoma 2015; 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Seto WK, Chan TS, Hwang YY, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol 2014; 32:3736–3743. [DOI] [PubMed] [Google Scholar]
- 17.Pei SN, Ma MC, Wang MC, et al. Analysis of hepatitis B surface antibody titers in B cell lymphoma patients after rituximab therapy. Ann Hematol 2012; 91:1007–1012. [DOI] [PubMed] [Google Scholar]
- 18.Chen DS, Hsu NH, Sung JL, et al. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. JAMA 1987; 257:2597–2603. [PubMed] [Google Scholar]
- 19.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 2007; 13 (2 Pt 1):559–565. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao LT, Chiou TJ, Liu JH, et al. Extended lamivudine therapy against hepatitis B virus infection in hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant 2006; 12:84–94. [DOI] [PubMed] [Google Scholar]
- 21.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346:235–242. [DOI] [PubMed] [Google Scholar]
- 22.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91:756–763. [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 24.Ennishi D, Maeda Y, Niitsu N, et al. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood 2010; 116:5119–5125. [DOI] [PubMed] [Google Scholar]
- 25.Fong TL, Di Bisceglie AM, Waggoner JG, et al. The significance of antibody to hepatitis C virus in patients with chronic hepatitis B. Hepatology 1991; 14:64–67. [DOI] [PubMed] [Google Scholar]
- 26.Shih CM, Lo SJ, Miyamura T, et al. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol 1993; 67:5823–5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao JH, Chen PJ, Lai MY, et al. Sequence analysis of pre-S/surface and pre-core/core promoter genes of hepatitis B virus in chronic hepatitis C patients with occult HBV infection. J Med Virol 2002; 68:216–220. [DOI] [PubMed] [Google Scholar]
- 28.Mikulska M, Nicolini L, Signori A, et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: risk factors and outcome. Clin Microbiol Infect 2014; 20:O694–O701. [DOI] [PubMed] [Google Scholar]
- 29.Koo YX, Tan DS, Tan IB, et al. Hepatitis B virus reactivation in a patient with resolved hepatitis B virus infection receiving maintenance rituximab for malignant B-cell lymphoma. Ann Intern Med 2009; 150:655–656. [DOI] [PubMed] [Google Scholar]
- 30.Dhedin N, Douvin C, Kuentz M, et al. Reverse seroconversion of hepatitis B after allogeneic bone marrow transplantation: a retrospective study of 37 patients with pretransplant anti-HBs and anti-HBc. Transplantation 1998; 66:616–619. [DOI] [PubMed] [Google Scholar]
- 31.Awerkiew S, Daumer M, Reiser M, et al. Reactivation of an occult hepatitis B virus escape mutant in an anti-HBs positive, anti-HBc negative lymphoma patient. J Clin Virol 2007; 38:83–86. [DOI] [PubMed] [Google Scholar]
- 32.Feeney SA, McCaughey C, Watt AP, et al. Reactivation of occult hepatitis B virus infection following cytotoxic lymphoma therapy in an anti-HBc negative patient. J Med Virol 2013; 85:597–601. [DOI] [PubMed] [Google Scholar]


