Abstract
The purpose of this study was to explore the association between zolpidem use and pyogenic liver abscess in Taiwan.
This was a population-based case-control study using the database of the Taiwan National Health Insurance Program since 2000 to 2011. We identified 1325 patients aged 20 to 84 years with the first-attack of pyogenic liver abscess as the cases, and 5082 patients without pyogenic liver abscess matched with sex, age, comorbidities, and index year of hospitalization for pyogenic liver abscess as the controls. Patients whose last remaining 1 tablet for zolpidem was noted ≤7 days before the date of admission for pyogenic liver abscess were defined as current use of zolpidem. Patients whose last remaining 1 tablet for zolpidem was noted >7 days before the date of admission for pyogenic liver abscess were defined as late use of zolpidem. Patients who never received 1 prescription for zolpidem were defined as never use of zolpidem. A multivariable unconditional logistic regression model was used to measure the odds ratio (OR) and 95% confidence interval (CI) to explore the association between zolpidem use and pyogenic liver abscess.
After adjustment for possible confounding variables, the adjusted OR of pyogenic liver abscess was 3.89 for patients with current use of zolpidem (95% CI 2.89, 5.23), when compared with those with never use of zolpidem. The adjusted OR decreased to 0.85 for those with late use of zolpidem (95% CI 0.70, 1.03), but without statistical significance.
Current use of zolpidem is associated with the increased risk of pyogenic liver abscess. Physicians should take the risk of pyogenic liver abscess into account when prescribing zolpidem.
INTRODUCTION
Zolpidem is a nonbenzodiazepine hypnotic agent, which is mainly indicated for the treatment of insomnia. Initially, zolpidem was widely prescribed by physicians because of its favorable pharmacological properties according to the manufacturer's prescribing information, including rapid onset of action, reduction of sleep latency, increase of total sleep duration, reduction of nighttime awakening, few next-day effects on daytime well-being and morning coordination, and almost devoid of abuse and dependence potential.1–5 However, the growing evidence discloses that zolpidem may cause delirium, nightmares, hallucinations, and a significant potential for abuse and dependence.6,7 Some observational studies have disclosed that zolpidem may be associated with comorbidities, including motor vehicle accidents, stroke, hip fracture, glaucoma, and acute pancreatitis.8–12 In addition, 2 observational studies have disclosed that zolpidem may be associated with some infections such as rhinitis, pharyngitis, bronchitis, and pneumonia.13,14 In spite of no direct evidence showing the mechanism of zolpidem effect on infections, hypnotic-related immune suppression,15,16 hypnotic-related impairment of clearance of oral secretion during sleep,17 and hypnotic-related gastroesophageal reflux during sleep and further resulting aspiration18,19 can partially explain the mechanism of some infections such as upper airway infections. However, the association between zolpidem use and pyogenic liver abscess has never been studied.
Pyogenic liver abscess is an infective condition of the liver, which is mainly caused by a wide variety of infective agents. Its clinical course might range from totally cured to severe and life-threatening. The mortality rate of pyogenic liver abscess can be up to 8.2% in Chen et al's20 observational study using the database of the Taiwan National Health Insurance Program. To date, a number of well-documented risk factors including biliary tract disease, cirrhosis, diabetes mellitus, and renal disease are found to be associated with pyogenic liver abscess,21–23 but zolpidem-related pyogenic liver abscess has not yet been studied.
Based on the aforementioned review, we make a plausible hypothesis that there could be a link between zolpidem use and pyogenic liver abscess because of zolpidem-related infections. If the link really exists, physicians should consider the risk of pyogenic liver abscess among zolpidem users. Given that zolpidem is the most widely prescribed nonbenzodiazepine hypnotic agent in Taiwan,24,25 we conducted a case-control study using the database of the Taiwan National Health Insurance Program to explore the association between zolpidem use and pyogenic liver abscess.
METHODS
Design and Study Population
This was a population-based case-control study using the database of the Taiwan National Health Insurance Program. This program began in March 1995, which covered 99% of the total 23 million people living in Taiwan.26 The diagnosis of disease is based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 codes). The database is available for public access.
The number of patient identification has been scrambled to ensure confidentiality. The database includes personal socio-demographic status, such as sex and date of birth, and information on ambulatory care, inpatient care, emergency care, dental care, and prescription drugs. The details of the program were well written in previous high-quality articles.27–30 This study was approved by the Ethics Review Board of China Medical University and Hospital in Taiwan (CMU-REC-101-012).
Participants
We used the inpatient claim dataset to identify patients aged 20 to 84 years with the first-attack of pyogenic liver abscess (ICD-9 code 572.0) during the period of 2000 to 2011 as the case group. The date of discharge with primary diagnosis of pyogenic liver abscess was defined as the index date. For each case of pyogenic liver abscess, 4 control patients aged 20 to 84 years without pyogenic liver abscess were randomly selected from the same database as the control group. Both case and control groups were matched with sex, age (every 5-year span), comorbidities, and index year of hospitalization for pyogenic liver abscess. Patients with prior diagnosis of amebic liver abscess (ICD-9 code 006.3) or liver transplantation (ICD-9 codes 996.82 and V42.7) before the index date were excluded from this study.
Other Medications and Comorbidities
History of prescriptions for benzodiazepines available in Taiwan was included in this study. To decrease biased results, patients who had prescriptions of other nonbenzodiazepine hypnotic agents including zopiclone, zaleplon, and eszopiclone were excluded from this study. Comorbidities potentially related to pyogenic liver abscess before the index date were included as follows: alcohol-related diseases, biliary stone, chronic kidney diseases, diabetes mellitus, as well as chronic liver diseases, including cirrhosis, Hepatitis B, Hepatitis C, and other chronic hepatitis. All comorbidities were diagnosed with ICD-9 codes.
Definition of Zolpidem Exposure
Because zolpidem has a short elimination half-life (approximately 2.1–2.4 hours) with no active metabolite, it does not cause an accumulating effect during repeated use.3,31 Therefore, we used 7 days as a cutoff point. Based on the prescription history, we can estimate the last remaining 1 tablet for zolpidem. Patients whose last remaining 1 tablet for zolpidem was noted ≤7 days before the date of admission for pyogenic liver abscess or those still having zolpidem tablets at the date of admission for pyogenic liver abscess were defined as current use of zolpidem. Patients whose last remaining 1 tablet for zolpidem was noted >7 days before the date of admission for pyogenic liver abscess were defined as late use of zolpidem. Patients who never received 1 prescription for zolpidem were defined as never use of zolpidem.
Statistical Analysis
The distributions of sex, age, zolpidem use, benzodiazepines use, and comorbidities were compared between the case group and the control group using the χ2 test for categorized variables and t test for continuous variables. Variables found significantly related to pyogenic liver abscess in the univariable unconditional logistic regression model were further included in the multivariable unconditional logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were measured to explore the risk of pyogenic liver abscess associated with zolpidem use, benzodiazepines use, and comorbidities. We also explore the association between average daily dose of current use of zolpidem and the risk of pyogenic liver abscess. The average daily dose of zolpidem was calculated by using the total prescribed dose divided by total number of days supplied. Two strengths of zolpidem are available in Taiwan for oral administration as 6.25 mg (extended-release tablet) and 10 mg. Therefore, we used 10 mg as a cutoff point. We classified patients with current use of zolpidem into 2 subgroups: high dose group with average daily dose >10 mg and low dose group with average daily dose ≤10 mg. All data processing and statistical analyses were performed with the SAS software version 9.2 (SAS Institute, Inc, Cary, NC). A 2-tailed P value of <0.05 was considered statistically significant.
RESULTS
Characteristics of the Study Population
Table 1 shows the distributions of sex, age, zolpidem use, benzodiazepines use, and comorbidities between the case group and the control group. This study consisted of 1325 cases with pyogenic liver abscess and 5082 controls without pyogenic liver abscess, with similar distributions of sex and age. The mean age (standard deviation) of the study patients was 59.5 ± 14.5 years for the case group and 59.3 ± 14.5 years for the control group (t test, P = 0.60). The case group had higher proportions of current use of zolpidem (7.4% vs 1.8%), ever use of benzodiazepines (82.0% vs 75.2%), alcohol-related diseases (7.09% vs 5.45%), biliary stone (19.0% vs 13.0%), chronic liver diseases (33.5% vs 29.8%), and diabetes mellitus (45.4% vs. 39.8%) than the control group, with statistical significance.
TABLE 1.
Characteristics Between Pyogenic Liver Abscess Cases and Controls
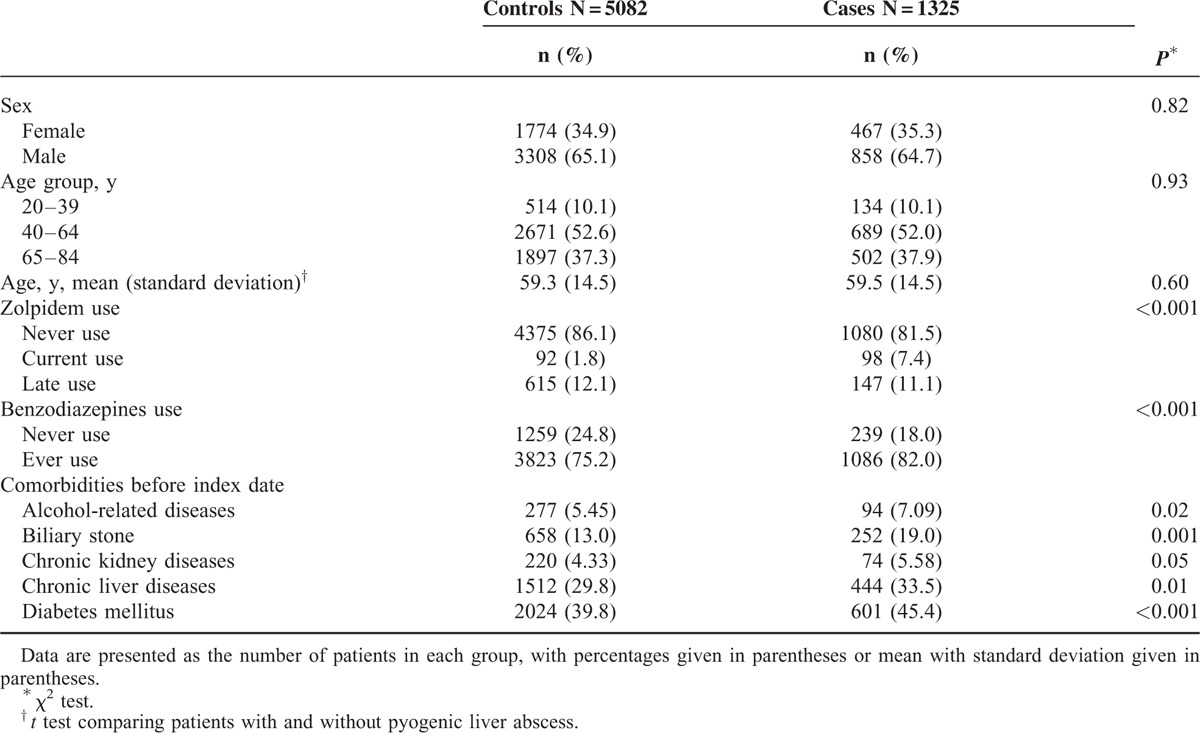
Risk of Pyogenic Liver Abscess Associated With Zolpidem Use and Other Factors
Table 2 shows the risk of pyogenic liver abscess associated with zolpidem use, benzodiazepines use, and other comorbidities. After adjustment for possible confounding factors, the multivariable unconditional logistic regression model disclosed that the adjusted OR of pyogenic liver abscess was 3.89 for patients with current use of zolpidem (95% CI 2.89, 5.23), when compared with those with never use of zolpidem. The adjusted OR decreased to 0.85 for those with late use of zolpidem (95% CI 0.70, 1.03), but without statistical significance. In addition, benzodiazepines use (adjusted OR 1.32, 95% CI 1.13, 1.55), biliary stone (adjusted OR 1.57, 95% CI 1.33, 1.85), and diabetes mellitus (adjusted OR 1.21, 95% CI 1.06, 1.37) were other factors significantly associated with pyogenic liver abscess.
TABLE 2.
OR and 95% CI of Pyogenic Liver Abscess Associated With Zolpidem Use, Benzodiazepines Use, and Other Comorbidities
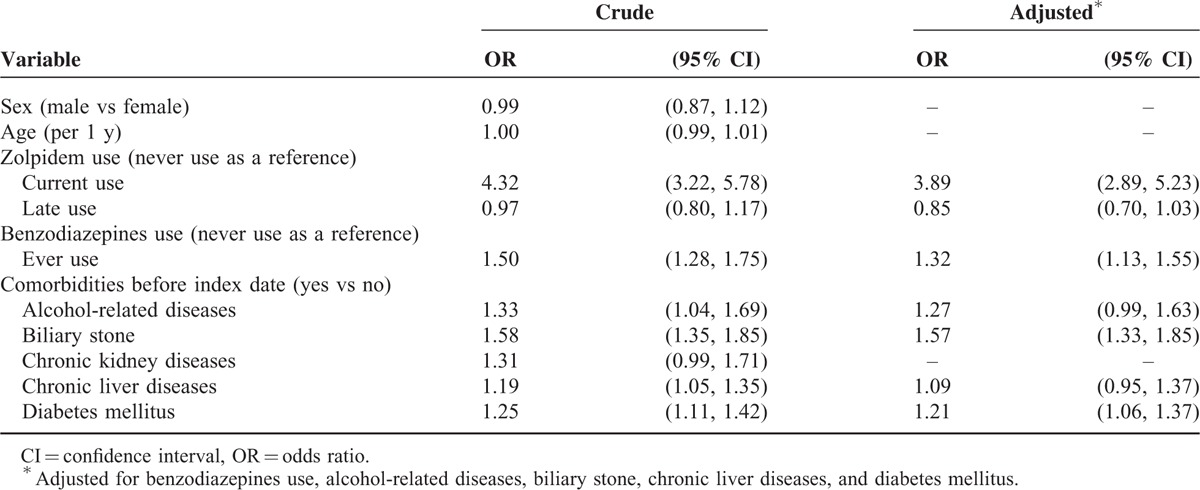
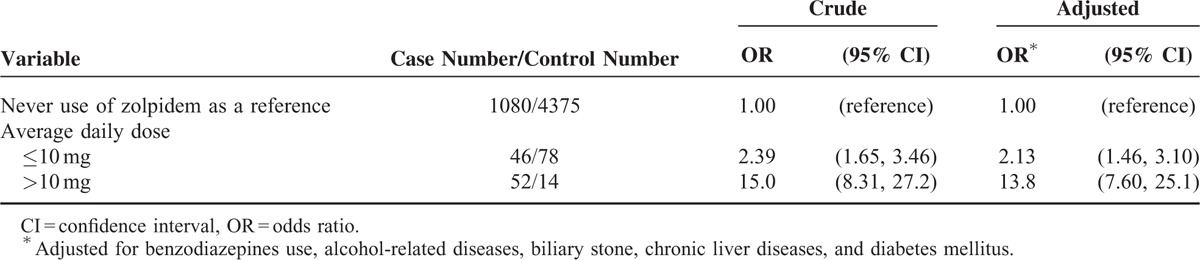
Average Daily Dose of Current Use of Zolpidem and Risk of Pyogenic Liver Abscess
Table 3 shows the association between average daily dose of current use of zolpidem and the risk of pyogenic liver abscess. After adjustment for possible confounding factors, the adjusted OR of pyogenic liver abscess was 2.13 in low dose group with average daily dose ≤10 mg (95% CI 1.46, 3.10, P value of test for trend <0.001), when compared with those with never use of zolpidem. The adjusted OR increased to 13.8 in high dose group with average daily dose >10 mg (95% CI 7.60, 25.1, P value of test for trend <0.001).
TABLE 3.
OR and 95% CI of Pyogenic Liver Abscess Associated With Average Daily Dose of Current Use of Zolpidem
DISCUSSION
This is the first population-based case-control study discloses that people with current use of zolpidem were associated with the increased odds of pyogenic liver abscess (adjusted OR 3.89), but those with late use of zolpidem did not have the increased odds. Given zolpidem only being a short elimination half-life with no active metabolite and no accumulating effect,3,31 our findings suggest that only people continuing use of zolpidem would have the risk. Those who had ever used zolpidem but not using it now would not have the risk. In additional analysis, we noted that people with average daily dose of zolpidem >10 mg had higher odds of pyogenic liver abscess than those with average daily dose ≤10 mg (adjusted OR 13.8 vs 2.13). This finding means that there is a dose-dependent effect of zolpidem on risk of pyogenic liver abscess. In addition, we also noted that among people with current use of zolpidem, use of extended-release zolpidem was not associated with the increased odds of pyogenic liver abscess. Only use of nonextended-release zolpidem was associated with the increased odds of pyogenic liver abscess (table not shown).
To date, no prospective clinical trial discloses that zolpidem can induce infections. Only 2 observational studies have disclosed that zolpidem might be associated with some infections,13,14 but pyogenic liver abscess was not included. One case report disclosed multiple aseptic cutaneous abscesses on the forearms and feet induced by self-injection of powdered zolpidem in a young female addict.32 The U.S. Food and Drug Administration has disclosed that since 2002 to 2012, 13 people (0.03%) had liver abscess among 43,174 people reporting to have side effects when taking zolpidem, but the causal–effect relationship was not confirmed.33 Therefore, the potential mechanism of the association between zolpidem use and pyogenic liver abscess cannot be totally determined by this observational study and the available literature. As we know, zolpidem is mainly used to treat patients with insomnia. The literature discloses that insomnia is associated with the decreased immune function,34,35 which would further increase the probability of infection. Therefore, the observed association of this study may be confounded by the association between insomnia and pyogenic liver abscess. Because the number of insomnia patients without using any hypnotic agent was too small in this study, it is considerably difficult to explore whether insomnia patients without using any hypnotic agent could have an association with pyogenic liver abscess. Similarly, it is also very difficult to include patients with only zolpidem use but without diagnosis of insomnia for analysis. Owing to no definite evidence disclosing that zolpidem can induce infections, whether insomnia is really associated with pyogenic liver abscess or people with current use of zolpidem have other unfound factors to be associated with pyogenic liver abscess cannot be completely illustrated. It indicates a future research direction on this issue. In addition, benzodiazepines such as diazepam are associated with immune suppression, which may further increase infection risk.15,16 We think that zolpidem may have a similar effect on immune suppression like benzodiazepines, which further increase the risk of pyogenic liver abscess.
Some limitations should be discussed in this present study. First, due to the natural limitation of the claim database, we did not know whether people actually took zolpidem of not. The next more rational step was to use prescriptions for analysis. Second, although a differential misclassification of ICD-9 code 572.0 for pyogenic liver abscess may be present due to the natural limitation of the claim database, we included patients only with primary discharge diagnosis of pyogenic liver abscess for analysis. Therefore, the diagnosis accuracy of ICD-9 code 572.0 for pyogenic liver abscess should be reliable. Third, although a cohort study could be performed using this robust database, there might be an immortal time bias. That is why we performed a case-control study instead of a cohort study. Fourth, although the cases and controls were matched with comorbidities, some comorbidities still could not be totally controlled (Table 1). We made an additional analysis to decrease the confounding effects caused by comorbidities. Even in absence of biliary stone and diabetes mellitus, people with current use of zolpidem alone were still associated with the increased odds of pyogenic liver abscess (adjusted OR 6.12, 95% CI 3.65, 10.3). Fifth, no any epidemiological study focuses on this topic. We cannot compare our findings with others. The causal–effect relationship between zolpidem use and pyogenic liver abscess cannot be confirmed in a case-control study. A further prospective research is needed to confirm our findings. Sixth, the literature shows that nonalcoholic fatty liver disease is associated with the increased risk of drug-induced liver injury through mitochondrial dysfunction.36,37 No specific ICD-9 code for nonalcoholic fatty liver disease is available. We cannot include nonalcoholic fatty liver disease for analysis. Further research is needed to illustrate whether nonalcoholic fatty liver disease also makes the liver more vulnerable to invasion of infective agents.
Some strengths of this study should be addressed. Because there is a relative paucity of information on this topic, this article provides the updated evidence to physicians. The methodology and analysis seem to be relatively reasonable. The results are of clinical importance.
We conclude that current use of zolpidem is associated with the increased risk of pyogenic liver abscess, with a dose-dependent effect. Physicians should take the risk of pyogenic liver abscess into account when prescribing zolpidem.
Footnotes
Abbreviations: ICD-9 codes = International Classification of Diseases, Ninth Revision, Clinical Modification.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006), Tseng-Lien Lin Foundation in Taichung in Taiwan, Taiwan Brain Disease Foundation in Taipei in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Specific author contributions—K-FL and S-WL substantially contributed to the conception of the article. They planned and conducted this study. They initiated the draft of the article and critically revised the article.
C-LL and W-CC conducted the data analysis and critically revised the article.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000; 59:865–889. [DOI] [PubMed] [Google Scholar]
- 2.Hajak G, Muller WE, Wittchen HU, et al. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction 2003; 98:1371–1378. [DOI] [PubMed] [Google Scholar]
- 3.Swainston Harrison T, Keating GM. Zolpidem: a review of its use in the management of insomnia. CNS Drugs 2005; 19:65–89. [DOI] [PubMed] [Google Scholar]
- 4.Dang A, Garg A, Rataboli PV. Role of zolpidem in the management of insomnia. CNS Neurosci Ther 2011; 17:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenblatt DJ, Roth T. Zolpidem for insomnia. Expert Opin Pharmacother 2012; 13:879–893. [DOI] [PubMed] [Google Scholar]
- 6.Toner LC, Tsambiras BM, Catalano G, et al. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol 2000; 23:54–58. [DOI] [PubMed] [Google Scholar]
- 7.Victorri-Vigneau C, Gerardin M, Rousselet M, et al. An update on zolpidem abuse and dependence. J Addict Dis 2014; 33:15–23. [DOI] [PubMed] [Google Scholar]
- 8.Yang YH, Lai JN, Lee CH, et al. Increased risk of hospitalization related to motor vehicle accidents among people taking zolpidem: a case-crossover study. J Epidemiol 2011; 21:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang WS, Tsai CH, Lin CC, et al. Relationship between zolpidem use and stroke risk: a Taiwanese population-based case-control study. J Clin Psychiatry 2013; 74:e433–e438. [DOI] [PubMed] [Google Scholar]
- 10.Lin FY, Chen PC, Liao CH, et al. Retrospective population cohort study on hip fracture risk associated with zolpidem medication. Sleep 2014; 37:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai SW, Lin CL, Liao KF. Increased relative risk of acute pancreatitis in zolpidem users. Psychopharmacology (Berl) 2015; 232:2043–2048. [DOI] [PubMed] [Google Scholar]
- 12.Ho YH, Chang YC, Huang WC, et al. Association between zolpidem use and glaucoma risk: a Taiwanese population-based case-control study. J Epidemiol 2015; 25:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joya FL, Kripke DF, Loving RT, et al. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med 2009; 5:377–383. [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CY, Chou FH, Huang YS, et al. The association between zolpidem and infection in patients with sleep disturbance. J Psychiatr Res 2014; 54:116–120. [DOI] [PubMed] [Google Scholar]
- 15.Covelli V, Munno I, Decandia P, et al. Effects of benzodiazepines on the immune system. Acta Neurol (Napoli) 1991; 13:418–423. [PubMed] [Google Scholar]
- 16.Zavala F. Benzodiazepines, anxiety and immunity. Pharmacol Ther 1997; 75:199–216. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol 2006; 115:334–339. [DOI] [PubMed] [Google Scholar]
- 18.Fass R, Quan SF, O’Connor GT, et al. Predictors of heartburn during sleep in a large prospective cohort study. Chest 2005; 127:1658–1666. [DOI] [PubMed] [Google Scholar]
- 19.Barbero GJ. Gastroesophageal reflux and upper airway disease. Otolaryngol Clin North Am 1996; 29:27–38. [PubMed] [Google Scholar]
- 20.Chen YC, Lin CH, Chang SN, et al. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000–2011. J Microbiol Immunol Infect 2014. [DOI] [PubMed] [Google Scholar]
- 21.Krige JE, Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system. BMJ 2001; 322:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molle I, Thulstrup AM, Vilstrup H, et al. Increased risk and case fatality rate of pyogenic liver abscess in patients with liver cirrhosis: a nationwide study in Denmark. Gut 2001; 48:260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 2008; 14:1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su TP, Chen TJ, Hwang SJ, et al. Utilization of psychotropic drugs in Taiwan: an overview of outpatient sector in 2000. Zhonghua Yi Xue Za Zhi (Taipei) 2002; 65:378–391. [PubMed] [Google Scholar]
- 25.Hsiao F-Y, Hsieh P-H, Gau C-S. Ten-year trend in prescriptions of z-hypnotics among the elderly: a nationwide, cross-sectional study in Taiwan. J Clin Gerontol Geriatr 2013; 4:37–41. [Google Scholar]
- 26.National Health Insurance Research Database. Taiwan. http://nhird.nhri.org.tw/en/Background.html [cited in 2015 June]. [Google Scholar]
- 27.Lai SW, Muo CH, Liao KF, et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011; 106:1697–1704. [DOI] [PubMed] [Google Scholar]
- 28.Hung SC, Liao KF, Lai SW, et al. Risk factors associated with symptomatic cholelithiasis in Taiwan: a population-based study. BMC Gastroenterol 2011; 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao KF, Lai SW, Li CI, et al. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol 2012; 27:709–713. [DOI] [PubMed] [Google Scholar]
- 30.Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore) 2010; 89:295–299. [DOI] [PubMed] [Google Scholar]
- 31.Darcourt G, Pringuey D, Salliere D, et al. The safety and tolerability of zolpidem—an update. J Psychopharmacol 1999; 13:81–93. [DOI] [PubMed] [Google Scholar]
- 32.Scrivener Y, Maradeix S, Konare H, et al. Multiple abscesses induced by self injection of zolpidem. Ann Dermatol Venereol 2005; 132:990–992. [DOI] [PubMed] [Google Scholar]
- 33.eHealthMe study from FDA and social media reports. Review: could zolpidem cause liver abscess? http://www.ehealthme.com/print/ds14688387 [cited in 2015 June]. [Google Scholar]
- 34.Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med 2003; 1:227–247. [DOI] [PubMed] [Google Scholar]
- 35.Savard J, Laroche L, Simard S, et al. Chronic insomnia and immune functioning. Psychosom Med 2003; 65:211–221. [DOI] [PubMed] [Google Scholar]
- 36.Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res 2007; 37:410–415. [DOI] [PubMed] [Google Scholar]
- 37.Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol 2008; 22:335–353. [DOI] [PubMed] [Google Scholar]


