Abstract
Spinal canal stenosis is a dynamic phenomenon that becomes apparent during spinal loading. Current diagnostic procedures have considerable short comings in diagnosing the disease to full extend, as they are performed in supine situation. Upright MRI imaging might overcome this diagnostic gap.
This study investigated the lumbar neuroforamenal diameter, spinal canal diameter, vertebral body translation, and vertebral body angles in 3 different body positions using upright MRI imaging.
Fifteen subjects were enrolled in this study. A dynamic MRI in 3 different body positions (at 0° supine, 80° upright, and 80° upright + hyperlordosis posture) was taken using a 0.25 T open-configuration scanner equipped with a rotatable examination bed allowing a true standing MRI.
The mean diameter of the neuroforamen at L5/S1 in 0° position was 8.4 mm on the right and 8.8 mm on the left, in 80° position 7.3 mm on the right and 7.2 mm on the left, and in 80° position with hyperlordosis 6.6 mm (P < 0.05) on the right and 6.1 mm on the left (P < 0.001).
The mean area of the neuroforamen at L5/S1 in 0° position was 103.5 mm2 on the right and 105.0 mm2 on the left, in 80° position 92.5 mm2 on the right and 94.8 mm2 on the left, and in 80° position with hyperlordosis 81.9 mm2 on the right and 90.2 mm2 on the left.
The mean volume of the spinal canal at the L5/S1 level in 0° position was 9770 mm3, in 80° position 10600 mm3, and in 80° position with hyperlordosis 9414 mm3.
The mean intervertebral translation at level L5/S1 was 8.3 mm in 0° position, 9.9 mm in 80° position, and 10.1 mm in the 80° position with hyperlordosis.
The lordosis angle at level L5/S1 was 49.4° in 0° position, 55.8° in 80° position, and 64.7 mm in the 80° position with hyperlordosis.
Spinal canal stenosis is subject to a dynamic process, that can be displayed in upright MRI imaging. The range of anomalies is clinically relevant and dynamic positioning of the patient during MRI can provide essential diagnostic information which are not attainable with other methods.
INTRODUCTION
Degenerative spondylolisthesis is a spinal pathology frequently diagnosed in the elderly.1 The progressive loss of height in a motion segment coupled with subluxation of the facet joints leads to changes in biomechanical forces and hypertrophy of the ligamentum flavum and spondylophyte formation around the facet joints. Together the combination of a loss of height in the intervertebral space and the apposition of osseous and ligamentary structures result in progressive compression of the nerves passing through affected canals. This compression can be exacerbated by bulging-disc syndrome and spondylophytes in the vertebral endplates. The clinical symptoms are low back and radicular lower-limb pain and intermittent spinal claudication. This cascade of anatomical and pathological anomalies most frequently occur in the lower lumbar region, especially segments L3/4, L4/5, and L5/S1.2,3 If conservative therapy fails, operative treatment, that is, segment decompression has to be considered. However, a satisfactory surgical outcome requires the indication for a specific surgical intervention preceded by differentiated diagnostics and accurate identification of the motion segment in question. Today, diagnosis of neuroforaminal stenosis or spinal stenosis relies on radiologic upright imaging on 2 levels as well as magnetic resonance tomography (MRI). As an alternative to MRI, computed tomography (CT) in combination with a myelography can be performed, however, due to its invasiveness and radiation exposure, MRI has become the diagnostic gold standard.4,5 CT myelography however is still performed in patients unable to undergo MRI. A drawback of conventional MRI imaging is the fact that images are taken in supine position and can therefore not directly display aforementioned dynamic anomalies.6,7 The dynamics in spinal-column width in patients suffering spinal canal stenosis while moving from a supine to a standing position were illustrated by Zander and Lander.8 This phenomenon correlates well with clinical findings in these patients, as they frequently report decreased pain, when changing from standing to supine position. However, gravity's effect on neuroforamen in the presence of degenerative spondylolysis in the lower lumbar region has attracted little attention, so far. Many radiologic images (both CT and MRI) of patients suffering from stenosis of the spinal canal fail to reveal any pathology.9,10 This fact underlines the need for additional diagnostic procedures, that enable us to visualize these effects. The possibility of dynamic radiological examinations with the upright MRI can close this diagnostic gap. However, data revealing dynamic anatomic changes in response to a load are scarce. Previous investigations of spinal canal imaging under a load are mostly cadaver studies.11,12 There is a paucity of in vivo data on structural changes in the lumbar spine, and those that do exist only address specific anatomic structures. Just one study has examined dynamic changes in the spinal canal.13
The aim of this study was therefore the radiological evaluation of the diameter of the lumbar neuroforamen, the diameter of the spinal canal, the translation of the vertebral bodies, and the angle of the vertebral bodies, that is, of L5/S1 in 3 different positions.
MATERIALS AND METHODS
Fifteen subjects with chronic back pain were enrolled in this study. All were scheduled for transforaminal lumbar interbody fusion (TLIF) L5/S1 after having been diagnosed with degenerative spondylolisthesis L5/S1 via conventional X-ray, segment infiltration, and probatory corset. Exclusion criteria were previous surgical interventions and any malignancies.
A dynamic MRI in 3 different body positions (at 0° supine, 80° upright, and 80° upright + hyperlordosis posture) was taken using a 0.25 T open-configuration scanner (G-Scan, Esaote, Genoa, Italy) equipped with a rotatable examination bed allowing a true standing MRI (Figure 1A and B). Gradient supports ±20 mT/m with a slew rate of 25 mT/m/ms. Phased array dedicated receiving coils were used. Sagittal MR examinations included a 2D FSE T2 sequence (TR = 3350 ms, TE = 120 ms, FOV = 310 × 310 mm2, M = 224 × 208, TH = 4 mm, TA = 5′28″) and a 3D HYCE (balanced steady state sequence, TR = 10 ms, TE = 5 ms, FOV = 290 × 290 × 100 mm3, M = 232 × 206 × 28, TA = 5′29″).
FIGURE 1.
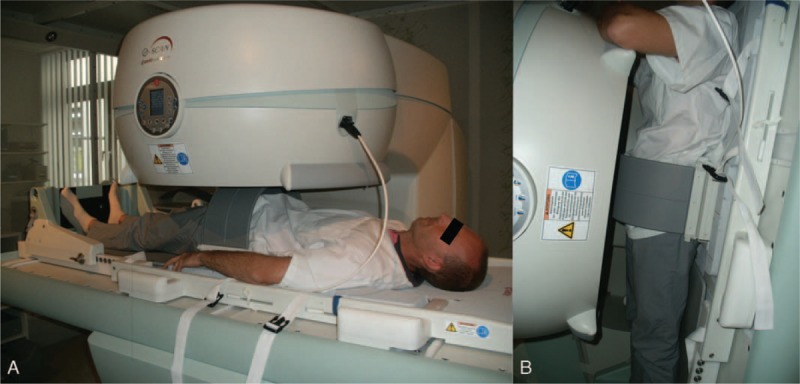
(A + B) Dynamic MRI in supine (0°) and weight bearing (80°) positions with a volunteer.
Images taken during these different positions were quantitatively evaluated by measuring the diameter of the neuroforamen and that of the spinal canal at the level of L5/S1.
The Medical Image Viewer Impax, Agfa HealthCare was used for standardized reorientation of the images (Figure 2). This was done to avoid measurement errors due to partial volume effects caused by differences in the patients’ positioning.
FIGURE 2.
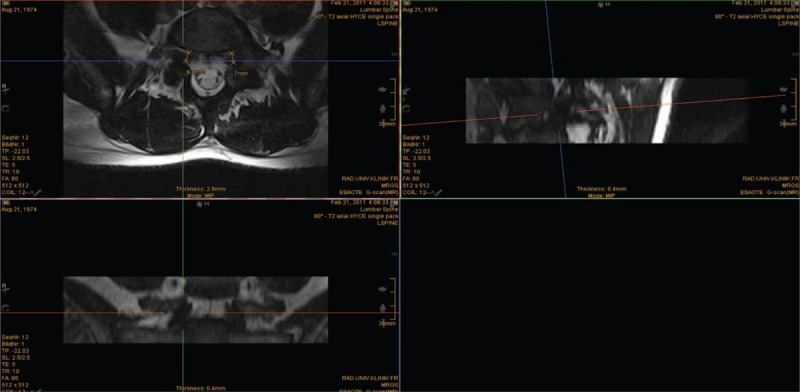
Measurement of the neuroforaminal diameter L5/S1 in weight bearing (80°) position with standardized reorientation of the images.
In the sagittal view, the slice was oriented along the ground plate of L5. In the axial view the slice was oriented alongside the middle of the vertebral body and spinal process. Comparative measurements of the spinal canal volume, spinal canal diameter, the neuroforaminal diameter and its area were taken in all the prescribed patient positions.
Moreover, intervertebral body translation and the lordosis angle were calculated (Figure 3).
FIGURE 3.
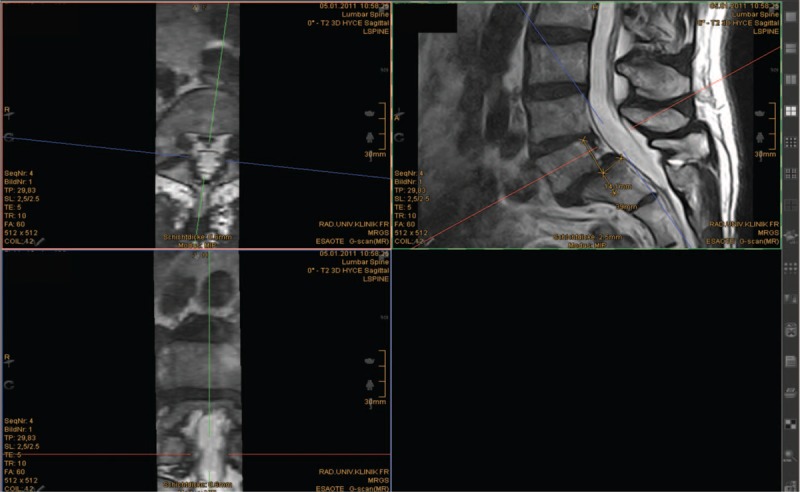
Measurement of the intervertebral body translation L5/S1 in supine (0°) position with standardized reorientation of the images.
Statistical Evaluation
For statistical evaluation we employed SPSS for Mac (version 22, SPSS, Inc., Chicago, Il). An one-way analysis of variance14 was used to assess statistical differences. The post hoc Bonferroni test was used to identify differences between each position. The level of significance was set at P < 0.05.
RESULTS
The mean diameter of the neuroforamen at L5/S1 in 0° position was 8.4 ± 1.6 mm on the right and 8.8 ± 1.8 mm on the left, in 80° position 7.3 ± 1.4 mm on the right and 7.2 ± 1.8 mm on the left, and in 80° position with hyperlordosis 6.6 ± 1.5 mm (P < 0.05) on the right and 6.1 ± 1.6 mm on the left (P < 0.001) (Figure 4A + B).
FIGURE 4.
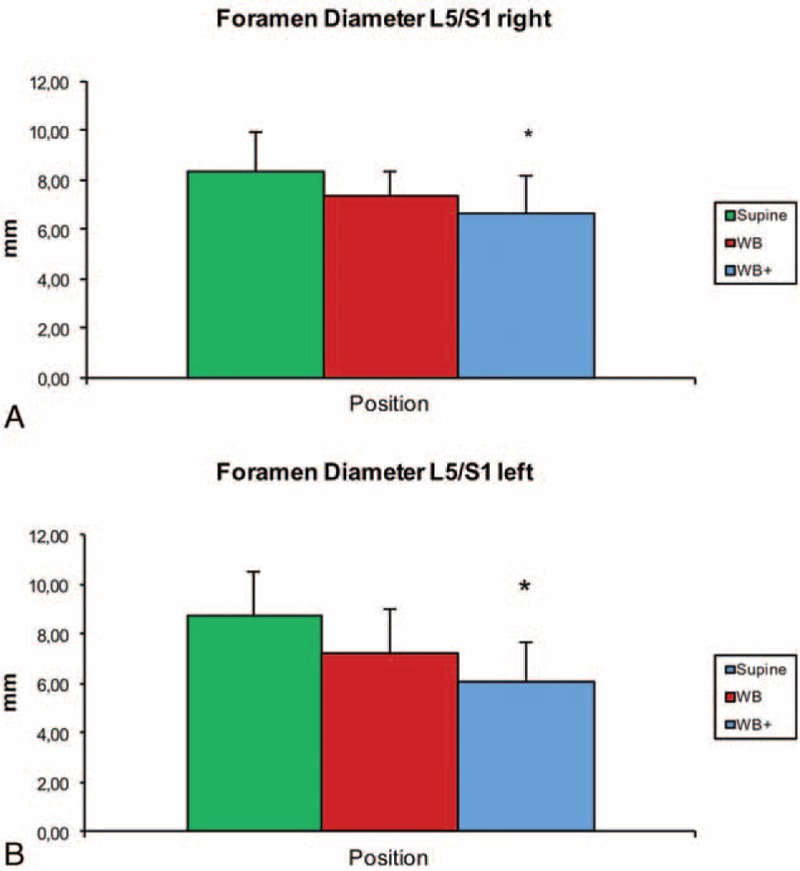
(A + B) Mean diameter and standard deviation (error bar) of the neuroforamen at L5/S1 in supine (0°), weight bearing (WB) and hyperlordosis (WB+) position (∗P < 0.005).
The mean area of the neuroforamen at L5/S1 in 0° position was 103.5 ± 22.2 mm2 on the right and 105.0 ± 25.8 mm2 on the left, in 80° position 92.5 ± 27.9 mm2 on the right and 94.8 ± 38.1 mm2 on the left, and in 80° position with hyperlordosis 81.9 ± 28.5 mm2 on the right and 90.2 ± 31.1 mm2 on the left.
The mean volume of the spinal canal at the L5/S1 level in 0° position was 9770 ± 3900 mm3, in 80° position 10600 ± 3900 mm3, and in 80° position with hyperlordosis 9414 ± 4700 mm3.
The mean intervertebral translation at level L5/S1 was 8.3 ± 4.4 mm in 0° position, 9.9 ± 4.9 mm in 80° position and 10.1 ± 5.1 mm in the 80° position with hyperlordosis.
The lordosis angle at level L5/S1 was 49.4 ± 11.3° in 0° position, 55.8 ± 13.3° in 80° position, and 64.7 mm ± 16.3° in the 80° position with hyperlordosis.
DISCUSSION
The main findings of the present study are that significant narrowing of the neuroforaminal diameter develops during the change from a lying to standing position or to a standing position with hyperlordosis. This effect can be displayed in a upright MRI and should be included into diagnostics of chronic lower back pain.
Segmental instability in the spinal column is defined as an anterior–posterior translation of the opposing vertebrae by more than 3 mm,15,16 whereby a translation exceeding 3 mm is considered pathological and is associated with lower back pain and sciatica.17 Patients suffering from a degenerative spinal disease such as spondylolisthesis complain of worsening pain when standing for a long time or carrying a load; this is because of a worsening of an existing spondylolisthesis in an upright position. Nevertheless, the imaging procedures performed to visualize a known spinal anomaly such as MRI or CT are usually carried out with the patient in supine position. As lumbar lordosis is reduced in supine position, the patient's lumbar pain also tends to lessen. Our data confirm the surveillance that lumbar lordosis diminishes when moving from a standing to lying position. Radiologic imaging of the spinal column in an upright position enables functional assessment under an axial load.18,19 Here too, our data confirm the assumption that an increasing axial load on the spinal column leads to a reduction in spinal canal volume and to progressive listhesis of the lumbar vertebrae. We were surprised to observe that changing from a supine position to an 80° upright stance triggered an initial expansion in the spinal canal at the L5/S1 level. A repeated change in position to an 80° upright stance in conjunction with lumbar hypomochlion, however, led to a reduction in spinal canal volume. This reveals that the pain experienced by patients with hyperlordotic spondylolisthesis can be aggravated by an increasing load on the facet joints, as well as by a worsening 360° restriction of the spinal canal.
Depending on its stage and the amount of pain it causes, treatment for spondylolisthesis can involve surgery. Bony decompression is clinical routine nowadays in patients suffering from a spinal canal stenosis caused by spondylolisthesis—an intervention designed to expand spinal canal volume. However, additional reposition of the vertebrae remains highly controversial.20,21 A randomized, double-blind trial by Audat et al20 examining clinically symptomatic patients suffering from Meyerding stage 1 and 2 spondylolisthesis demonstrated that the outcome of patients who had undergone fixation versus decompression in the affected motion segment were nearly identical, whether the sliding vertebrae had been repositioned or not.
Due to the more beneficial biomechanical relationships within the spinal motion segment created thereby, other authors recommend repositioning.22,23 As a study comparing the sagittal alignment of the spinal column in patients presenting spondylolysis and spondylolisthesis with a normal control group's sagittal alignment revealed, patients with spondylolysis and spondylolisthesis present exacerbated lumbar lordosis, which in turn alters the biomechanics in the spinal column and facet joints.22 Spondylolisthesis thus correlates negatively with spinal canal volume. Theoretically speaking, repositioning the sliding vertebrae in conjunction with spinal-cord decompression should create expansion in the spinal cord.
Considering other spinal column structures and their behavior in association with degenerative diseases, several experimental cadaver studies employing MRI and CT come to mind. These specifically examined the effect of a load on spinal-column structures and the related anatomical changes in the neuroforamen. These in vitro cadaver investigations demonstrated in lumbar spinal-column segments that a load on the spinal column caused by axial rotation, extension, or lateral deflection leads to the constriction of neural pathways that does not occur when there is no load.24–28 Our data confirm this observation. This effect also applies to the diameter of the neuroforamen and its corresponding volume. Also apparent is that the effect of increasing constriction in the neuroforamen is much worse at L4/5 than at L5/S1 when changing from a supine to standing position.
Our investigation has demonstrated that the change from a supine to an 80° upright position results in worsening lumbar lordosis, which in turn exacerbates lumbar hypomochlion. A certain degree of lumbar lordosis is needed, as it enables impacts affecting the discs to be absorbed and deflected. Specifically, the effect of lordosis is that it creates increased pressure on the dorsal parts of the disc and thus to ventral displacement of gel-like elements such as the nucleus pulposus, thereby preventing the disc from slipping in a dorsal direction.27,28
Considering the spinal column's dorsal structures, it again becomes clear how relevant the change in position from a supine to upright position is. The shear forces occurring in an upright position are absorbed primarily by the facet joints,29 whereas the facet joints absorb just 16% of the compression forces affecting the spinal column.30
Disc degeneration and the discs’ accompanying loss of height can raise the facet joints’ compression load up to 70%, eventually causing the facet joints to degenerate and leading to facet-joint arthrosis, which in turn plays a major role in constricting the adjacent neuroforamen.
LIMITATIONS
This study provides an investigation of the dynamic structures of the lumbar spine in 3 different body positions. In our point of view, the gathered information is essential to understand and evaluate the pathogenesis of degenerative diseases of the spine. Nevertheless, some limitations need to be recognized; at first, the number of investigated subjects is limited. However, the present study size is in line with previously reported investigations.13,31,32 Moreover, we could demonstrate significant changes in the neuroforaminal diameter that allow to draw conclusions conclude to general public of. However, further investigations on intervertebral translation and the impact on positioning on the spinal canal volume should be carried out in a bigger population. Moreover, upright MRI was carried out in 80° not in 90°. The quasi full up-right position was chosen as the overlordosis position was not possible in the full 90° upright position as we wanted the patients to stand naturally and not to be binded against the patients bed of the scanner. Last but not least, investigations were carried out at low field. Therefore spatial resolution is lower compared to the high-field environment. However, weight-bearing MRI of the spine is so far not possible at high-field. Moreover several studies could show that with careful adjustment of slice orientation and sequence design high accuracy during functional MRI of the musculoskeletal system can be achieved.33–35
CONCLUSION
Pathologies of the spinal column like spinal canal stenosis or (degenerative) spondylolisthesis are subject to dynamic processes that can be displayed in upright MRI by positioning the patient dynamically. The range of anomalies is clinically relevant, and dynamic positioning of the patient during upright MRI can provide essential diagnostic information to the physician that is not attainable with other methods. We consider upright MRI to be superior to supine MRI for diagnosing degenerative spinal cord diseases.
Acknowledgement
The article processing charge was funded by the German Research Foundation (DFG) and the Albert Ludwigs University Freiburg in the funding programme Open Access Publishing.
Footnotes
Abbreviations: CT = computed tomography, MRI = magnetic resonance tomography.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Iguchi T, Wakami T, Kurihara A, et al. Lumbar multilevel degenerative spondylolisthesis: radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech 2002; 15:93–99. [DOI] [PubMed] [Google Scholar]
- 2.Epstein NE, Epstein JA, Carras R, et al. Far lateral lumbar disc herniations and associated structural abnormalities. An evaluation in 60 patients of the comparative value of CT, MRI, and myelo-CT in diagnosis and management. Spine (Phila Pa 1976) 1990; 15:534–539. [DOI] [PubMed] [Google Scholar]
- 3.Jenis LG, An HS, Gordin R. Foraminal stenosis of the lumbar spine: a review of 65 surgical cases. Am J Orthop 2001; 30:205–211. [PubMed] [Google Scholar]
- 4.Kent DL, Haynor DR, Longstreth WT, Jr, et al. The clinical efficacy of magnetic resonance imaging in neuroimaging. Ann Intern Med 1994; 120:856–871. [DOI] [PubMed] [Google Scholar]
- 5.Maus T. Imaging the back pain patient. Phys Med Rehabi Clin N Am 2010; 21:725–766. [DOI] [PubMed] [Google Scholar]
- 6.Amundsen T, Weber H, Lilleas F, et al. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976) 1995; 20:1178–1186. [DOI] [PubMed] [Google Scholar]
- 7.Benoist M. The natural history of lumbar degenerative spinal stenosis. Joint Bone Spine 2002; 69:450–457. [DOI] [PubMed] [Google Scholar]
- 8.Zander DR, Lander PH. Positionally dependent spinal stenosis: correlation of upright flexion-extension myelography and computed tomographic myelography. Can Assoc Radiol J 1998; 49:256–261. [PubMed] [Google Scholar]
- 9.Geisser ME, Haig AJ, Tong HC, et al. Spinal canal size and clinical symptoms among persons diagnosed with lumbar spinal stenosis. Clin J Pain 2007; 23:780–785. [DOI] [PubMed] [Google Scholar]
- 10.Zeifang F, Schiltenwolf M, Abel R, et al. Gait analysis does not correlate with clinical and MR imaging parameters in patients with symptomatic lumbar spinal stenosis. BMC Musculoskelet Disord 2008; 9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am 1995; 77:32–38. [PubMed] [Google Scholar]
- 12.Mayoux-Benhamou MA, Revel M, Aaron C, et al. A morphometric study of the lumbar foramen. Influence of flexion-extension movements and of isolated disc collapse. Surg Radiol Anat 1989; 11:97–102. [DOI] [PubMed] [Google Scholar]
- 13.Miao J, Wang S, Park WM, et al. Segmental spinal canal volume in patients with degenerative spondylolisthesis. Spine J 2013; 13:706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiebaut M, Kisseleva-Romanova E, Rougemaille M, et al. Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol Cell 2006; 23:853–864. [DOI] [PubMed] [Google Scholar]
- 15.Kauppila LI, Eustace S, Kiel DP, et al. Degenerative displacement of lumbar vertebrae. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976) 1998; 23:1868–1873.discussion 1864–1873. [DOI] [PubMed] [Google Scholar]
- 16.Vogt MT, Rubin D, Valentin RS, et al. Lumbar olisthesis and lower back symptoms in elderly white women. The Study of Osteoporotic Fractures. Spine (Phila Pa 1976) 1998; 23:2640–2647. [DOI] [PubMed] [Google Scholar]
- 17.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res 1982; 110–123. [PubMed] [Google Scholar]
- 18.Jinkins JR, Dworkin JS, Damadian RV. Upright, weight-bearing, dynamic-kinetic MRI of the spine: initial results. Eur Radiol 2005; 15:1815–1825. [DOI] [PubMed] [Google Scholar]
- 19.Wildermuth S, Zanetti M, Duewell S, et al. Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology 1998; 207:391–398. [DOI] [PubMed] [Google Scholar]
- 20.Audat ZM, Darwish FT, Al Barbarawi MM, et al. Surgical management of low grade isthmic spondylolisthesis; a randomized controlled study of the surgical fixation with and without reduction. Scoliosis 2011; 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehdian SM, Arun R, Jones A, et al. Reduction of severe adolescent isthmic spondylolisthesis: a new technique. Spine (Phila Pa 1976) 2005; 30:E579–E584. [DOI] [PubMed] [Google Scholar]
- 22.Roussouly P, Gollogly S, Berthonnaud E, et al. Sagittal alignment of the spine and pelvis in the presence of L5-s1 isthmic lysis and low-grade spondylolisthesis. Spine (Phila Pa 1976) 2006; 31:2484–2490. [DOI] [PubMed] [Google Scholar]
- 23.Labelle H, Roussouly P, Chopin D, et al. Spino-pelvic alignment after surgical correction for developmental spondylolisthesis. Eur Spine J 2008; 17:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonstrom N, Lindahl S, Willen J, et al. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res 1989; 7:115–121. [DOI] [PubMed] [Google Scholar]
- 25.Ferreiro Perez A, Garcia Isidro M, Ayerbe E, et al. Evaluation of intervertebral disc herniation and hypermobile intersegmental instability in symptomatic adult patients undergoing recumbent and upright MRI of the cervical or lumbosacral spines. Eur J Radiol 2007; 62:444–448. [DOI] [PubMed] [Google Scholar]
- 26.Alyas F, Sutcliffe J, Connell D, et al. Morphological change and development of high-intensity zones in the lumbar spine from neutral to extension positioning during upright MRI. Clin Radiol 2010; 65:176–180. [DOI] [PubMed] [Google Scholar]
- 27.Haughton VM, Lim TH, An H. Intervertebral disk appearance correlated with stiffness of lumbar spinal motion segments. AJNR Am J Neuroradiol 1999; 20:1161–1165. [PMC free article] [PubMed] [Google Scholar]
- 28.Weishaupt D, Schmid MR, Zanetti M, et al. Positional MR imaging of the lumbar spine: does it demonstrate nerve root compromise not visible at conventional MR imaging? Radiology 2000; 215:247–253. [DOI] [PubMed] [Google Scholar]
- 29.Hutton WC, Toribatake Y, Elmer WA, et al. The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine (Phila Pa 1976) 1998; 23:2524–2537. [DOI] [PubMed] [Google Scholar]
- 30.Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br 1980; 62:358–362. [DOI] [PubMed] [Google Scholar]
- 31.Shymon S, Hargens AR, Minkoff LA, et al. Body posture and backpack loading: an upright magnetic resonance imaging study of the adult lumbar spine. Eur Spine J 2014; 23:1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong W, Driscoll SJ, Tsai TY, et al. In vivo dynamic changes of dimensions in the lumbar intervertebral foramen. Spine J 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauch F, Jung C, Huth J, et al. Changes in the lumbar spine of athletes from supine to the true-standing position in magnetic resonance imaging. Spine (Phila Pa 1976) 2010; 35:1002–1007. [DOI] [PubMed] [Google Scholar]
- 34.Izadpanah K, Weitzel E, Vicari M, et al. Influence of knee flexion angle and weight bearing on the Tibial Tuberosity-Trochlear Groove (TTTG) distance for evaluation of patellofemoral alignment. Knee Surg Sports Traumatol Arthrosc 2014; 22:2655–2661. [DOI] [PubMed] [Google Scholar]
- 35.Izadpanah K, Winterer J, Vicari M, et al. A stress MRI of the shoulder for evaluation of ligamentous stabilizers in acute and chronic acromioclavicular joint instabilities. J Magn Reson Imaging 2013; 37:1486–1492. [DOI] [PubMed] [Google Scholar]


