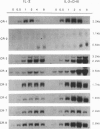
Abstract
Clonal expansion of antigen-reactive T lymphocytes is driven by the lymphokine interleukin 2 (IL-2). To further elucidate the mechanisms of IL-2 action, we have utilized a differential hybridization procedure to clone IL-2-induced immediate-early genes from an IL-2-stimulated human T-cell cDNA library. To increase the frequency of IL-2-induced transcripts represented in the library, the protein synthesis inhibitor cycloheximide was included during the 2-hr IL-2 stimulation to superinduce gene expression, and the uridine analogue 4-thiouridine was utilized to enable selective purification of newly synthesized transcripts. From the enriched library, we have isolated eight IL-2-induced genes, six of which represent previously unrecognized human sequences. Northern blot analysis revealed that the induction of seven of the genes is specific to the IL-2-mediated G1 "progression" phase of the cell cycle, in that only one gene is also induced during the T-cell receptor-triggered G0-G1 "competence" phase. These results indicate that the effects of IL-2 are mediated by the specific induction of a number of immediate-early genes and provide a means with which to further delineate the mechanisms whereby IL-2 stimulates T-lymphocyte proliferation and differentiation. The methods described in this report should also be of general utility in the dissection of the signaling pathways activated by diverse cytokine receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollahi A., Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Sequence and expression of a cDNA encoding MyD118: a novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene. 1991 Jan;6(1):165–167. [PubMed] [Google Scholar]
- Alexander D. R., Cantrell D. A. Kinases and phosphatases in T-cell activation. Immunol Today. 1989 Jun;10(6):200–205. doi: 10.1016/0167-5699(89)90325-3. [DOI] [PubMed] [Google Scholar]
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Haemopoietic receptors and helical cytokines. Immunol Today. 1990 Oct;11(10):350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry F., Weil D., Yu J., Dautry-Varsat A. Regulation of pim and myb mRNA accumulation by interleukin 2 and interleukin 3 in murine hematopoietic cell lines. J Biol Chem. 1988 Nov 25;263(33):17615–17620. [PubMed] [Google Scholar]
- Dickinson L. A., Joh T., Kohwi Y., Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992 Aug 21;70(4):631–645. doi: 10.1016/0092-8674(92)90432-c. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Gullberg M., Smith K. A. Regulation of T cell autocrine growth. T4+ cells become refractory to interleukin 2. J Exp Med. 1986 Feb 1;163(2):270–284. doi: 10.1084/jem.163.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Johnson K. W., Smith K. A. Molecular cloning of a novel human cdc2/CDC28-like protein kinase. J Biol Chem. 1991 Feb 25;266(6):3402–3407. [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Complexity of the immediate early response of myeloid cells to terminal differentiation and growth arrest includes ICAM-1, Jun-B and histone variants. Oncogene. 1990 Mar;5(3):387–396. [PubMed] [Google Scholar]
- Merida I., Gaulton G. N. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Interleukin 2-induced lymphocyte proliferation is independent of increases in cytosolic-free calcium concentrations. J Immunol. 1985 Apr;134(4):2431–2435. [PubMed] [Google Scholar]
- Mills G. B., Girard P., Grinstein S., Gelfand E. W. Interleukin-2 induces proliferation of T lymphocyte mutants lacking protein kinase C. Cell. 1988 Oct 7;55(1):91–100. doi: 10.1016/0092-8674(88)90012-8. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Stewart D. J., Mellors A., Gelfand E. W. Interleukin 2 does not induce phosphatidylinositol hydrolysis in activated T cells. J Immunol. 1986 Apr 15;136(8):3019–3024. [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Hsu J. C., Melby A. E., Bravo R., Taub R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol. 1991 Jan;11(1):381–390. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Papathanasiou M. A., Kerr N. C., Robbins J. H., McBride O. W., Alamo I., Jr, Barrett S. F., Hickson I. D., Fornace A. J., Jr Induction by ionizing radiation of the gadd45 gene in cultured human cells: lack of mediation by protein kinase C. Mol Cell Biol. 1991 Feb;11(2):1009–1016. doi: 10.1128/mcb.11.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle D. E., Wojchowski D. M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath D. E., Podolin P. L., Comber P. G., Prystowsky M. B. cDNA cloning and characterization of interleukin 2-induced genes in a cloned T helper lymphocyte. J Biol Chem. 1990 Jul 25;265(21):12671–12678. [PubMed] [Google Scholar]
- Saltzman E. M., White K., Casnellie J. E. Stimulation of the antigen and interleukin-2 receptors on T lymphocytes activates distinct tyrosine protein kinases. J Biol Chem. 1990 Jun 15;265(17):10138–10142. [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Stern J. B., Smith K. A. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986 Jul 11;233(4760):203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- Stetler G. L., Thorner J. Molecular cloning of hormone-responsive genes from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1144–1148. doi: 10.1073/pnas.81.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Telerman A., Amson R., Zakut-Houri R., Givol D. Identification of the human pim-1 gene product as a 33-kilodalton cytoplasmic protein with tyrosine kinase activity. Mol Cell Biol. 1988 Apr;8(4):1498–1503. doi: 10.1128/mcb.8.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B., Rapp U., App H., Greene M., Dobashi K., Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valge V. E., Wong J. G., Datlof B. M., Sinskey A. J., Rao A. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell. 1988 Oct 7;55(1):101–112. doi: 10.1016/0092-8674(88)90013-x. [DOI] [PubMed] [Google Scholar]
- Woodford T. A., Schlegel R., Pardee A. B. Selective isolation of newly synthesized mammalian mRNA after in vivo labeling with 4-thiouridine or 6-thioguanosine. Anal Biochem. 1988 May 15;171(1):166–172. doi: 10.1016/0003-2697(88)90138-8. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Hazum S., Givol D., Telerman A. The cDNA sequence and gene analysis of the human pim oncogene. Gene. 1987;54(1):105–111. doi: 10.1016/0378-1119(87)90352-0. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuidzinas A., Mamon H. J., Roberts T. M., Smith K. A. Interleukin-2-triggered Raf-1 expression, phosphorylation, and associated kinase activity increase through G1 and S in CD3-stimulated primary human T cells. Mol Cell Biol. 1991 May;11(5):2794–2803. doi: 10.1128/mcb.11.5.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]