Abstract
Takotsubo cardiomyopathy (TTC) causes sudden cardiac death and has garnered increased attention worldwide in recent years. However, few studies have clearly classified the risk factors for this disease, including gender, race and morbidity, as well as the physical and mental stressors that can exacerbate the disease, particularly in young patients. To better analyze the characteristics of young TTC patients, we performed a systematic review of reported cases involving young patients.
A computer-assisted search was performed using prominent electronic medical information sources to identify literature published between January 1965 and December 2013. Relevant studies containing clinical data of young TTC patients were included.
Ninety-six records that included information about 104 cases were ultimately selected for our review. Several of the following results were noted: First, physical stress was more likely to exacerbate TTC than was mental stress in young patients. Second, more female than male TTC patients were noted among both young patients and the general population. Third, ethnicity appears to play no role in the disease, as no significant differences were noted among individuals of different races with respect to clinical characteristics, morbidity or stressors. Fourth, the clinical manifestations of TTC were similar to those of other cardiac diseases, including coronary heart disease. However, TTC may be detected using the combination of echocardiography and ventriculography.
Clinicians should consider TTC if young patients present with symptoms similar to those of coronary heart disease so that harmful treatments such as coronary artery stent placement may be avoided. Moreover, the answers to questions regarding the clinical diagnostic criteria, etiology, pathophysiology, and the management of this syndrome in youth remain unclear; therefore, further research is needed.
INTRODUCTION
Among the 12 million people who die from cardiovascular disease worldwide each year, half die of sudden cardiac death (SCD). Thus, SCD is one of the primary emergent events that leads to cardiovascular death. Acute coronary syndrome (ACS) is one of the primary causes of SCD.1 Meta analyses indicate that among the 1% to 2% of all admissions that occur due to ACS, left ventricular dysfunction, electrocardiographic changes, and myocardial enzyme elevations suggestive of an acute myocardial infarction (AMI), a reversible trend was noted, and subsequent coronary angiography was normal.2 For these patients, the use of ventriculography revealed an apical ballooning appearance and a hypercontractile base of the heart. This disease is called Takotsubo cardiomyopathy (TTC). However, due to the limited knowledge regarding this disease and the inability of clinicians to identify it, many cases of TTC are misdiagnosed; therefore, its true prevalence is underestimated.3,4
TTC, also known as stress-induced cardiomyopathy, left ventricular apical ballooning syndrome, transient ventricular ballooning syndrome, ampulla cardiomyopathy, and broken heart syndrome, is characterized by transient left ventricular systolic dysfunction and apical ballooning that resembles the shape of a traditional Japanese octopus trap, the rationale for the name “Takotsubo.”5 Following its inclusion among the cardiomyopathies by the International Society of Cardiology, TTC has gradually gained more recognition in recent years. The clinical manifestations of TTC and AMI are so similar that TTC may be easily misdiagnosed as an AMI. However, TTC differs significantly from AMI with respect to treatment and prognosis. More than 95% of patients with TTC have favorable prognoses that entail the full recovery of left ventricle (LV) function; however, serious complications such as heart failure, malignant arrhythmia, and death have also been reported.6,7 As catecholamine-mediated cardiotoxicity is widely accepted as the primary pathophysiological mechanism of TTC,2,8–10 beta receptor blockers and angiotensin-converting enzyme inhibitors (ACEI) are commonly chosen to treat the disease rather than thrombolytic agents, coronary artery stents, coronary bypass, or long-term antiplatelet or lipid-lowering drugs.11
Physical and mental stressors are recognized as the leading causes of TTC.9,10 With the increasing pressure of work and life in modern society, the morbidity of TTC in large cities is gradually increasing. TTC has drawn increased attention as a new mechanism of SCD. Under these circumstances, an early diagnosis and the administration of appropriate and timely treatment may avoid unnecessary coronary angiography, avert fatal complications, and improve the survival rates of SCD patients.1 Although many countries have reported cases of TTC in last 10 years,3,5,12 including Japan, Korea, America, and Australia, only a limited number of studies has focused on young patients, who make up the largest proportion of the metropolitan population. No randomized controlled studies have been performed in this cohort either in China or aboard. Therefore, to better understand the prevalence, risk factors, clinical characteristics, and prognosis of TTC in young patients, we performed a systematic review of the existing literature on this topic.
METHODS
A computer-assisted search was performed using the CBMDISC, PUBMED, EMBASE, SCI, and Cochrane Library databases to identify relevant literature published between January 1965 and December 2013. We used the search terms “stress cardiomyopathy,” “Takotsubo,” “Tako-tsubo,” “apical ballooning,” “ampulla cardiomyopathy,” “amphora cardiomyopathy,” and “broken heart syndrome,” paired with the terms “adult,” “infant,” or “child.” The criteria for the inclusion of publications in this study were as follows: reports describing original data that contained complete individual patient information; patient age ≤40 years old; transient dyskinesis or akinesis of the left ventricular apex, midventricular wall-motion abnormalities, basal ballooning, and a pattern of regional wall-motion abnormalities beyond the range of a single epicardial coronary arterial distribution, as noted via echocardiography or left ventriculography; and coronary angiography demonstrating the absence of acute plaque ruptures or signs of obstructive coronary disease (normal coronary arteries or luminal stenosis <50%). The generated list of articles obtained via the literature search was checked manually by 2 investigators, and the study eligibility was confirmed by all authors. Publications were selected for further detailed review when the titles or abstracts indicated that they were likely to meet the inclusion criteria. Furthermore, we analyzed the references of these papers to identify additional relevant articles. We chose the latest publication when several publications were found that described the same case series, although we included data from earlier publications if necessary. For each selected study, 2 reviewers independently extracted data, including data regarding the demographic characteristics, risk factors, clinical presentation, complications, treatment and prognosis of TTC. Differences were resolved by consultation or by a third independent reviewer. Due to the heterogeneity of the collected data, we conducted a narrative synthesis of the reported cases and case series instead of a formal meta-analysis. The purpose of our study was to identify the baseline characteristics, risk factors, treatment and prognosis of young patients with TTC. Cases with patients who died were analyzed based on their clinical data.
Stata 7.0 (StataCorp, College Station, TX) was used to perform all statistical evaluations. Quantitative variables are presented as means ± standard deviations, and categorical data are presented as absolute values and percentages. Continuous variables were compared using Student t test and the Chi-square test. Fisher exact test was used to compare categorical data. Any P ≤ 0.05 was considered statistically significant.
RESULTS
Search Results
Using the retrieval strategy described above, a total of 858 articles were identified. Articles were screening by reading the titles and abstracts to exclude any articles that did not meet our inclusion criteria as well as to exclude reviews and repeat articles; 397 articles were ultimately selected. Based on our exclusion criteria, we removed 277 papers that included patients older than 40 years of age. Nineteen articles with incomplete clinical data were also excluded, as were 5 additional articles without any data regarding coronary angiography. Ninety-six records, including 104 cases, were selected for our research (Figure 1).13–108
FIGURE 1.
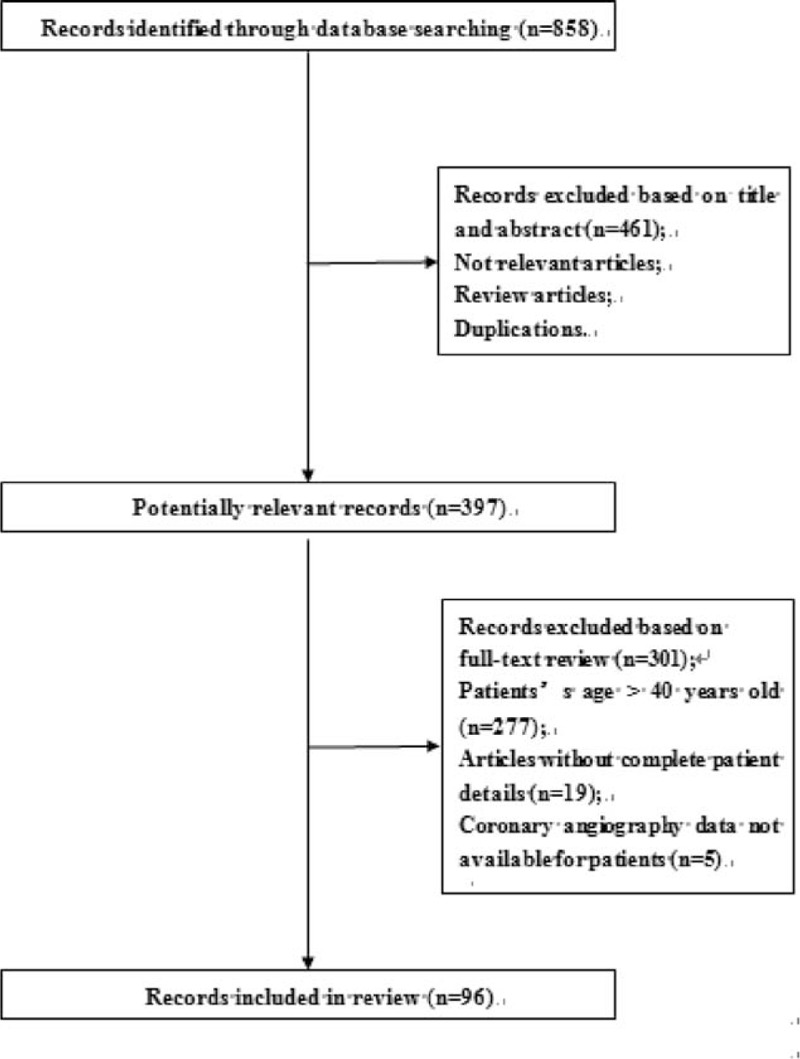
Case series selection process.
Baseline Characteristics
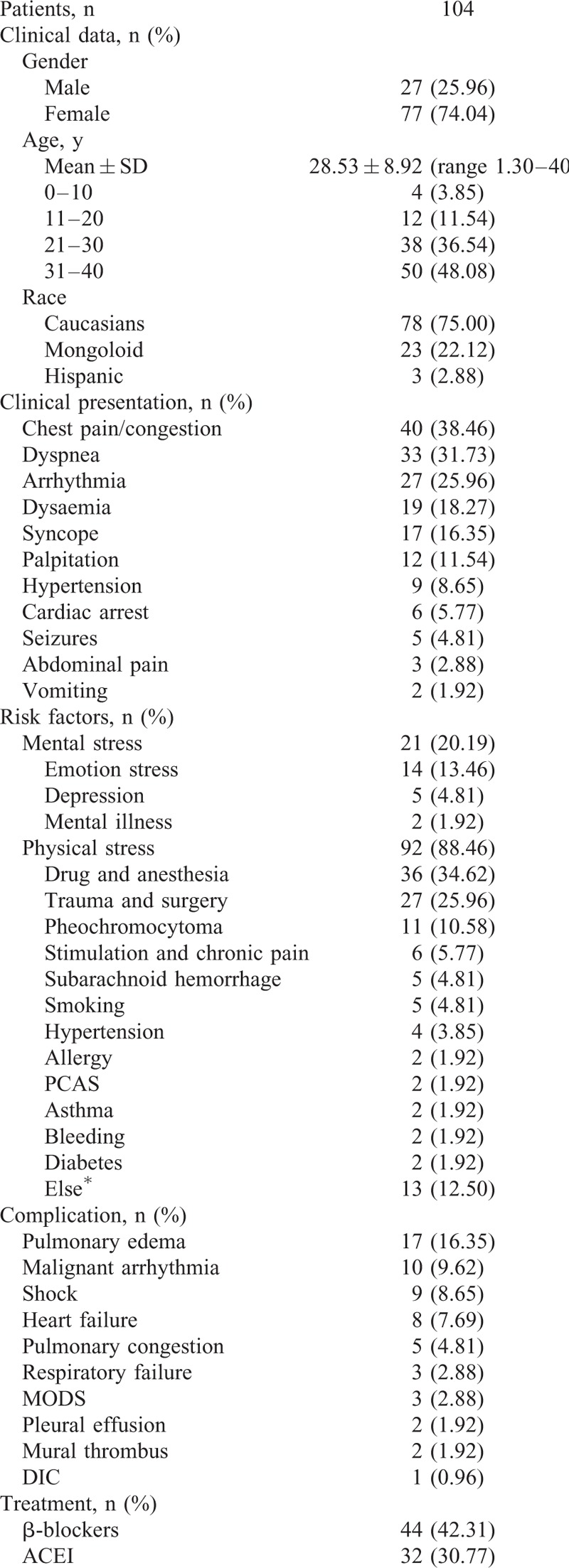
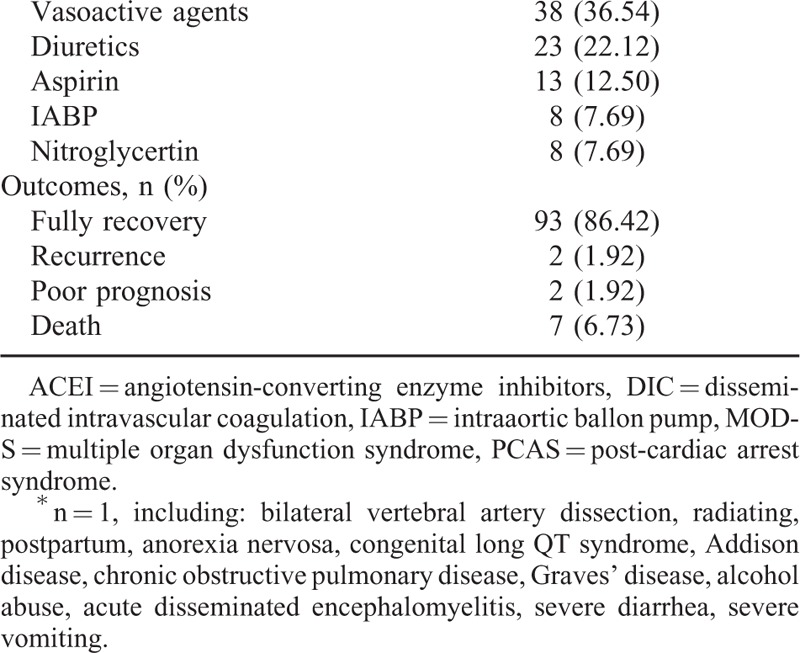
We undertook a systematic review of the reported cases that included information regarding the clinical characteristics of TTC in the young patients. A total of 104 cases were selected for our study. The basic characteristics of these patients are described in detail in Table 1 . The average age of the patients was 28.53 ± 8.92 years old. Among these patients, the majority were 31 to 40 years old (48.08%) and were female (74.04%), similar to the general population.5,109 Caucasians represented a significant percentage of the patients (75.00%), followed by people of Asian origin (22.12), whereas Hispanic individuals accounted for only 2.88% of patients. The most common presenting symptoms were chest pain and chest congestion (38.46%). Dyspnea (31.73%) and cardiac arrhythmia (25.96%) were the second and third most common clinical manifestations. More than 10% of patients experienced an episode of syncope during the progression of their illnesses, and other patients experienced cardiac arrest, seizures, or gastrointestinal symptoms. Most interestingly, physical stress accounted for 88.46% of cases of TTC among young patients. Among these patients, drugs, anesthesia (34.62%), trauma, surgery (25.96%), and pheochromocytoma (10.58%) were the most common physical stressors. Chronic pain, subarachnoid hemorrhage, smoking, high blood pressure, allergic reactions, asthma and the other physical stressors that induced TTC were less common among young individuals. Mental stress (20.19%) also played a role in the etiology of TTC among young patients. Emotional stress represented the majority (13.46%) of mental stress that caused TTC; depression and mental illness ranked second and third, respectively. The most common complications associated with TTC were as follows: pulmonary edema (16.35%), malignant arrhythmia (9.62%), and shock (8.65%). Current treatments for TTC among youth primarily included β-blockers (42.31%), ACEI (30.77%), vasoactive agents (36.54%), diuretics (22.12%), aspirin (12.50%), nitroglycerin (7.69%), intraaortic balloon pumps (7.69%), and symptomatic and supportive treatments for patients with concurrent pericardial effusions, mural thrombi and additional ailments. As in the general patient population TTC has an excellent prognosis among young people. Among the 104 cases studied, 93 had a favorable prognosis, and patients experienced full recovery of their cardiac function. Two (1.92%) cases recurred,34,110 and 2 (1.92%) cases had a poor prognosis.83,105 Unfortunately, there were 7 cases (6.73%) in which patients died from TTC, and no clear explanation for their deaths was found24,27,37,52,98,106,111; further research and analyses are needed to elucidate the mechanisms underlying this phenomenon.
TABLE 1.
Demographic and Clinical Characteristics of TTC Patients
Stress
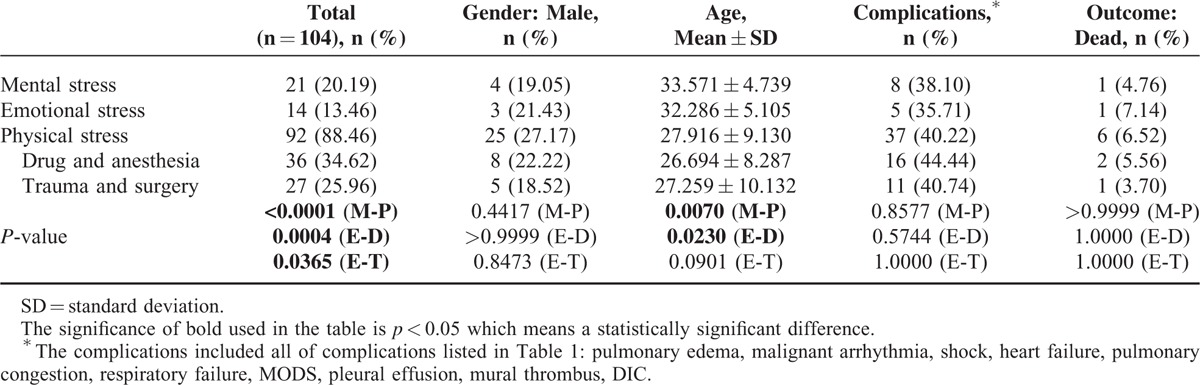
As stated previously, most cases of apical ballooning are preceded by stressful events, either mental or physical in origin. This relationship holds true in young patients. In our study, we compared the clinical characteristics of young patients exposed to different stressors (Table 2). Physical stress was more likely to exacerbate TTC than was mental stress (88.46% vs. 20.19%, P < 0.0001) among young patients, a finding that differed from the findings of previous reports involving the general population.5,109 Patients who suffered from physical stress were much younger than those who suffered from mental stress (27.916 ± 9.130 vs. 33.571 ± 4.739, P < 0.05). The results pertaining to both morbidity (19.05% vs. 27.17%, P = 0.4417) and mortality (4.76% vs. 6.52%, P > 0.9999) did not differ significantly with respect to exposure to different stressors, results similar to those pertaining to the rates of complications (38.10% vs. 40.22%, P = 0.8577). Meanwhile, the gender ratio was the same with respect to the numbers of men and women who experienced both mental and physical stress.
TABLE 1 (Continued).
Demographic and Clinical Characteristics of TTC Patients
Race
We compared the clinical characteristics of Caucasian TTC patients with Asian and Hispanic TTC patients (Table 3) and observed no significant differences with respect to race for the following characteristics: age (30.348 ± 8.505 vs. 28.016 ± 9.024, P = 0.2708), gender (female: 86.96% vs. 70.37%, P = 0.1829), mortality (0% vs. 8.64%, P = 0.3230), stress (mental stress: 26.09% vs. 18.52%, P = 0.6145; physical stress: 78.26% vs. 91.36%, P = 0.1722), or complications (43.38% vs. 38.27%, P = 0.8094).
TABLE 2.
Number and Percentages of Patients in Which TTC, Complications, and Outcome Were Associated With Patient Mental and Physical Stress
Prognosis
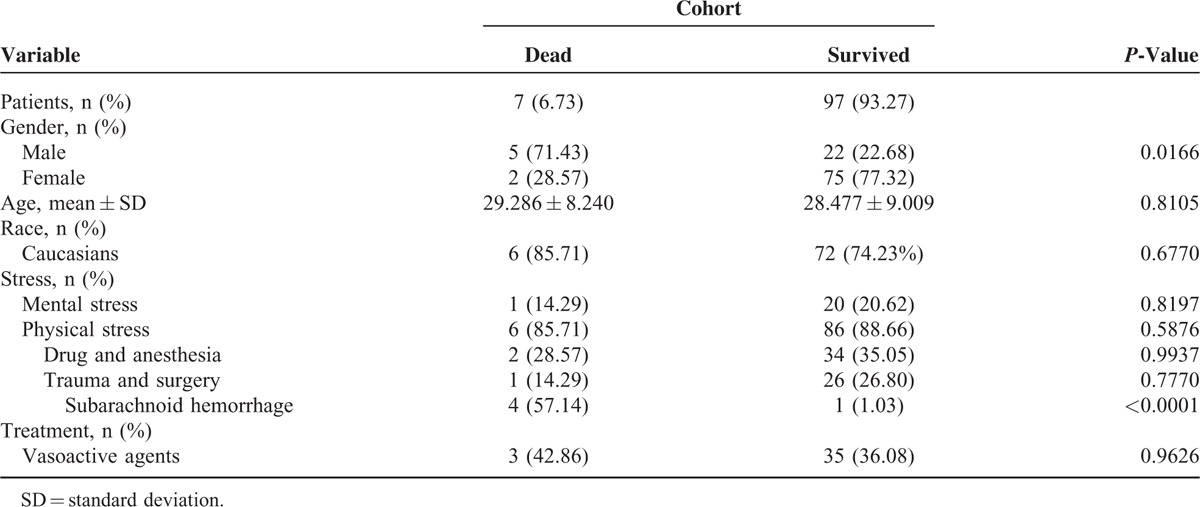
As they represent the main body of social productivity, young patients’ prognoses are particularly noteworthy. With respect to the 7 cases of death included in our study, we performed a detailed analysis of the baseline characteristics of the affected individuals (Table 4) as well as a series of statistical calculations (Table 5).24,27,37,52,98,106,111 The results indicated that the mortality rate of the male patients was significantly higher (71.43% vs. 28.57%, P < 0.05) among young patients. The majority of the patients who died were Caucasian (85.71%).24,27,37,52,98,106,111 The average age of the patients who died did not differ significantly from that of the patients who survived (29.286 ± 8.240 vs. 28.477 ± 9.009, P = 0.8105). Common clinical presentations of the patients who died included syncope (57.14%),24,98,106,111 cardiac arrest (28.57%),37,52 and seizure (14.29%).27 Systematic complications included shock (28.57%),27,52 multiple organ dysfunction syndrome (MODS) (14.29%),27 and disseminated intravascular coagulation (DIC) (14.29%).27 Vasoactive agents were used to treat 3 of the patients in question (42.86%),52,98,111 and a similar proportion of surviving patients were also treated with vasoactive agents (36.08%). With respect to predisposing factors, no significant difference were noted regarding the incidences of mental stress (14.29% vs. 20.62%, P = 0.8197) or physical stress (85.71% vs. 88.66%, P = 0.5876) between the decreased patients and the surviving patients. The most prominent form of mental stress was emotional stress (14.29%); the most prominent physical stressors included subarachnoid hemorrhage (57.14%),37,98,106,111 drugs and anesthesia (28.57%).27,111 The most interesting result observed in our study was that the majority of the youth who suffered a subarachnoid hemorrhage later died (1.03% vs. 57.14%, P < 0.0001).
TABLE 3.
Clinical Characteristics of Patients by Ethnicity
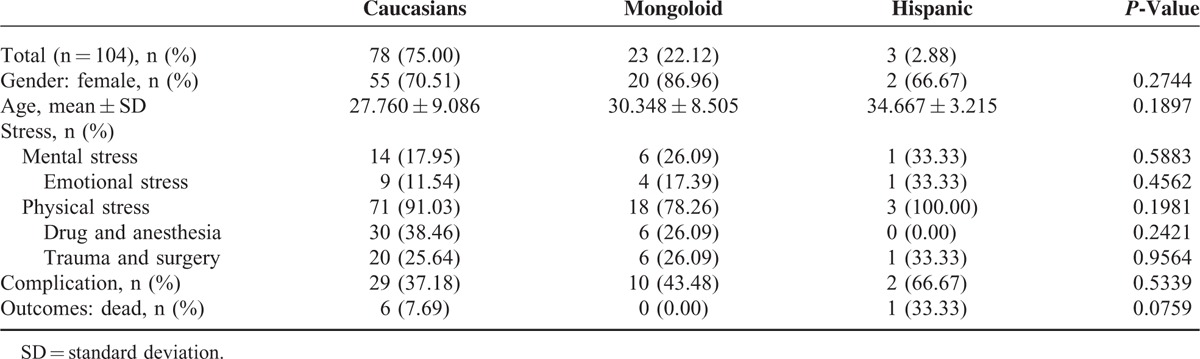
TABLE 4.
Characteristics of Patients Who Died
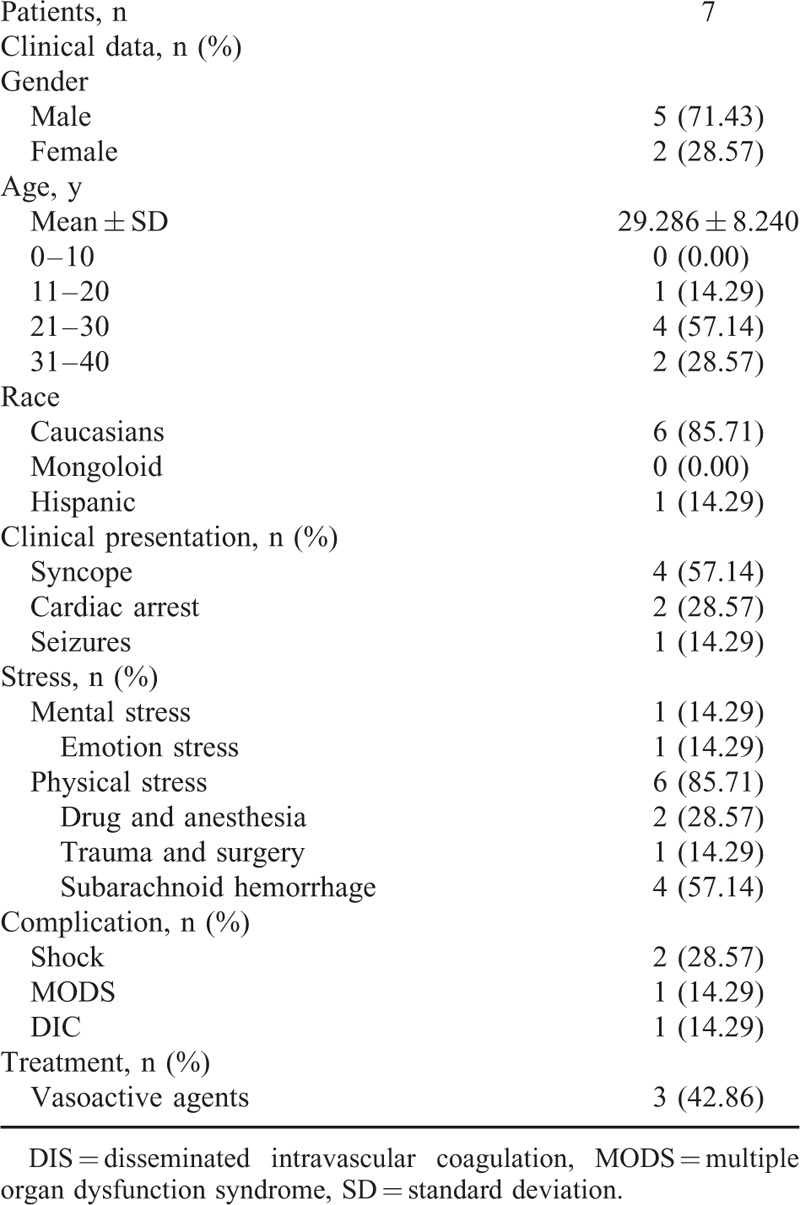
TABLE 5.
Comparison of Characteristics of Patients Who Died and Those Who Survived
DISCUSSION
TTC is a newly recognized disease that was first described in 1991 by Dote et al in Japan.3,5,12 Following the original description, several case series involving other ethnic groups, including studies featuring Caucasian and Hispanic patients, have been published, suggesting that this syndrome is a worldwide problem and represents a new phenotype of SCD. Most of the studies involving patients with TTC have been conducted in the general population or with postmenopausal women,4,5 whereas the clinical characteristics, risk factors, morbidity and mortality of young patients with TTC has been relatively unexplored. However, young people are always under either physical stress or mental stress, often as a result of an unhealthy lifestyle, particularly in larger cities. Therefore, this particular cohort warrants attention. To shed light on the complexity of this entity, a comprehensive analysis of the clinical characteristics, particularly stressors triggering exacerbations, and the underlying risk factors for death in young patients suffering from TTC was performed.
Stress
Either mental or physical stress was noted to be a trigger in the majority of young patients, as previously described in a general cohort.9,112 However, this finding prompts questions concerning the unique clinical characteristics and underlying pathophysiology of the disease. It is unclear why only certain individuals exposed to stressors suffer from TTC. What type of stress is the primary culprit? What type of stress plays a more important role? What amount of a particular type of stress results in disease?
One interesting result noted by our study was that compared with the general population, young patients appeared to suffer from TTC as a result of physical stress rather than mental stress. In our cohort, the number of patients who suffered from physical stress was much higher than the number of patients who suffered from mental stress (88.46% vs. 20.19%, P < 0.0001). Drugs, trauma, anesthesia, surgery, chronic pain, subarachnoid hemorrhage, smoking, allergic reactions, high blood pressure, asthma, and other physical stressors were common triggers of TTC. Compared with mental triggers, physical stressors have been speculated to be associated with more acute catecholamine surges that cause more significant cardiac stress and may explain why physical stressors are more often noted among youth with TTC.113 Chronic mental stress has also been associated with increased odds of developing TTC among young patients; TTC has been shown to cause structural changes in the cerebrum, alterations of the hypothalamic–pituitary–adrenal axis, and chronic and repetitive catecholamine release.113,114 The factor that predisposes patients suffering from chronic mental stress to TTC is believed to be a large emotional burden, such as that incurred via the death of a loved one, sudden bankruptcy, or unexpected unemployment. Either physical or mental stress, as well as either endogenous or exogenous catecholamine surges, may precipitate an exacerbation of TTC, which appears to be a response to whole-body stress that affects the cardiocirculatory syndrome and other organ systems. Therefore, stress avoidance is the key to reducing the morbidity of TTC among young patients.
Gender
Male patients accounted for a small minority (26%) of patients with TTC in our cohort, a finding that is consistent with studies involving the general population. The precise reason for the predominance of female patients is unclear. Previous studies have suggested that estrogen exerts a desensitizing effect on the myocardial response to catecholamines; therefore, elderly women lacking the protection of estrogen may be vulnerable to a rebound cardiac reaction to stress-reduced catecholamine release.3,115–117 However, this mechanism does not explain the large numbers of young female patients with TTC. In our study, the proportion of young female patients was significantly higher than the proportion of males. Previous research has suggested that estrogen replacement may not mitigate the risk of developing TTC.5,116 These findings suggest that other mechanisms in addition to estrogen deficiency may lead to TTC. Compared with male patients, the smaller left ventricular outflow tracts and reduced left ventricular volumes observed in female patients may explain the above-noted predominance.3,5 Therefore, a smaller left ventricular volume and a lower density of adrenergic receptors in the cardiomyocyte membrane may predispose individuals to developing an outflow tract obstruction, as these individuals possess less protection against a severe catecholamine storm because the saturation of a smaller number of adrenoreceptors takes less time.
Disease is characterized by an unbalanced state of biology–society–psychology. With respect to biology, gender affects cardiomyocyte contractility.118–120 Cardiomyocyte contractility studies have demonstrated that estrogen and testosterone exhibit contrasting inotropic actions and modulate Ca2+ handling differently.118 Golden et al reported that the androgen receptor protein is present in cardiac myocytes and exerts a positive inotropic effect in response to testosterone in cardiac myocytes.119 Mellor et al demonstrated that myocytes from female patients with elevated AngII levels appeared to be more susceptible to contractility deficits, which may explain the female predominance among TTC patients.120 Both social and psychological factors may play a role in the development of TTC. As a result of biological evolution, males and females exist in different physiological and social states; therefore, different psychological and physiological reactions to the same stress event occur as a result of natural selection and are affected synthetically by sex hormones and gender selection.114,121,122 In most societies, men have been trained to withstand greater pressures than women. Long-term evolution has resulted in a higher threshold for stress in males than in females. Therefore, compared with females, males demonstrate stronger adaptability and tolerance to stress.121,123 Due to their fragility and sensitive character traits, women possess a lesser capacity for mental adaptation; therefore, they respond with greater sensitivity to stressful events.123 Research with animal models has demonstrated that compared with male monkeys, female monkeys’ emotional states are more susceptible to environmental changes, similar to what has been observed in both rats and humans.124 Kelly et al121 demonstrated that males successfully withstood negative subjective experiences during a psychological test under stressful conditions, whereas females faired significantly worse; women were more irritable, timid, and confused, indicating that gender affects stress responses. This response differential may represent an important theoretical basis that explains why females have previously demonstrated increased susceptibility to psychosomatic diseases. Several studies on animals have verified this hypothesis. Faverjon et al reported that under chronic stress, increased levels of acetylcholine are released in female rats.125 Female animals are proposed to be characterized by sensitization to chronic stress, whereas male animals exhibit habituation.114 Additionally, studies have demonstrated that physical stress improves the sensitivity of the hypothalamic–pituitary–adrenocortical (HPA) axis in females, which may be another reason for the high morbidity of TTC in females. These findings are consistent with those of our research, as we found that young patients with TTC suffered from physical stress more frequently than mental stress.123
Race
We noted a much higher percentage of Caucasians among the young patients included in our study compared with other races (Table 1 ). The precise reason for this difference has not been well explained and may be related to economic conditions, living environments, lifestyle, dietary habits, and hereditary factors. A previous study reported that the incidence of hyperlipidemia and diabetes, both risk factors for cardiovascular disease, is much higher in Caucasians, which may explain the increased morbidity of TTC in this population.126 Williams and Lawler127 analyzed the stress–illness relationship of low-income women in a biracial group (African Americans and Caucasian Americans) and reported that race may affect stress; under high stress conditions, Caucasian women were more likely to suffer from illnesses.
We compared clinical characteristics among Caucasians, Asians, and Hispanics with respect to age, gender, stress, complications, and mortality; there were no significant differences in these parameters among Caucasians, Asians, and Hispanics in young patients with TTC. Whether the high incidence of TTC in Caucasians is due to their greater vulnerability to stress and whether the similarities in the demographic characteristics noted among different races depend on their genetic backgrounds remain to be determined. Thus, further investigation is warranted.
Clinical Characteristics
The overall clinical symptoms and outcomes among young patients with TTC noted by our study were similar to the clinical symptoms and outcomes noted in the general population.5 Chest pain, chest congestion, and dyspnea were the most common presenting clinical symptoms; additionally, cardiac arrhythmia, syncope, cardiac arrest, seizures, and gastrointestinal symptoms were reported. Severe complications, including pulmonary edema, malignant arrhythmia, and shock, were also noted in young patients at frequencies comparable with those of the general population.12
Diagnosis and Treatment
TTC is often misdiagnosed as other cardiovascular diseases, particularly in young patients. Differentiating transient LV apical ballooning syndrome from ACS or other cardiovascular diseases is difficult but necessary. Misdiagnosis results in treatment with thrombolytic agents and may place patients at unnecessary risk of bleeding. Cardiac echocardiography and ventriculography may be used to confirm the diagnosis.128 Early diagnosis and timely and appropriate treatment may not only avoid the need for coronary angiography but may also avert fatal complications and improve the survival rates of young patients with TTC. Significant debate surrounds the treatment for TTC, as several treatments, including the manipulation of intravenous fluids and the use of β-blockers, ACEI, aspirin, diuretics, vasoactive agents, nitroglycerin, or intraarterial balloon pumps, have been suggested.129,130 It may be wise for doctors to adhere to the “primum non nocere” principle while awaiting information regarding evidence-based therapeutic methods for treating TTC. Most importantly, the treatment of this syndrome should be performed based on the unique characteristics of each patient.
Prognosis
Most TTC patients, including young patients, recover following transient cardiac dysfunction. Although the abnormal kinetics of TTC typically reverse or improve within 4 to 5 weeks in young patients, and although the prognosis is favorable in this population, some patients develop complications such as pulmonary edema, malignant arrhythmia, shock, or death.3,5,6 The overall in-hospital mortality rate was 6.73% among the young patients included in our study, a finding consistent with the mortality rates ranged from 0% to 8% in previous studies on the general population.7,109,131 It is intriguing that the mortality rate noted in male patients was significantly higher (71.43% vs. 28.57%, P < 0.05) among young patients compared with the general population. Gender appears to play a role in disease prognosis.
A high risk of in-hospital mortality was noted in patients with an underlying critical illness, a finding corroborated by a study published by Song et al132 and illustrated by our study, as the majority of the youth who died suffered a previous subarachnoid hemorrhage (57.14% vs. 1.03%, P < 0.0001). A significantly higher prevalence of all types of critical illnesses was noted in males compared with females and may have contributed to the higher mortality rate.133 The precise mechanism underlying the higher mortality rate noted among young males remains unclear. Further study is necessary to determine the demographic and clinical predictors of mortality in the setting of TTC. Interestingly, the self-healing mechanisms that characterize TTC have provided us with new insight into cardiovascular disease as a whole.
Pathophysiology
The mechanism of TTC has not been clearly elucidated. Adrenergic overstimulation, β-adrenoceptor (β-AR) stimulus trafficking, epinephrine-specific β2AR-Gi signaling, catecholamine-induced myocardial toxicity, coronary artery spasm, microvascular dysfunction, outflow tract obstruction, and the direct toxic effects of catecholamines on the myocardium have been reported as possible mechanisms that underlie the development of TTC.5,9,117,134,135 The numbers of evidence-based studies and animal experiments are limited. Additional studies must be undertaken to elucidate the underlying mechanisms of TTC. We need more basic and clinical research to determine if takotsubo patients share any causal disease factors (eg, inherited genetic predisposition), and if clear diagnostic criteria can be identified that define this disease, and distinguish it from other similar disorders of cardiac ventricular function.
LIMITATIONS
There were several limitations to our study. First, the absence of comparison groups limited the accuracy of our evidence and data interpretation; our paper was based on retrospective observational studies and included only case reports and case-series reports describing young patients with TTC. Second, not all data were included in the study; we removed those case series lacking comprehensive demographic and clinical data. Third, not all cases of TTC were included due to both investigator and publication bias in spite of careful electronic and manual searches.
CONCLUSION
In conclusion, we conducted a systematic review of the reported cases of TTC in young patients in an attempt to better characterize the clinical and demographic characteristics of TTC patients in this cohort. TTC is a reversible phenomenon that has been associated with catecholamine surges and acute norepinephrine toxicity subsequent to stressful events. The findings of our study are as follows: First, physical stress is more likely to exacerbate TTC compared with mental stress among young patients. Second, females are more predominantly affected than males, both among young people and in the general population. Other parameters, including smaller left ventricular outflow tracts, reduced left ventricular volumes, and female psychological traits may play a pivotal role in the pathogenesis of TTC in young patients. Third, different human ethnic origins may not affect disease severity; therefore, individuals of different races do not experience significantly different disease characteristics, morbidity, or stressors. Fourth, TTC is not characterized by typical clinical symptoms; therefore, clinical characteristics alone are insufficient to make a diagnosis, and both echocardiography and ventriculography are important methods of disease detection. Thus, clinicians should be alert when evaluating young patients who present with symptoms similar to those of acute cardiomyopathy so that harmful treatments, including the use of thrombolytic drugs, coronary artery stents, bypass operations, long-term antiplatelet, and lipid-lowering drugs, may be avoided.
Additionally, the answers to questions regarding the clinical diagnostic criteria, etiology, pathophysiology, and management of this syndrome remain unclear; further research is necessary to answer these questions.
Acknowledgment
Thanks go to all the persons contributing to our work, especially Professor Liyan Long, who made a lot of effort to literature retrieval.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitors, ACS = acute coronary syndrome, AMI = acute myocardial infarction, DIC = disseminated intravascular coagulation, HPA = hypothalamic–pituitary–adrenocortical, IABP = intraaortic ballon pump, MODS = multiple organ dysfunction syndrome, PCAS = post-cardiac arrest syndrome, SCD = sudden cardiac death, TTC = Takotsubo cardiomyopathy.
The research was supported by grant from Chinese National Natural Science Fund (no. 30900543 to Dr. H.Y.Z.). Beijing National Natural Science Fund (no. 7152136 to Dr. H.Y.Z.) and Clinical Science Fund of PLA General Hospital (no. 2013FC-TSYS-1028 to Dr. H.Y.Z.).
YW, LX, and XS contributed equally to the work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008; 51:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118:2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol 2008; 124:283–292. [DOI] [PubMed] [Google Scholar]
- 4.Zeb M, Sambu N, Scott P, et al. Takotsubo cardiomyopathy: a diagnostic challenge. Postgrad Med J 2011; 87:51–59. [DOI] [PubMed] [Google Scholar]
- 5.Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006; 27:1523–1529. [DOI] [PubMed] [Google Scholar]
- 6.de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol 2008; 131:18–24. [DOI] [PubMed] [Google Scholar]
- 7.Singh K, Carson K, Shah R, et al. Meta-Analysis of Clinical Correlates of Acute Mortality in Takotsubo Cardiomyopathy. Am J Cardiol 2014; 113:1420–1428. [DOI] [PubMed] [Google Scholar]
- 8.Skolnick AH, Michelin K, Nayar A, et al. Transient apical ballooning syndrome precipitated by dobutamine stress testing. Ann Intern Med 2009; 150:501–502. [DOI] [PubMed] [Google Scholar]
- 9.Nef HM, Mollmann H, Akashi YJ, et al. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol 2010; 7:187–193. [DOI] [PubMed] [Google Scholar]
- 10.Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008; 5:22–29. [DOI] [PubMed] [Google Scholar]
- 11.Fazio G, Pizzuto C, Barbaro G, et al. Chronic pharmacological treatment in takotsubo cardiomyopathy. Int J Cardiol 2008; 127:121–123. [DOI] [PubMed] [Google Scholar]
- 12.Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004; 141:858–865. [DOI] [PubMed] [Google Scholar]
- 13.Bonnemeier H, Ortak J, Burgdorf C, et al. The artichoke heart”: the inverse counterpart of left ventricular apical ballooning. Resuscitation 2007; 72:342–343. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Youn HJ, Lee WH, et al. A case of anorexia nervosa complicated with strongly suspected stress-induced cardiomyopathy and mural thrombus. Korean Circ J 2011; 41:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SY, Lee SE, Choi JW, et al. A case of transient left ventricular apical ballooning syndrome in a child: clinical features and imaging findings. Int J Cardiovasc Imaging 2010; 26:345–351. [DOI] [PubMed] [Google Scholar]
- 16.Takizawa M, Kobayakawa N, Uozumi H, et al. A case of transient left ventricular ballooning with pheochromocytoma, supporting pathogenetic role of catecholamines in stress-induced cardiomyopathy or takotsubo cardiomyopathy. Int J cardiol 2007; 114:e15–e17. [DOI] [PubMed] [Google Scholar]
- 17.Bajolle F, Basquin A, Lucron H, et al. Acute ischemic cardiomyopathy after extreme emotional stress in a child. Congenit Heart Dis 2009; 4:387–390. [DOI] [PubMed] [Google Scholar]
- 18.Michel J, Pegg T, Porter D, et al. Atypical variant stress (Takotsubo) cardiomyopathy associated with gastrointestinal illness: rapid normalisation of LV function. N Z Med J 2012; 125:85–87. [PubMed] [Google Scholar]
- 19.Fulcher J, Wilcox I. Basal stress cardiomyopathy induced by exogenous catecholamines in younger adults. Int J Cardiol 2013; 168:e158–e160. [DOI] [PubMed] [Google Scholar]
- 20.Seifert T, Klein E, Legat-Wallner S, et al. Bilateral vertebral artery dissection and infratentorial stroke complicated by stress-induced cardiomyopathy. N Z Med J 2008; 79:480–481. [DOI] [PubMed] [Google Scholar]
- 21.Pant S, Deshmukh A, Mehta K, et al. Burden of arrhythmias in patients with takotsubo cardiomyopathy (apical ballooning syndrome). Int J cardiol 2013; 170:64–68. [DOI] [PubMed] [Google Scholar]
- 22.Cherian J, Angelis D, Filiberti A, et al. Can takotsubo cardiomyopathy be familial. Int J Cardiol 2007; 121:74–75. [DOI] [PubMed] [Google Scholar]
- 23.Facciorusso A, Amico C, Vigna C, et al. Cardiac arrest caused by Barlow's syndrome or by stress cardiomyopathy. Int J cardiol 2010; 140:e16–e18. [DOI] [PubMed] [Google Scholar]
- 24.D’Errico S, Neri M, Nieddu A, et al. Cardiac beta1-adrenoceptor expression in two stress-induced cardiomyopathy-related deaths. Forensic Sci Int 2011; 207:e8–e11. [DOI] [PubMed] [Google Scholar]
- 25.Di PG, Daniele GP, Antonini-Canterin F, et al. Cardiogenic shock with basal transient left ventricular ballooning (Takotsubo-like cardiomyopathy) as first presentation of pheochromocytoma. J Cardiovasc Med (Hagerstown) 2010; 11:507–510. [DOI] [PubMed] [Google Scholar]
- 26.Schimpf R, Meinhardt J, Borggrefe M, et al. Catecholaminergic polymorphic ventricular tachycardia and midventricular Takotsubo cardiomyopathy: a novel association. Herzschrittmacherther Elektrophysiol 2013; 24:63–66. [DOI] [PubMed] [Google Scholar]
- 27.Champion S, Malissin I, Cleophax C, et al. Chloroquine poisoning-associated inverted Tako-tsubo cardiomyopathy. Clin Toxicol (Phila) 2012; 50:721–722. [DOI] [PubMed] [Google Scholar]
- 28.Bonnemeier H, Ortak J, Burgdorf C, et al. “The artichoke heart”: the inverse counterpart of left ventricular apical ballooning. Resuscitation 2007; 72:342–343. [DOI] [PubMed] [Google Scholar]
- 29.Subramaniam A, Cooke JC, Ernest D. ‘Inverted’ tako-tsubo cardiomyopathy due to exogenous catecholamines. Crit Care Resusc 2010; 12:104–108. [PubMed] [Google Scholar]
- 30.Revelo AE, Pallavi R, Espana-Schmidt C, et al. ‘Stoned’ people can get stunned myocardium: a case of heroin withdrawal precipitating Tako-Tsubo cardiomyopathy. Int J Cardiol 2013; 168:e96–e98. [DOI] [PubMed] [Google Scholar]
- 31.Nolan S, Ionescu A. Accidental cold water immersion: an unusual cause of stress cardiomyopathy in a patient with Marfan's syndrome and aortic exostent. Int J Cardiol 2011; 151:e98–e99. [DOI] [PubMed] [Google Scholar]
- 32.Pujadas S, Punti J, Carreras F, et al. Atypical form of Tako-Tsubo cardiomyopathy. J Cardiovasc Magn Reson 2007; 9:697–699. [DOI] [PubMed] [Google Scholar]
- 33.Eitel I, Schuler G, Gutberlet M, et al. Biventricular stress-induced (Tako-tsubo) cardiomyopathy with left midventricular and right apical ballooning. Int J Cardiol 2012; 151:e63–e64. [DOI] [PubMed] [Google Scholar]
- 34.Kurt IH. Coronary artery disease mimicking Tako-tsubo cardiomyopathy: a case report. Cases J 2009; 2:6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Rosa G, Pardeo M, Di RC, et al. Neurogenic stunned myocardium presenting as left ventricular hypertrabeculation in childhood: a variant of Takotsubo cardiomyopathy. Pediatr Crit Care Med 2011; 12:e420–e423. [DOI] [PubMed] [Google Scholar]
- 36.Win CM, Pathak A, Guglin M. Not takotsubo: a different form of stress-induced cardiomyopathy—a case series. Congest Heart Fail 2011; 17:38–41. [DOI] [PubMed] [Google Scholar]
- 37.Marechaux S, Fornes P, Petit S, et al. Pathology of inverted Takotsubo cardiomyopathy. Cardiovasc Pathol 2008; 17:241–243. [DOI] [PubMed] [Google Scholar]
- 38.Dewachter P, Mouton-Faivre C. Possible link between apical ballooning syndrome during anaphylaxis and inappropriate administration of epinephrine. Mayo Clin Proc 2010; 85:397–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaqub Y, Jenkins LA, Nugent KM, et al. Postpartum depression and apical ballooning syndrome (takotsubo syndrome). J Obstet Gynaecol Can 2009; 31:736–739. [DOI] [PubMed] [Google Scholar]
- 40.Deshmukh A, Kumar G, Pant S, et al. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J 2012; 164:66–71. [DOI] [PubMed] [Google Scholar]
- 41.Cherian J, Angelis D, Filiberti A, et al. Recurrence of stress-induced (takotsubo) cardiomyopathy. Cardiology 2007; 108:144–146. [DOI] [PubMed] [Google Scholar]
- 42.Kaoukis A, Panagopoulou V, Mojibian HR, et al. Reverse Takotsubo cardiomyopathy associated with the consumption of an energy drink. Circulation 2012; 125:1584–1585. [DOI] [PubMed] [Google Scholar]
- 43.Movahed MR, Mostafizi K. Reverse or inverted left ventricular apical ballooning syndrome (reverse Takotsubo cardiomyopathy) in a young woman in the setting of amphetamine use. Echocardiography 2008; 25:429–432. [DOI] [PubMed] [Google Scholar]
- 44.Ramaraj R, Movahed MR. Reverse or inverted takotsubo cardiomyopathy (reverse left ventricular apical ballooning syndrome) presents at a younger age compared with the mid or apical variant and is always associated with triggering stress. Congest Heart Fail 2010; 16:284–286. [DOI] [PubMed] [Google Scholar]
- 45.Law C, Khaliq A, Guglin M. Reversible cardiogenic shock due to catecholamine-induced cardiomyopathy: a variant of takotsubo. Am J Emerg Med 2013; 31:1621.e1–1621.e3. [DOI] [PubMed] [Google Scholar]
- 46.Jalan P, Dhakal L, Pandav V, et al. Status migrainosus as a potential stressor leading to takotsubo cardiomyopathy. Cephalalgia 2012; 32:1140–1143. [DOI] [PubMed] [Google Scholar]
- 47.Hasdemir C, Vuran O, Yuksel A, et al. Stress cardiomyopathy (Tako-Tsubo) associated with sustained polymorphic ventricular tachycardia. Pacing Clin Electrophysiol 2013; 36:e111–e114. [DOI] [PubMed] [Google Scholar]
- 48.Abraham J, Mudd JO, Kapur NK, et al. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol 2009; 53:1320–1325. [DOI] [PubMed] [Google Scholar]
- 49.Olivotti L, Moshiri S, Nicolino A, et al. Stress cardiomyopathy and arrhythmic storm in a 14-year-old boy. J Cardiovasc Med (Hagerstown) 2010; 11:519–521. [DOI] [PubMed] [Google Scholar]
- 50.Niu J, Gilliland MG, Jin Z, et al. MCP-1 and IL-1β expression in the myocardia of two young patients with Type 1 diabetes mellitus and fatal diabetic ketoacidosis. Exp Mol Pathol 2014; 96:71–79. [DOI] [PubMed] [Google Scholar]
- 51.Al-Moghairi A, Al-Harfi Z, Al-Shouli S. Stress-induced cardiomyopathy (Tako-Tsubo) in a premenopausal woman: a case report from Saudi Arabia. J Saudi Heart Assoc 2010; 22:219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachotti PLJ, Cardoso CMN, Vissoci RF, et al. Stress-induced cardiomyopathy (takotsubo cardiomyopathy) after liver transplantation-report of two cases. Transplant Proc 2012; 44:2497–2500. [DOI] [PubMed] [Google Scholar]
- 53.Chen WT, Lin CH, Hsieh MH, et al. Stress-induced cardiomyopathy caused by heat stroke. Ann Emerg Med 2012; 60:63–66. [DOI] [PubMed] [Google Scholar]
- 54.Sun RH, Hu BC, Li Q. Stress-induced cardiomyopathy complicated by multiple organ failure following cephalosporin-induced anaphylaxis. Intern Med (Tokyo, Japan) 2011; 51:895–899. [DOI] [PubMed] [Google Scholar]
- 55.Suk EH, Kim DH, Kweon TD, et al. Stress-induced cardiomyopathy following cephalosporin-induced anaphylactic shock during general anesthesia. Can J Anaesth 2009; 56:432–436. [DOI] [PubMed] [Google Scholar]
- 56.Schultz T, Shao Y, Redfors B, et al. Stress-induced cardiomyopathy in Sweden: evidence for different ethnic predisposition and altered cardio-circulatory status. Cardiology 2012; 122:180–186. [DOI] [PubMed] [Google Scholar]
- 57.Redfors B, Shao Y, Omerovic E. Stress-induced cardiomyopathy in a patient with chronic spinal cord transection at the level of C5: endocrinologically mediated catecholamine toxicity. Int J Cardiol 2012; 159:e61–e62. [DOI] [PubMed] [Google Scholar]
- 58.Pfister S, Wagar P, Casserly IP, et al. Stress-related cardiomyopathy in a 31-year-old woman. AANA J 2010; 78:406–411. [PubMed] [Google Scholar]
- 59.Freitas HF, Renault R, Ribeiro ES, et al. Sudden cardiac arrest due to puerperal transient left ventricular apical ballooning syndrome. Int J Cardiol 2011; 149:e12–e13. [DOI] [PubMed] [Google Scholar]
- 60.Guerci P, Novy E, Vial F, et al. Sulprostone for postpartum hemorrhage in a parturient with a history of Tako-tsubo cardiomyopathy. J Clin Anesth 2013; 25:327–330. [DOI] [PubMed] [Google Scholar]
- 61.Crimi E, Baggish A, Leffert L, et al. Acute reversible stress-induced cardiomyopathy associated with cesarean delivery under spinal anesthesia. Circulation 2008; 117:3052–3053. [DOI] [PubMed] [Google Scholar]
- 62.Parodi G, Antoniucci D. Transient left ventricular apical ballooning syndrome after inadvertent epidural administration of potassium chloride. Int J Cardiol 2008; 124:e14–e15. [DOI] [PubMed] [Google Scholar]
- 63.Grilo LS, Pruvot E, Grobéty M, et al. Takotsubo cardiomyopathy and congenital long QT syndrome in a patient with a novel duplication in the Per-Arnt-Sim (PAS) domain of hERG1. Heart Rhythm 2010; 7:260–265. [DOI] [PubMed] [Google Scholar]
- 64.Vergez M, Pirracchio R, Mateo J, et al. Tako Tsubo cardiomyopathy in a patient with multiple trauma. Resuscitation 2009; 80:1074–1077. [DOI] [PubMed] [Google Scholar]
- 65.Gundara JS, Lee JC, Ip J, et al. Takotsubo cardiomyopathy complicating thyroidectomy for Graves’ disease. Thyroid 2012; 22:975–976. [DOI] [PubMed] [Google Scholar]
- 66.Volman MN, Ten Kate RW, Tukkie R. Tako Tsubo cardiomyopathy, presenting with cardiogenic shock in a 24-year-old patient with anorexia nervosa. Neth J Med 2011; 69:129–131. [PubMed] [Google Scholar]
- 67.Billingham ME. Endomyocardial biopsy detection of acute rejection in cardiac allograft recipients. Heart Vessels Suppl 1985; 1:86–90. [DOI] [PubMed] [Google Scholar]
- 68.Caudron J, Rey N, Dacher JN. Midventricular Takotsubo cardiomyopathy associated with ventricular fibrillation during general anaesthesia in a 34-year-old woman: insight from cardiac computed tomography and magnetic resonance imaging. Arch Cardiovasc Dis 2012; 105:329–331. [DOI] [PubMed] [Google Scholar]
- 69.Park JY, Kim SJ. Inverted-Takotsubo cardiomyopathy secondary to adrenal mass. Arch Cardiovasc Dis 2012; 105:396–397. [DOI] [PubMed] [Google Scholar]
- 70.Collen J, Bimson W, Devine P. A variant of Takotsubo cardiomyopathy: a rare complication in the electrophysiology lab. J Invasive Cardiol 2008; 20:E310–E313. [PubMed] [Google Scholar]
- 71.Jo YY, Park S, Choi YS. Extracorporeal membrane oxygenation in a patient with stress-induced cardiomyopathy after caesarean section. Anaesth Intensive Care 2011; 39:954–957. [DOI] [PubMed] [Google Scholar]
- 72.Krpata DM, Barksdale EM., Jr Trauma induced left ventricular apical ballooning syndrome in a 15 year old: a rare case of Tako-tsubo cardiomyopathy. J Pediatr Surg 2013; 48:876–879. [DOI] [PubMed] [Google Scholar]
- 73.Von Bergen NH, Lyon JK, Edens RE. Takotsubo-like cardiomyopathy in a 17-year-old male with a pheochromocytoma. Pediatr Cardiol 2009; 30:184–187. [DOI] [PubMed] [Google Scholar]
- 74.Hernandez LE, Martinez Y, Chan KC. Takotsubo cardiomyopathy: an unusual cardiomyopathy at an unusual age. Cardiol Young 2010; 20:577–579. [DOI] [PubMed] [Google Scholar]
- 75.Berton E, Vitali-Serdoz L, Vallon P, et al. Young girl with apical ballooning heart syndrome. Int J Cardiol 2012; 161:e4–e6. [DOI] [PubMed] [Google Scholar]
- 76.Bortnik M, Verdoia M, Schaffer A, et al. Ventricular fibrillation as primary presentation of takotsubo cardiomyopathy after complicated cesarean delivery. World J Cardiol 2012; 4:214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sengupta S, Alsi V, Mohan V, et al. Unique phenotypes of typical and inverted Takotsubo cardiomyopathy in young females. Indian Heart J 2010; 62:348–350. [PubMed] [Google Scholar]
- 78.Jayaraman L, Sethi N, Sharma S, et al. Transient left ventricular apical ballooning post-pneumoperitoneum: takotsubo cardiomyopathy. A case report. Minerva Anestesiol 2010; 76:455–458. [PubMed] [Google Scholar]
- 79.Afonso L, Bachour K, Awad K, et al. Takotsubo cardiomyopathy: pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Minerva Anestesiol 2010; 76:455–458. [DOI] [PubMed] [Google Scholar]
- 80.Gemenetzis G, Gourgiotis S, Aravosita P, et al. Takotsubo cardiomyopathy: a hidden enemy of the hypovolemic patient. Am J Emerg Med 2013; 31:262e5–262e7. [DOI] [PubMed] [Google Scholar]
- 81.Mallick PN, Upadhaya SP, Das AK, et al. Takotsubo cardiomyopathy mimicking postoperative myocardial infarction in a young healthy patient. Indian J Anaesth 2013; 57:193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brezina P, Isler CM. Takotsubo cardiomyopathy in pregnancy. Obstet Gynecol 2008; 112:450–452. [DOI] [PubMed] [Google Scholar]
- 83.Barcin C, Kursaklioqlu H, Kose S, et al. Takotsubo cardiomyopathy in a patient with Addison disease: is apical ballooning always reversible. Int J Cardiol 2010; 138:e15–e17. [DOI] [PubMed] [Google Scholar]
- 84.Baleine J, Jacquot A, Novais AR, et al. Takotsubo cardiomyopathy in an adolescent girl. Arch Pediatr 2014; pii: S0929-693X(14)00081-5. [DOI] [PubMed] [Google Scholar]
- 85.Dundon BK, Puri R, Leong DP, et al. Takotsubo cardiomyopathy following lightning strike. Emerg Med J 2008; 25:460–461. [DOI] [PubMed] [Google Scholar]
- 86.Kodama S, Miyoshi K, Maruyama S, et al. Takotsubo cardiomyopathy complicated by high-grade atrioventricular block: a report of two cases. Exp Clin Cardiol 2009; 14:e35–e38. [PMC free article] [PubMed] [Google Scholar]
- 87.von Knobelsdorff-Brenkenhoff F, Abdel-Aty H, Schulz-Menger J. Takotsubo cardiomyopathy after nasal application of epinephrine—a magnetic resonance study. Int J Cardiol 2010; 145:308–309. [DOI] [PubMed] [Google Scholar]
- 88.Schoof S, Bertram H, Hohmann D, et al. Takotsubo cardiomyopathy in a 2-year-old girl: 3-dimensional visualization of reversible left ventricular dysfunction. J Am Coll Cardiol 2010; 55:e5. [DOI] [PubMed] [Google Scholar]
- 89.de Souza F, Altenburg Odebrecht Curi Gismondi R, Henriques Cunha Neto S, et al. Tako-tsubo-like cardiomyopathy and extra-adrenal pheochromocytoma: case report and literature review. Clin Res Cardiol 2008; 97:397–401. [DOI] [PubMed] [Google Scholar]
- 90.Deininger MH, Radicke D, Buttler J, et al. Tako-tsubo cardiomyopathy: reversible heart failure with favorable outcome in patients with intracerebral hemorrhage: case report. J Neurosurg 2006; 105:465–467. [DOI] [PubMed] [Google Scholar]
- 91.Yaghoubi AR, Ansarin K, Hashemzadeh S, et al. Tako-tsubo cardiomyopathy induced by emotional stress leading to severe mitral regurgitation, cardiogenic shock and cardiopulmonary arrest. Int J Cardiol 2009; 135:e85–e86. [DOI] [PubMed] [Google Scholar]
- 92.Berganzo K, Ciordia R, Gómez-Esteban JC, et al. Tako-tsubo cardiomyopathy in a patient with bilateral lesions in the dorsal medulla. Clin Auton Res 2011; 21:65–67. [DOI] [PubMed] [Google Scholar]
- 93.Santoro F, leva R, Spennati G, et al. Tako-Tsubo cardiomyopathy in a teen girl with pheochromocytoma. Int J Cardiol 2012; 160:e48–e49. [DOI] [PubMed] [Google Scholar]
- 94.Shoji T, Takatori E, Oyama R, et al. Tako-Tsubo cardiomyopathy caused immediately following cesarean section delivery of triplets: a case report. Gynecol Obstet Invest 2012; 74:84–88. [DOI] [PubMed] [Google Scholar]
- 95.Chung H, Kwon SW, Kim TH, et al. Synephrine-containing dietary supplement precipitating apical ballooning syndrome in a young female. Korean J Intern Med 2013; 28:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bridgman PG. Late presentation of a cardiomyopathy provoked by sudden emotional stress. Heart Lung Circ 2006; 15:383–385. [DOI] [PubMed] [Google Scholar]
- 97.Tiong K. Irukandji syndrome, catecholamines, and mid-ventricular stress cardiomyopathy. Eur J Echocardiogr 2009; 10:334–336. [DOI] [PubMed] [Google Scholar]
- 98.Manzanal A, Ruiz L, Madrazo J, et al. Inverted takotsubo cardiomyopathy. J Invasive Cardiol 2011; 23:E76–E78. [PubMed] [Google Scholar]
- 99.Litvinov IV, Kotowycz MA, Wassmann S, et al. Iatrogenic epinephrine-induced reverse Takotsubo cardiomyopathy: direct evidence supporting the role of catecholamines in the pathophysiology of the “broken heart syndrome”. Clin Res Cardiol 2009; 98:457–462. [DOI] [PubMed] [Google Scholar]
- 100.Riera M, Llompart-Pou JA, Carrillo A, et al. Head injury and inverted Takotsubo cardiomyopathy. J Trauma 2010; 68:E13–E15. [DOI] [PubMed] [Google Scholar]
- 101.Nojima Y, Kotani J-i. Global coronary artery spasm caused takotsubo cardiomyopathy. J Am Coll Cardiol 2010; 55:e17. [DOI] [PubMed] [Google Scholar]
- 102.Redfors B, Shao Y, Omerovic E. Fatal stress-induced cardiomyopathy in a young patient treated with adrenomimetics. Clin Res Cardiol 2012; 101:939–940. [DOI] [PubMed] [Google Scholar]
- 103.Cvorovic V, Stankovic I, Panic M, et al. Establishing the diagnosis of inverted stress cardiomyopathy in a patient with cardiac arrest during general anesthesia: a potential role of myocardial strain. Echocardiography 2013; 30:E161–E163. [DOI] [PubMed] [Google Scholar]
- 104.Higuchi H, Maeda S, Miyawaki T, et al. Dental management of a patient with takotsubo cardiomyopathy: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103:e26–e29. [DOI] [PubMed] [Google Scholar]
- 105.Panneerselvam A, Krishnamurthy AH, Bhat P, et al. Delayed contrast enhancement in MRI in takotsubo cardiomyopathy. BMJ Case Rep 2011; 2011: pii: bcr0620114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maréchaux S, Goldstein P, Girardie P, et al. Contractile pattern of inverted Takotsubo cardiomyopathy: illustration by two-dimensional strain. Eur J Echocardiogr 2009; 10:332–333. [DOI] [PubMed] [Google Scholar]
- 107.Mitchell JH, Hadden TB, Wilson JM, et al. Clinical features and usefulness of cardiac magnetic resonance imaging in assessing myocardial viability and prognosis in Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome). Am J Cardiol 2007; 100:296–301. [DOI] [PubMed] [Google Scholar]
- 108.Fabi M, Testa G, Gesuete V, et al. An unusual cardiomyopathy after physical stress in a child. Congenit Heart Dis 2013; 8:E45–E48. [DOI] [PubMed] [Google Scholar]
- 109.Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev 2005; 10:311–316. [DOI] [PubMed] [Google Scholar]
- 110.Strachinaru M, Tran-Ngoc E, Damry N, et al. An atypical evolution of tako-tsubo cardiomyopathy. Acta Cardiol 2013; 68:417–420. [DOI] [PubMed] [Google Scholar]
- 111.Redfors B, Shao Y, Omerovic E. Fatal stress-induced cardiomyopathy in a young patient treated with adrenomimetics. Clin Res Cardiol 2012; 101:939–940. [DOI] [PubMed] [Google Scholar]
- 112.Reimann M, Lohmann T, Ziemssen T. Stress-cardiomyopathy—a psychological phenomenon? MMW Fortschr Med 2013; 155:41–43. [DOI] [PubMed] [Google Scholar]
- 113.Redfors B, Shao Y, Omerovic E. Stress-induced cardiomyopathy (Takotsubo)—broken heart and mind. Vasc Health Risk Manag 2013; 9:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 2009; 162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Szardien S, Mollmann H, Willmer M, et al. Mechanisms of stress (takotsubo) cardiomyopathy. Heart Fail Clin 2013; 9:197–205. [DOI] [PubMed] [Google Scholar]
- 116.Kuo BT, Choubey R, Novaro GM. Reduced estrogen in menopause may predispose women to takotsubo cardiomyopathy. Gend Med 2010; 7:71–77. [DOI] [PubMed] [Google Scholar]
- 117.Ueyama T, Kasamatsu K, Hano T, et al. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann N Y Acad Sci 2008; 1148:479–485. [DOI] [PubMed] [Google Scholar]
- 118.Bell JR, Bernasochi GB, Varma U, et al. Sex and sex hormones in cardiac stress—mechanistic insights. J Steroid Biochem Mol Biol 2013; 137:124–135. [DOI] [PubMed] [Google Scholar]
- 119.Golden KL, Marsh JD, Jiang Y, et al. Acute actions of testosterone on contractile function of isolated rat ventricular myocytes. Eur J Endocrinol 2005; 152:479–483. [DOI] [PubMed] [Google Scholar]
- 120.Mellor KM, Curl CL, Chandramouli C, et al. Ageing-related cardiomyocyte functional decline is sex and angiotensin II dependent. Age (Dordr) 2014; 36:9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelly MM, Tyrka AR, Anderson GM, et al. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry 2008; 39:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 2010; 62:155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sandanger I, Nygard JF, Sorensen T, et al. Is women's mental health more susceptible than men's to the influence of surrounding stress. Soc Psychiatry Psychiatr Epidemiol 2004; 39:177–184. [DOI] [PubMed] [Google Scholar]
- 124.Palanza P. Animal models of anxiety and depression: how are females different. Neurosci Biobehav Rev 2001; 25:219–233. [DOI] [PubMed] [Google Scholar]
- 125.Faverjon S, Silveira DC, Fu DD, et al. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology 2002; 59:1356–1364. [DOI] [PubMed] [Google Scholar]
- 126.Napoli AM, Choo EK, Dai J, et al. Racial disparities in stress test utilization in an emergency department chest pain unit. Crit Pathw Cardiol 2013; 12:9–13. [DOI] [PubMed] [Google Scholar]
- 127.Williams D, Lawler KA. Stress and illness in low-income women: the roles of hardiness, John Henryism, and race. Women Health 2001; 32:61–75. [DOI] [PubMed] [Google Scholar]
- 128.Kawai S, Kitabatake A, Tomoike H. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J 2007; 71:990–992. [DOI] [PubMed] [Google Scholar]
- 129.Bietry R, Reyentovich A, Katz SD. Clinical management of takotsubo cardiomyopathy. Heart Fail Clin 2013; 9:177–186. [DOI] [PubMed] [Google Scholar]
- 130.Fazio G, Pizzuto C, Barbaro G, et al. Chronic pharmacological treatment in takotsubo cardiomyopathy. Int J Cardiol 2008; 127:121–123. [DOI] [PubMed] [Google Scholar]
- 131.Madhavan M, Rihal CS, Lerman A, et al. Acute heart failure in apical ballooning syndrome (TakoTsubo/stress cardiomyopathy) clinical correlates and Mayo Clinic risk score. J Am Coll Cardiol 2011; 57:1400–1401. [DOI] [PubMed] [Google Scholar]
- 132.Song BG, Hahn JY, Cho SJ, et al. Clinical characteristics, ballooning pattern, and long-term prognosis of transient left ventricular ballooning syndrome. Heart Lung 2010; 39:188–195. [DOI] [PubMed] [Google Scholar]
- 133.Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J 2012; 164:215–221. [DOI] [PubMed] [Google Scholar]
- 134.Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 2012; 126:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008; 5:22–29. [DOI] [PubMed] [Google Scholar]


