Supplemental Digital Content is available in the text
Abstract
Pancreaticoduodenectomy (PD) holds high postoperative morbidity. How to resolve this issue is challenged. An additional anastomosis (Braun enteroenterostomy) following PD may decrease the postoperative morbidity, but holds conflicting results. The objective of this study is to investigate the advantages and disadvantages of Braun enteroenterostomy in PD.
Clinical studies compared perioperative outcomes between the Braun group and the non-Braun group following PD before December 21, 2014 were retrieved and filtered from PubMed, EMBASE, Web of Science, the Cochrane Library, and Chinese electronic databases (VIP database, WanFang database, and CNKI database). Relevant data were extracted according to predesigned sheets. Blood loss, operating time, and postoperative mortality and morbidity were evaluated using odds ratio (OR), weighted mean difference, or standard mean difference (SMD).
Ten studies concerning 1614 patients were included. No significant differences between the Braun and the non-Braun group were identified in mortality (OR: 0.65, 95% confidence interval [CI]: 0.26–1.60), intraoperative blood loss (SMD: −0.035, 95% CI: −0.253 to 0.183), postoperative pancreatic fistula (POPF) (OR: 0.67, 95% CI: 0.35–1.67), bile leakage (OR: 0.537, 95% CI: 0.287–1.004), postoperative gastrointestinal hemorrhage (OR: 1.17, 95% CI: 0.578–2.385), intraabdominal abscesses (OR: 0.793, 95% CI: 0.444–1.419), wound complications (OR: 0.806, 95% CI: 0.490–1.325), and hospital stay (SMD: −0.098, 95% CI: −0.23 to 0.033). Braun enteroenterostomy extended operating time (SMD: 0.39, 95% CI: 0.02–0.78), but it was associated with lower reoperation rate (OR: 0.380, 95% CI: 0.149–0.968), lower morbidity rate (OR: 0.66, 95% CI: 0.49–0.91), lower clinically relevant delayed gastric emptying (Grades B and C) (OR: 0.375, 95% CI: 0.164–0.858), lower nasogastric tube reinsertion (OR: 0.436, 95% CI: 0.232–0.818), and less postoperative vomiting (OR: 0.444, 95% CI: 0.262–0.755).
Braun enteroenterostomy can be safely performed during PD. It is beneficial for patients and could be recommended in PD from the current published data.
PROSPERO registration number: CRD42015016198.
INTRODUCTION
Pancreaticoduodenectomy (PD) is the first choice of curative treatments for pancreatic cancer and periampullary adenocarcinoma. Since the first PD was reported in the 1930s,1 the operative mortality rate remained between 20% and 40% in the following 50 years. With the improvements of surgical techniques, instruments, and perioperative managements, the mortality rates of PD have dramatically reduced to <5%, while the postoperative morbidity rate remains high (30% to 50%),2 even up to 60%.3 Postoperative pancreatic fistula (POPF) and delayed gastric emptying (DGE), which always result in prolonged hospital stay and increased costs, are the 2 common postoperative complications after PD. Based on the definition of the International Study Group,4,5 the incidence of POPF is 14% to 60%,6,7 and the incidence of DGE is 38% to 57%.8–10 How to reduce the postoperative mortality and morbidity, including POPF and DGE, is ever a challenged issue.
The optimal way of digestive reconstructions to minimize POPF or DGE is controversial. Braun enteroenterostomy (BEE), first reported 100 years ago, might be a useful technique to decrease the morbidity rate, especially the incidence of DGE. It is an anastomosis between the afferent and efferent limbs, which is distal to a gastroenterostomy or duodenoenterostomy. It is designed to divert pancreatic juice and bile from the afferent limb, leading to decreased reflux into the stomach. It was reported that Braun jejunojejunostomy diverted jejunal contents and prevented postoperative alkaline reflux gastritis in Billroth II gastric resection, leading decreased postgastrectomy complications and offering an alternative resolution to intractable Mini-Gastric Bypass symptomatic dyspepsia/“bile reflux.”11 Regarding life quality, Wang et al12 reported an addition of Braun anastomosis to Billroth II in gastric cancer surgery prolonged patients’ survival. In theory, BEE following classic PD potentially stabilizes and prevents kinking at the gastroenterostomy, and delivers pancreatic and biliary juices away from the stomach, suggesting that BEE is a promising reconstruction possibly associated with lower DGE. However, conflicting results of clinical effects of BEE were reported. Zhang et al13 reported BEE following classic PD did not decrease DGE, while others3,14 showed BEE reduced the incidence of DGE. Therefore, the advantages and disadvantages of BEE during PD remain controversial.
Till now, no well-designed large-scale randomized controlled trials have been done to investigate outcomes of BEE following PD. Only several retrospective studies describe the relationships between BEE and the postoperative complications in PD, but hold inconsistent results. Abraham et al15 confirmed the pooling results of high-quality nonrandomized comparative trials were similar to those of randomized controlled trials when comparing surgical outcomes using meta-analysis. The purpose of this study is to evaluate possible associations between BEE and patient-relevant outcomes from PD through systematically pooling results, and to determine clinical impacts of BEE during PD.
MATERIALS AND METHODS
Search Strategy
PubMed, EMBASE, Web of Science, the Cochrane Library, and Chinese electronic databases (VIP database, WanFang database, and CNKI database) were systematically searched, and the final search date was December 21, 2014. The following combined terms were used: “Braun enteroenterostomy” or “Braun anastomosis,” and the language was limited to English or Chinese. The reference list was also manually checked to find pertinent articles.
Inclusion Criteria
All studies included in this meta-analysis must meet the following criteria: the surgical procedure was PD; the intervention group was BEE following PD; the control group was PD without BEE; and one of short- or long-term postoperative outcomes could be extracted.
Excluded Criteria
Studies with the following characteristics were excluded: animal researches, conference abstracts, letters, comments, editorials, expert opinions, reviews without original data, and non-English or non-Chinese language articles, duplicates and repeated series published by the same centre.
Data Extraction
Titles and abstracts were checked for the potentially eligible studies. Full articles were founded for the detailed evaluation. Regarding articles reported by the same institution, either the study with better quality or the more recent publication was included. All data extractions were performed separately by BX and Y-HZ. Disagreements were settled by discussion. The following data from each included study were extracted: first author, year of publication, details of where the studies were conducted, the study period, sample sizes, baseline characteristics of the studies, perioperative outcomes, hospital stay, duration of follow-ups. Perioperative outcomes included operative time, operative blood loss, reoperation rate, morbidity, mortality, etc.
Qualitative Assessment
The quality assessment of included studies was evaluated using Newcastle–Ottawa quality assessment scale (website URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). A score of 0 to 9 stars was used to assess the quality of each study. Studies labeled with 6 stars or greater were considered to be high quality.
Statistical Analysis
Estimating the mean and variance from the median, range, and the size of a sample was performed using Hozo's method.16 The point estimate of the odds ratio (OR), weighted mean difference, or standard mean difference (SMD) was considered statistically significant at P < 0.05. The I squared (I2) statistic and chi-squared (χ2) test were used to evaluate the heterogeneity; significance was identified at I2 > 50% and P < 10%, respectively. The random-effect model was used if there was significant heterogeneity between the studies; otherwise, the fixed-effect model was used; 0.5 was added to each cell of the 2 × 2 table for studies with 0 cells to avoid problems with computation of estimates and standard errors according to Cochrane Manual for data transforming. Publication bias was assessed by the funnel plot; Egger's test and Begg's test were used to detect the difference. Analysis of the main results was performed using Comprehensive Meta-Analysis (Version 2.0).
RESULTS
Selection and Characteristics of Studies
A total of 255 records pertinent to BEE were retrieved from PubMed, EMBASE, Web of Science, the Cochrane Library, and Chinese electronic databases (VIP database, WanFang database, and CNKI database). Two hundred twenty-one studies were excluded after screening the titles and abstracts because of irrelevant studies, case reports, review articles, abstracts, or duplicate reports. Thirty-four articles were founded for more detailed evaluation. Eleven eligible studies were determined through applying our inclusion criteria.3,13,14,17–24 Two of 11 were reported by the same institute and the same first author.20,24 Only 1 with more detailed data was selected20 to avoid statistical bias because of duplicate counting (Figure 1). Therefore, 10 studies regarding 1614 patients were included for our meta-analysis:3,13,14,17–23 6 in China,3,13,19,20,22,23 1 in USA,18 1 in Australia,21 1 in German,17 and 1 in Japan.14 The sample size of BEE following PD ranged from 21 to 347. Mean or median age varied from 50 to 70. Sex between the Braun group and the non-Braun group was comparable (OR: 1.06, 95% confidence interval [CI]: 0.82–1.37, P = 0.65), with low heterogeneity (I2 = 0%). The detailed characteristics of the included eligible studies were shown in Table 1. Surgical reconstruction, definition of DGE and POPF, and postoperative managements of the included studies were listed in Table 2.
FIGURE 1.
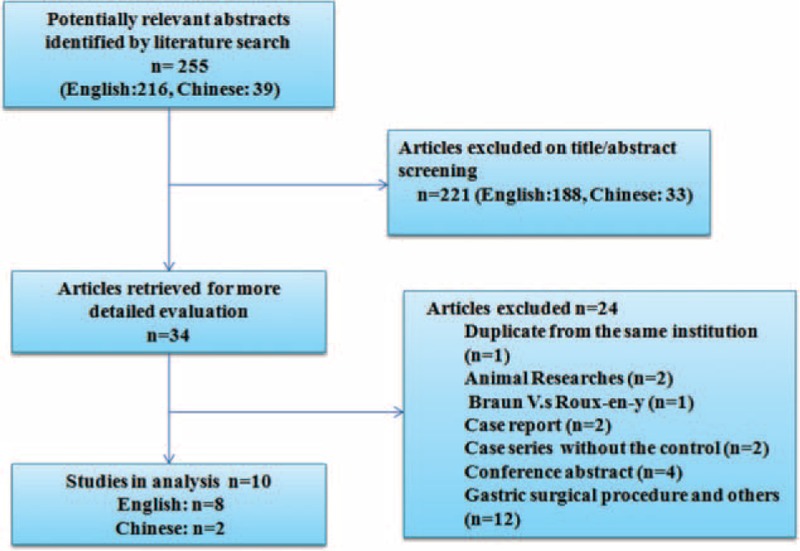
Flow-chart of identification of eligible studies.
TABLE 1.
Major Characteristics of the Included Studies
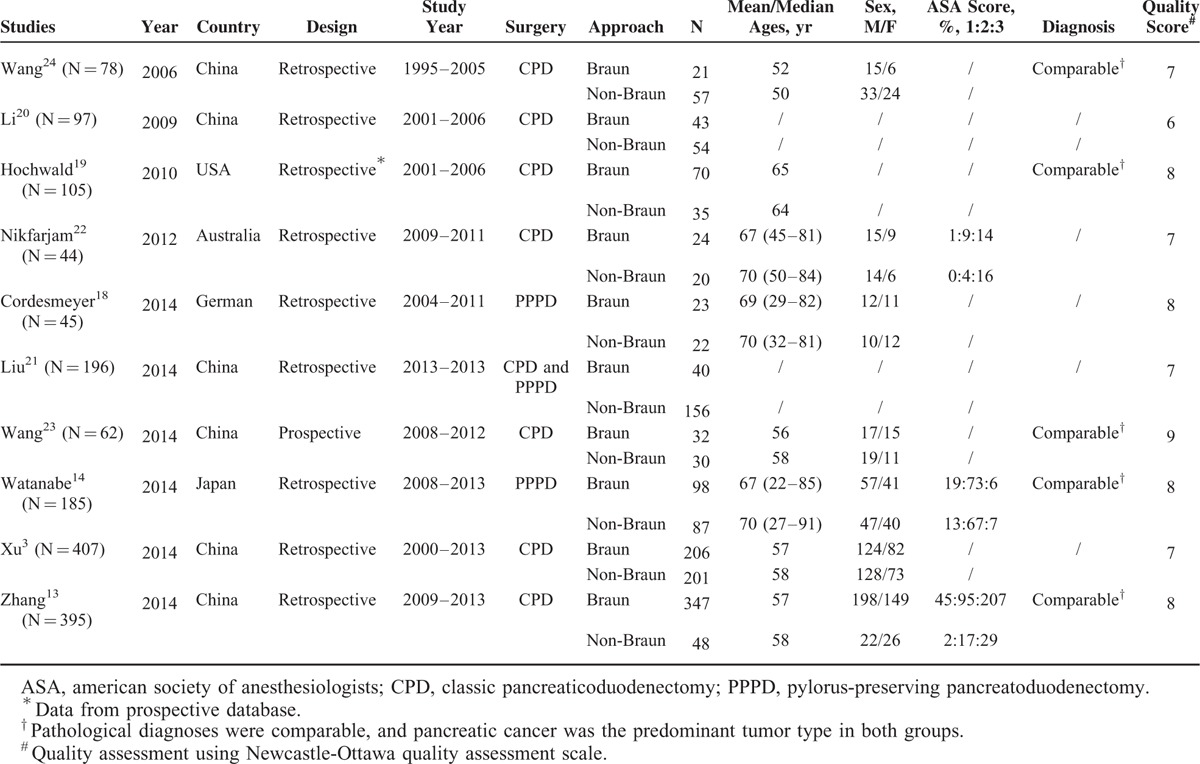
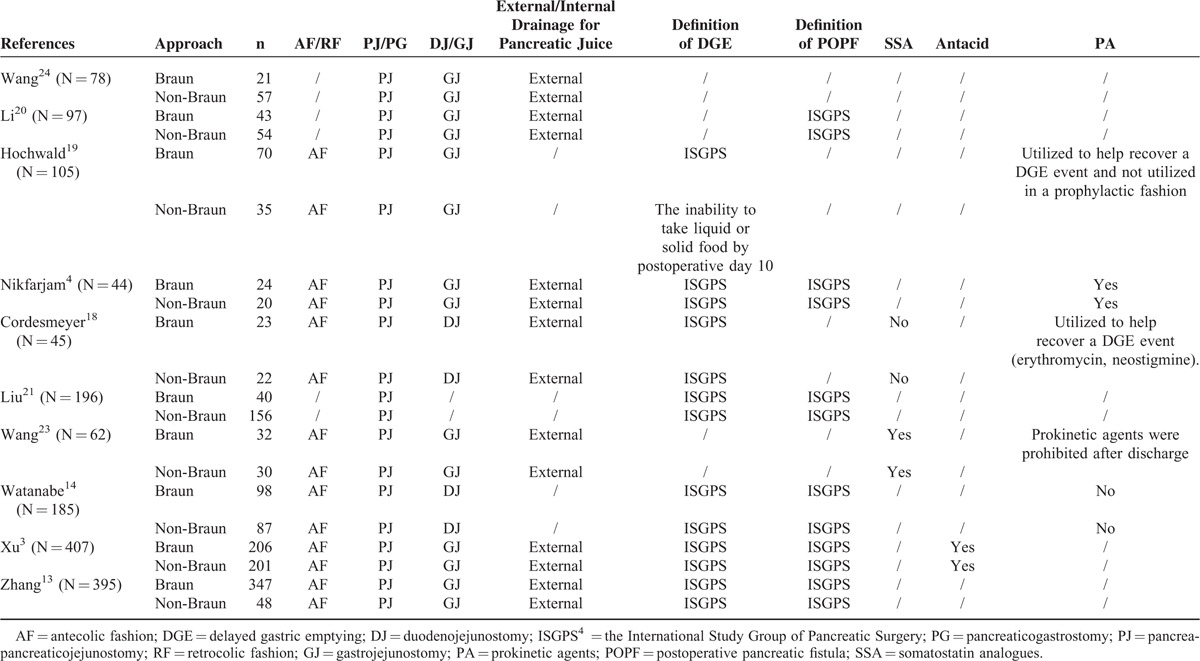
TABLE 2.
Surgical Reconstruction, Definition of DGE and POPF, and Postoperative Managements of the Included Studies
Qualitative Assessment
The Newcastle–Ottawa quality assessment scale was used to evaluate the quality of the included articles. The highest score in this scale is 9 stars. Studies labeled with 6 or more stars were regarded as high quality. All included studies had 6 stars or greater. Data from 2 studies were prospectively collected,18,22 and the other 8 studies were retrospective studies.3,13,14,17,19–21,23 Only 1 was Randomized controlled trial,22 but with low quality evaluated by Jadad scale. Details were given in Table S1, http://links.lww.com/MD/A373.
Intraoperative Outcomes and Postoperative Outcomes
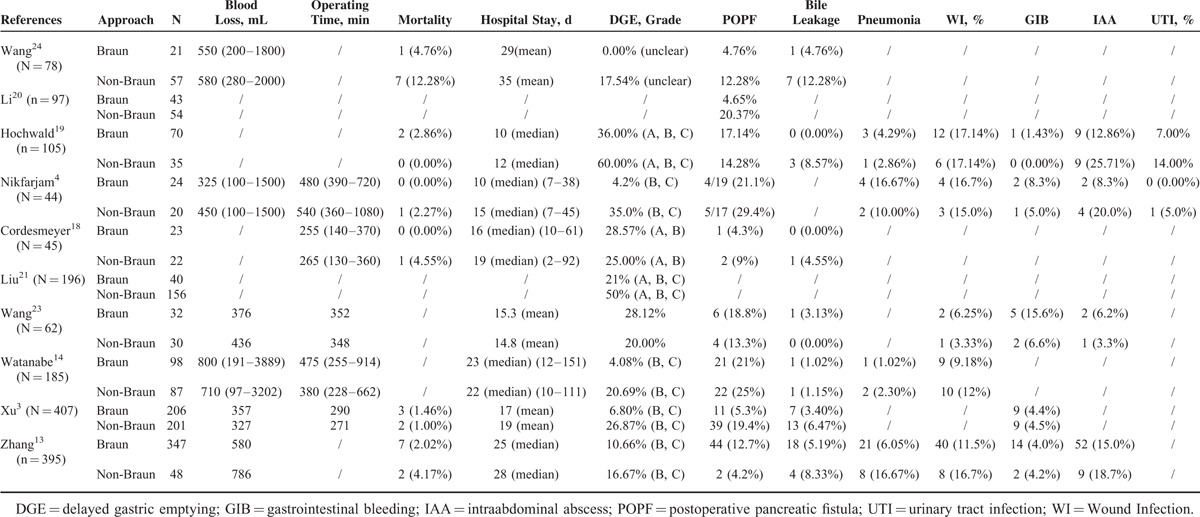
Intraoperative outcomes and postoperative outcomes were summarized in Table 3. The detailed evaluation comparing outcomes between the Braun group and the non-Braun group was analyzed as follows.
TABLE 3.
Surgical Outcomes
Operating Time (min)
There were 5 studies presented operating time,3,14,17,21,22 but the heterogeneity across the studies was significant (P < 0.01, I2 = 86.55%). Longer operating time was associated with BEE (SMD: 0.39, 95% CI: 0.02–0.78, P = 0.049). Publication bias was not observed from the funnel plot; Egger's test (P = 0.95) and Begg's test (P = 0.46) were not significant.
Intraoperative Blood Loss (mL)
Intraoperative blood loss was reported in 6 studies,3,13,14,21–23 and no significant difference between the Braun group and the non-Braun group was identified (SMD: −0.035, 95% CI: −0.253 to 0.183, P = 0.753). Median heterogeneity was identified across the studies (P = 0.046, I2 = 55.64%).
Mortality
There were 6 studies with the mortality rate,3,13,17,18,21,23 concerning 691 patients with BEE following PD. Thirteen of 691 died postoperatively in hospital because of the following: presumed aspiration18 (1), postoperative bleeding3,13,18 (4), Multiple Organ Dysfunction Syndrome3 (4), interstitial pneumonia and infection13 (2), cardiogenic shock (1), and the cause un-reported23 (1). No death was directly attributed to the BEE. Pooling results revealed no significant difference in mortality between the Braun group and the non-Braun group (OR: 0.65, 95% CI: 0.26–1.60, P = 0.35) (Figure 2A). No significant heterogeneity between the studies was noted (P = 0.774, I2 = 0.00%). The funnel plot showed symmetrical.
FIGURE 2.
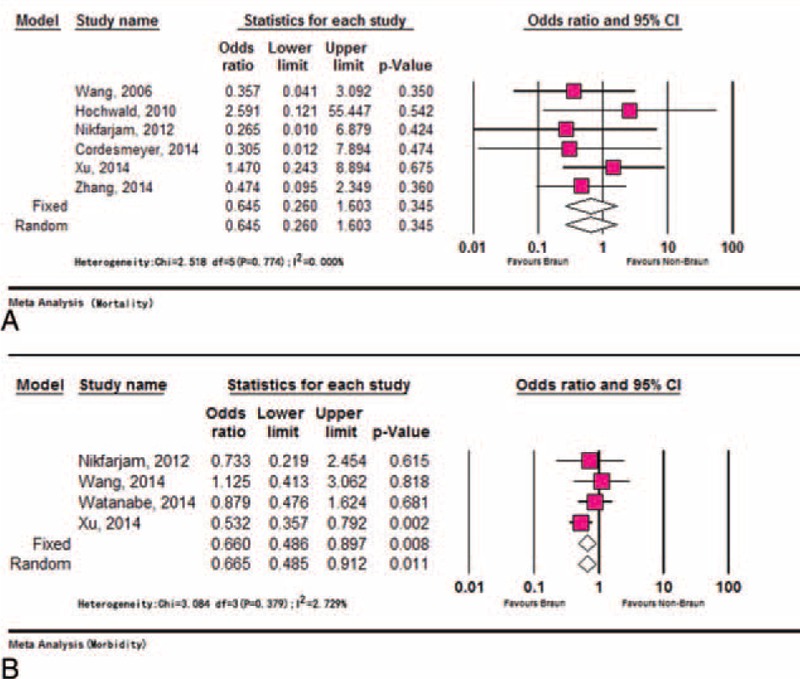
Meta-analysis of mortality and morbidity. (A) Comparable mortality rates between the Braun and the non-Braun group and (B) lower morbidity rate in Braun group compared with non-Braun group.
Morbidity
Postoperative morbidity regarding 360 BEE was reported in 4 studies.3,14,21,22 Lower morbidity rate was linked to BEE compared with non-BEE (OR: 0.66, 95% CI: 0.49–0.91, P = 0.01). No significant heterogeneity was identified across the studies (P = 0.38, I2 = 2.73%) (Figure 2B). No publication bias was detected using Egger's test (P = 0.252) and Begg's test (P = 0.734).
Postoperative Pancreatic Fistula
There was significant heterogeneity (P = 0.02, I2 = 57.06%) across the studies,3,13,14,17–19,21–23 which reported the incidence of POPF because of the different definition of pancreatic fistula and the different grade reported. Using random models, the difference in respect to the incidence of POPF between the Braun group and the non-Braun group was not statistically significant (OR: 0.67, 95% CI: 0.35–1.67, P = 0.22). Random effects model was used to combine studies within each subgroup that was divided according to the definition of pancreatic fistula, and results revealed no significant difference was identified regardless of the different definition: OR was 0.567 (95% CI: 0.26–1.26, P = 0.16) for the group with the definition of International Study Group of Pancreatic Surgery (ISGPS), and OR was 0.911 (95% CI: 0.31–2.68, P = 0.86) for the group with the unclear definitions. Grades B and C POPF were reported separately in 2 studies,14,21 and no significant difference was identified between the Braun group and the non-Braun group: grade B (OR: 0.80, 95% CI: 0.31–2.07, P = 0.64) and grade C (OR: 0.73, 95% CI:0.32–1.67, P = 0.46).
Bile Leakage
The incidence of postoperative bile leakage was low, and 13 of 797 cases suffered from bile leakage in 7 studies.3,13,14,17,18,22,23 There was no significant heterogeneity (P = 0.92, I2 = 0.00%) between the studies.3,13,14,17,18,22,23 No significant difference was identified between the Braun group and the non-Braun group regarding the incidence of postoperative bile leakage (OR: 0.537, 95% CI: 0.287–1.004, P = 0.052). Publication bias was not noted using Egger's test (P = 0.97) and Begg's test (P = 1.00).
DGE and Postoperative Gastrointestinal Recovery
The definition of DGE was unclear in 2 studies,22,23 and the definition from the ISGPS4 was applied in the other studies.3,13,14,17,18,20,21 Five studies17,18,20,22,23 reported the total incidence of DGE (Grades A, B, and C) after PD. There was no significant difference regarding the total incidence of DGE between the Braun and the non-Braun group using random models (OR: 0.922, 95% CI: 0.350–2.424, P = 0.869; heterogeneity: P = 0.008, I2 = 71.117%). Clinically relevant delayed gastric emptying (CR-DGE) was reported by 7 studies3,13,14,17,18,20,21 involving 1369 patients (Braun group: 806 vs non-Braun group: 563). The incidence of CR-DGE in the Braun group and the non-Braun group was 9.45% (95% CI: 6.31–13.915) and 21.445% (95% CI: 16.00–28.73), respectively. Lower DGE was occurred in BEE following PD compared with standard PD (OR: 0.375, 95% CI: 0.164–0.858, P = 0.020; heterogeneity: P < 0.01, I2 = 75.862%). The DGE grade B or C was reported separately in 5 studies,3,13,14,17,21 and results showed lower DGE grade B trended in the Braun group, but no statistical significance was identified when compared with the non-Braun group (OR: 0.349, 95% CI: 0.119–1.021, P = 0.055; heterogeneity: P = 0.082, I2 = 51.742%). Dramatically lower DGE grade C was identified in the Braun group (OR: 0.300, 95% CI: 0.174–0.519, P < 0.0001; heterogeneity: P = 0.664, I2 = 0.00%) (Figures 3 and 4). Other characteristics with respect to the postoperative gastrointestinal recovery were also evaluated: postoperative time to remove nasogastric tube13,18,22 (SMD: −0.013, 95% CI: −0.231 to 0.204, P = 0.904; heterogeneity: P = 0.766, I2 = 0.00%), days for staring liquid meals14,18 (SMD: −0.089, 95% CI: −0.324 to 0.147, P = 0.460; heterogeneity: P = 0.608, I2 = 0.00%), nasogastric tube reinsertion13,14,18 (OR: 0.436, 95% CI: 0.232–0.818, P = 0.010; heterogeneity: P = 0.818, I2 = 0.00%) and postoperative vomiting13,18 (OR: 0.444, 95% CI: 0.262–0.755, P = 0.003; heterogeneity: P = 0.316, I2 = 53.60%). No publication bias was detected using Egger's test and Begg's test when analyzing above variables.
FIGURE 3.
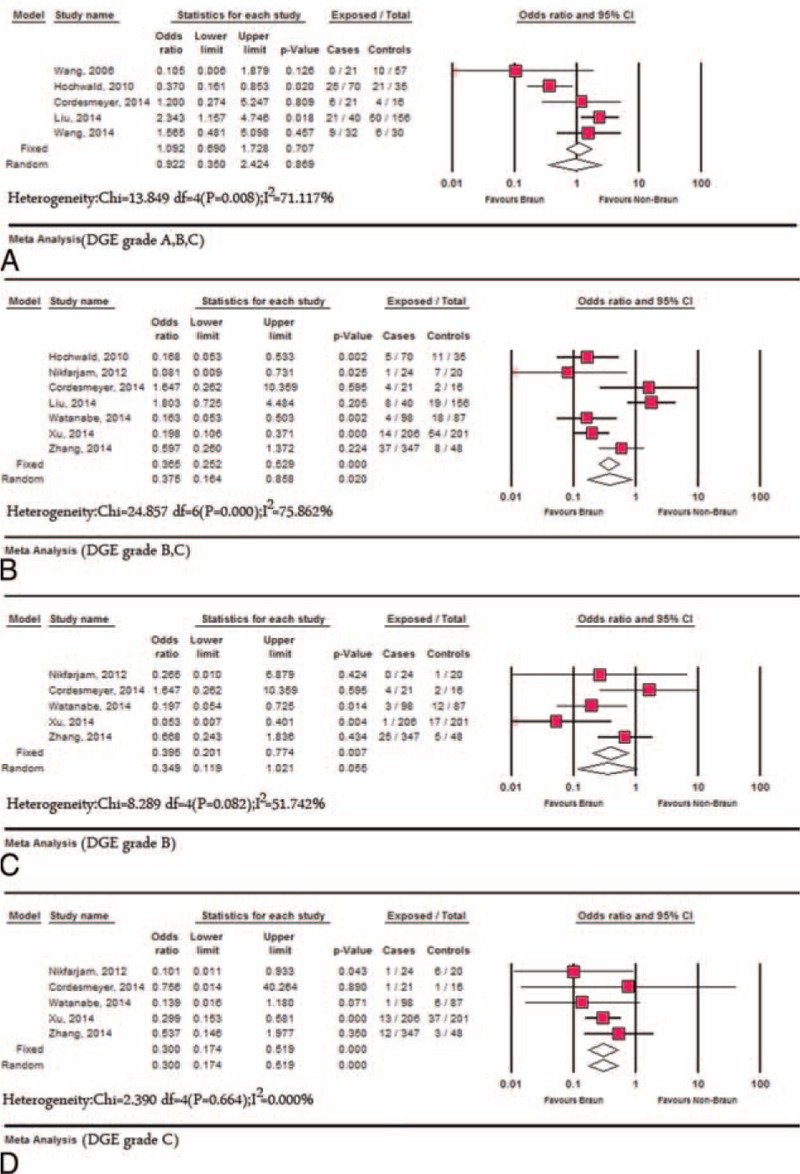
Meta-analysis of DGE between Braun and the non-Braun group. (A) The overall incidence of DGE; (B) the incidence of clinically relevant-DGE; (C) the incidence of DGE grade B; and (D) the incidence of DGE grade C. DGE = delayed gastric emptying.
FIGURE 4.
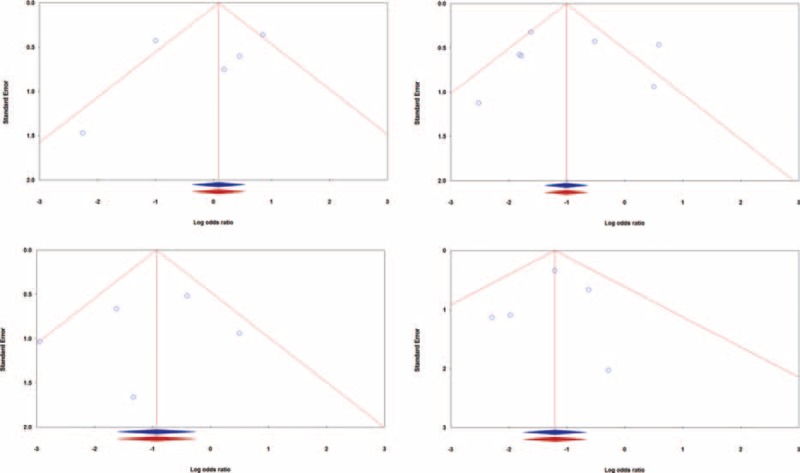
Funnel plots of standard errors by Log odds ratio for analysis of the studies. (A) The overall incidence of DGE (Egger's test: P = 0.506, Begg's test: P = 0.462); (B) the incidence of clinically relevant-DGE (Egger's test: P = 0.915, Begg's test: P = 0.548); (C) the incidence of DGE grade B (Egger's test: P = 0.623, Begg's test: P = 0.806); and (D) the incidence of DGE grade C (Egger's test: P = 0.762, Begg's test: P = 0.806). Blue, observed studies; red, imputed studies. DGE = delayed gastric emptying.
Other Postoperative Complications
Four studies3,13,18,22 reported postoperative gastrointestinal hemorrhage, and no significant difference with respect to the incidence of gastrointestinal bleeding between the Braun and the non-Braun group was identified (OR: 1.17, 95% CI: 0.578–2.385, P = 0.658), and no significant heterogeneity between the studies was found (P = 0.789, I2 = 0.00%). There was also no significant difference between the Braun group and the non-Braun group with respect to the following complications: intraabdominal abscesses (OR: 0.793, 95% CI: 0.444–1.419, P = 0.436; heterogeneity: P = 0.855, I2 = 0.00%) and wound complications (OR: 0.806, 95% CI: 0.490–1.325, P = 0.396; heterogeneity: P = 0.963, I2 = 0.00%).
Reoperation
There were 5 studies13,14,17,21,22 that reported the incidence of reoperation. The pooled incidence of reoperation in the Braun group concerning 524 patients was 3.16% (95% CI: 1.89–5.22), while the incidence of the non-Braun group was 7.80% (95% CI: 2.52–21.47). Lower incidence of reoperation was identified in the Braun group compared with the non-Braun group (OR: 0.380, 95% CI: 0.149–0.968, P = 0.043); no significant heterogeneity between the studies was identified (P = 0.512, I2 = 0.00%) (Figure 5). No significant publication bias was detected using Egger's test (P = 0.260) and Begg's test (P = 0.806).
FIGURE 5.
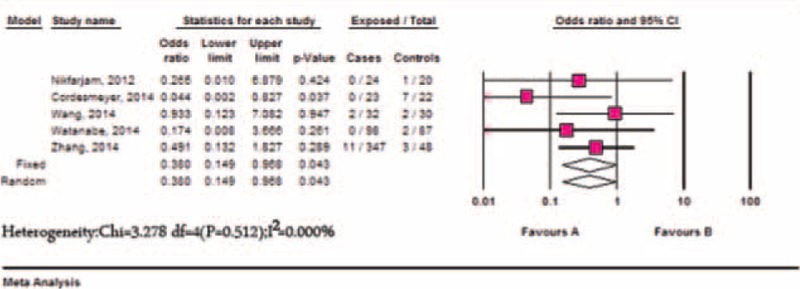
Meta-analysis of reoperation between the Braun and the non-Braun group.
Hospital Stay
Hospital stay was extracted from 6 studies.3,13,14,17,21,22 No significant difference was observed when comparing the hospital stay between the Braun and the non-Braun groups (SMD: −0.098, 95% CI: −0.23 to 0.033, P = 0.14), and there was no statistically significant heterogeneity between the included studies (P = 0.33, I2 = 12.72%).
DISCUSSION
PD was once discouraged because of high mortality (20% to 40%) in 1930 to 1960.18 It now becomes safe with <5% mortality; however, its morbidity rate remains high. To minimize postoperative morbidity and get a better life quality, much has been done to improve PD, including BEE. The clinical effects of BEE following PD have not been systematically reviewed. We demonstrate an additional BEE following PD decreases postoperative morbidity, reoperation, nasogastric tube reinsertion, and postoperative vomiting, especially the CR-DGE. Therefore, BEE is beneficial for patients and could be recommended in PD.
BEE between the afferent and efferent limbs is one of digestive reconstructions, and it is distal to a gastroenterostomy or duodenoenterostomy in PD. An additional BEE to PD is a safe surgical procedure without the additional resection extend; however, it might extend the operating time. Our meta-analysis showed a longer operating time was associated with BEE, BEE did not contribute to any direct postoperative complications,18 and no death was directly linked to the BEE. POPF and bile leakage are known to be the most common complications after PD. It was reported that a lower incidence of POPF was associated with BEE,3,19 while others14,17,18,22 showed BEE did not affect POPF. Our pooling data revealed no significant difference was identified with respect to the incidence of POPF between the Braun group and the non-Braun group. Most studies3,13,14,17 supported the incidence of postoperative bile leakage was not associated with BEE, as was similar in our study. In addition, our analysis also showed BEE did not increase the following morbidities: intraabdominal abscesses, postoperative gastrointestinal hemorrhage and wound infections, while it reduced the postoperative morbidity rate.
Despite improvements of surgical techniques and perioperative managements, the reported incidence of DGE is high, up to 60%.3 The exact mechanism of DGE is unclear. It might result from any potential obstructions at the level of gastroenterostomy: anastomotic edema or stenosis, gastric irritant effects, limb volvulus, and adhesions. BEE potentially stabilizes the gastroenterostomy and prevents kinking, which might reduce limb volvulus. Moreover, BEE diverts food, pancreatic juices and biles from the afferent limb, which decrease anastomotic edema and mucosal irritation through reducing pancreatiobiliary loop press and bile reflux. It was reported that the patients with gastric cancer underwent BEE had decreased DGE. In pancreatic surgery, whether BEE can reduce DGE is controversial. Some reported BEE did not reduce DGE,13,22 while others showed BEE played a role in lower DGE.3,14,18,21,23 There was no significant difference regarding the total incidence of DGE (A, B, and C) between the Braun group and the non-Braun group in our meta-analysis, but we prefer CR-DGE to total DGE (A, B, and C) for evaluating the relationship between BEE and DGE. Using the incidence of total DGE might bias the impacts of BEE on DGE, because a major part of DGE is DGE grade A that could be affected by subjective factors, such as the surgeon's preference for removal of nasogastric tubes and starting meals. Our studies showed the pooled incidence of CR-DGE in the Braun group and the non-Braun group was 9.45% (95% CI: 6.31–13.915) and 21.445% (95% CI: 16.00–28.73), respectively. BEE significantly reduced the incidence of CR-DGE. Further analysis showed BEE dramatically decreased DGE grade C. In addition, BEE also reduced nasogastric tube reinsertion and postoperative vomiting during postoperative gastrointestinal recovery. Therefore, BEE is acceptable considering its safety and benefits.
The limitations of this present study include the retrospective nature of the design. Most of studies are retrospective, and only 1 study is referred to randomly allocate patients into BEE and non-BEE group, but no detailed information is offered concerning randomization, concealment of allocation, double blinding, and withdrawals and dropouts. Some pooling results base on the original data with significant heterogeneity, so it should be with caution when applying these relevant outcomes in clinic. BEE can reduce bile reflux gastritis; therefore, it may improve postoperative life quality. Wang et al12 reported an addition of Braun anastomosis to Billroth II in gastric cancer procedures prolonged patients’ survival without increasing the surgical complications and mortality. However, there are no data regarding these long-term outcomes in the studies included to evaluate the survival and life quality, which should be added into the future trials.
In conclusion, an additional BEE is associated with decreased CR-DGE rather than increasing postoperative morbidity and mortality. BEE can be safely performed during PD. It is beneficial for patients and could be recommended in PD from current published data. However, further well-designed, larger randomized controlled trials that assess clinical impacts of BEE are needed.
Footnotes
Abbreviations: BEE = Braun enteroenterostomy, CR-DGE = clinically relevant delayed gastric emptying, DGE = delayed gastric emptying, ISGPS = International Study Group of Pancreatic Surgery, OR = odds ratio, PD = pancreaticoduodenectomy, POPF = postoperative pancreatic fistula, SMD = standard mean difference, WMD = weighted mean difference.
Y-WZ and W-YZ have contributed equally to this work.
Supported by National Natural Science Foundation of China (No. 81001007); Fudan University Youth Fund (2012); and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Chinese Ministry of Education.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.jpgn.org).
REFERENCES
- 1.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg 1935; 102:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014; 259:773–780. [DOI] [PubMed] [Google Scholar]
- 3.Xu B, Meng H, Qian M, et al. Braun enteroenterostomy during pancreaticoduodenectomy decreases postoperative delayed gastric emptying. Am J Surg 2015; 209:1036–1042. [DOI] [PubMed] [Google Scholar]
- 4.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007; 142:761–768. [DOI] [PubMed] [Google Scholar]
- 5.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138:8–13. [DOI] [PubMed] [Google Scholar]
- 6.Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB 2014; 16:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu QY, Zhang WZ, Xia HT, et al. Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol 2014; 20:17491–17497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto Y, Yamamoto Y, Hata S, et al. Analysis of risk factors for delayed gastric emptying (DGE) after 387 pancreaticoduodenectomies with usage of 70 stapled reconstructions. J Gastrointest Surg 2011; 15:1789–1797. [DOI] [PubMed] [Google Scholar]
- 9.Rayar M, Sulpice L, Meunier B, et al. Enteral nutrition reduces delayed gastric emptying after standard pancreaticoduodenectomy with child reconstruction. J Gastrointest Surg 2012; 16:1004–1011. [DOI] [PubMed] [Google Scholar]
- 10.Nikfarjam M, Kimchi ET, Gusani NJ, et al. A reduction in delayed gastric emptying by classic pancreaticoduodenectomy with an antecolic gastrojejunal anastomosis and a retrogastric omental patch. J Gastrointest Surg 2009; 13:1674–1682. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge R. Braun side to side enteroenterostomy: a simple 30 minute laparoscopic resolution of dyspepsia bile reflux symptoms following mini-gastric bypass. Obes Surg 2011; 21:1061. [Google Scholar]
- 12.Wang F, Zu HL, Jiang H, et al. Clinical investigation of combined Billroth II with Braun anastomosis for patients with gastric cancer. Hepato-Gastroenterology 2014; 61:1812–1816. [PubMed] [Google Scholar]
- 13.Zhang XF, Yin GZ, Liu QG, et al. Does Braun enteroenterostomy reduce delayed gastric emptying after pancreaticoduodenectomy? Medicine 2014; 93:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe Y, Ohtsuka T, Kimura H, et al. Braun enteroenterostomy reduces delayed gastric emptying after pylorus-preserving pancreatoduodenectomy: a retrospective review. Am J Surg 2015; 209:369–367. [DOI] [PubMed] [Google Scholar]
- 15.Abraham NS, Byrne CJ, Young JM, et al. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 2010; 63:238–245. [DOI] [PubMed] [Google Scholar]
- 16.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordesmeyer S, Lodde S, Zeden K, et al. Prevention of delayed gastric emptying after pylorus-preserving pancreatoduodenectomy with antecolic reconstruction, a long jejunal loop, and a jejuno-jejunostomy. J Gastrointest Surg 2014; 18:662–673. [DOI] [PubMed] [Google Scholar]
- 18.Hochwald SN, Grobmyer SR, Hemming AW, et al. Braun enteroenterostomy is associated with reduced delayed gastric emptying and early resumption of oral feeding following pancreaticoduodenectomy. J Surg Oncol 2010; 101:351–355. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Dong M, Zhou JP, et al. Analysis of pancreatic leaking-related risk factors after pancreaticoduodenectomy. Chin J Surg 2009; 47:752–754. [PubMed] [Google Scholar]
- 20.Liu QY, Li L, Xia HT, et al. Risk factors of delayed gastric emptying following pancreaticoduodenectomy. ANZ J Surg 2014; http://www.ncbi.nlm.nih.gov/pubmed/25312402 [DOI] [PubMed] [Google Scholar]
- 21.Nikfarjam M, Houli N, Tufail F, et al. Reduction in delayed gastric emptying following non-pylorus preserving pancreaticoduodenectomy by addition of a Braun enteroenterostomy. J Pancreas 2012; 13:488–496. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Su A, Zhang Y, et al. Reduction of alkaline reflux gastritis and marginal ulcer by modified Braun enteroenterostomy in gastroenterologic reconstruction after pancreaticoduodenectomy. J Surg Res 2014; 189:41–47. [DOI] [PubMed] [Google Scholar]
- 23.Wang YG, Ye QF, Qi HZ, et al. Pancreatoduodenectomy appending Braun's anastomosis for periampullary tumour. J Clin Res 2006; 23:1768–1770. [Google Scholar]
- 24.Liu QY, Li L, Xia HT, et al. Risk factors of delayed gastric emptying after pancreaticoduodenectomy. Chin J Hepatobiliary Surg 2014; 20:719–722. [Google Scholar]


