Abstract
The aims of the study were to understand the racial/ethnic differences in cost of care and mortality in Medicare elderly with advanced stage prostate cancer.
This retrospective, observational study used SEER-Medicare data. Cohort consisted of 10,509 men aged 66 or older and diagnosed with advanced-stage prostate cancer between 2001and 2004. The cohort was followed retrospectively up to 2009. Racial/ethnic variation in cost was analyzed using 2 part-models and quantile regression. Step-wise GLM log-link and Cox regression was used to study the association between race/ethnicity and cost and mortality. Propensity score approach was used to minimize selection bias.
Pattern of cost and mortality varies between racial/ethnic groups. Compared with other racial/ethnic groups, non-Hispanic white patients had higher unadjusted costs in treatment and follow-up phases. Quintile regression results indicated that in treatment phase, Hispanics had higher costs in the 95th quantile and non-Hispanic blacks had lower cost in the 95th quantile, compared with non-Hispanic white men. In terminal phase non-Hispanic blacks and Hispanics had higher cost. After controlling for treatment, all-cause and prostate cancer-specific mortality was not significant for non-Hispanic black men, compared with non-Hispanic white men. However, for Asians, mortality remained significantly lower compared with non-Hispanic white men.
In conclusion, relationship between race/ethnicity, cost of care, and mortality is intricate. For non-Hispanic black men, disparity in mortality can be attributed to treatment differences. To reduce racial/ethnic disparities in prostate cancer care and outcomes, tailored policies to address underuse, overuse, and misuse of treatment and health services are necessary.
INTRODUCTION
Prostate cancer is the most prevalent cancer among elderly men in the U.S. with an estimated 233,000 new cases diagnosed in 2014.1 The median age at diagnosis of prostate cancer is 66 years and majority of prostate cancer survivors are 70 years or older.1 Annual Medicare expenditure is higher for prostate cancer than for any other cancer in elderly men and is expected to grow with the aging of the U.S. population.2 Over the years, management of prostate cancer has undergone significant changes, especially for advanced disease stage. Most men with advanced prostate cancer are elderly and often have multiple comorbidities that require complex care.3 Thus, caring for advanced stage prostate cancer is challenging due to the uncertainty regarding best course of treatment, associated costs, and outcomes.
Non-Hispanic black men have higher incidence of prostate cancer, higher mortality related to this disease, and are more likely to be diagnosed with advanced disease stage.1–4 Research shows that race/ethnicity is an important predictor of treatment for prostate cancer.4,5 However, limited information is available regarding racial/ethnic disparity in cost of care and mortality for advanced stage prostate cancer.4,6 Assessment of cost of care and mortality can yield valuable insight into intensity of care. Such information is crucial for development of healthcare policies aimed at improving the quality of care for Medicare elderly from all racial/ethnic groups.7–9 The objective of this study was to analyze the racial/ethnic differences in cost of care and 5-year mortality (prostate cancer-specific and all-cause), in elderly men with advanced stage prostate cancer, after adjusting for socio-demographic attributes, clinical attributes, and prostate cancer treatment. We hypothesized that cost of care and mortality will vary between racial/ethnic groups, racial/ethnic disparity in cost of care will exist at all levels of expenditure, and treatment can explain disparity in outcomes.
METHODS
Design Overview
We used SEER-Medicare linked data of the National Cancer Institute which bring together administrative claims and clinical tumor registry data for Medicare enrollees.10 SEER program collects data on cancer incidence, treatment and mortality from 16 SEER sites and encompasses 26% of the US population. Except for individuals enrolled in health maintenance organizations or those without Part B coverage, Medicare claims provides information about health services utilization for US residents aged 65 and older or for those disabled persons younger than 65 years. Of persons diagnosed with cancer at age 65 or older, and enrolled in SEER registries, 93% have Medicare claims match. SEER data also provide tumor characteristics that are essential to adjust for prostate cancer severity.10 This study was reviewed and approved by the Institutional Review Board of University of Pennsylvania.
Setting and Participants
We created a cohort of all non-Hispanic black, Asian, Hispanic, and non-Hispanic white men, aged 66 years or older, and diagnosed with advanced prostate cancer between 2001 and 2004. Eligible patients had no prior history of other cancer and had continuous coverage. Those who were alive at 5-year follow-up had continuous coverage of Part A and Part B and no HMO over the 5-year follow-up period. Those who had died within 5 years of diagnosis had continuous Part A and Part B coverage and no HMO until the time of death. From the SEER Patient Entitlement and Diagnosis Summary (PEDSF) file, advanced stage prostate cancer cases were identified by selecting regional or distant codes for the variable “Summary stage 2000.” This variable is derived from Collaborative Stages (CS) for 2004+ and extent of disease (EOD) before that, and is used in most SEER publications.11 We excluded those younger than 66 years of age at the time of prostate cancer diagnosis to ensure that we have at least 1 year of prediagnosis claims available to determine comorbidity scores. The longitudinal nature of the SEER-Medicare linked data enabled us to follow retrospectively each patient in our cohort for 1 year before and up to 5 years post his prostate cancer diagnosis. We defined treatment phase as the 1-year period after diagnosis of cancer, and follow-up phase as the period of 4 years posttreatment phase. For those who died during treatment or follow-up phase, we defined the 1-year period before death as the terminal phase.12–14
Outcome Variables
Direct Medical Care Cost
Direct medical care costs are the reimbursements made by Medicare12,14 and include reimbursements for inpatient hospitalizations, outpatient hospital visits, hospice care, home health agency, durable medical equipment, and physician and provider services.
Mortality
We used all-cause mortality data reported both by SEER and by Medicare. For SEER reported mortality, we constructed date of death using the SEER month and year of death. Since SEER does not report day of death, we assigned the middle of the month as the day of death. We coded a patient as deceased if SEER and/or Medicare reported so. Time to death was the time between prostate cancer diagnosis and death. We censored those patients who were alive at the end of 5-year follow-up. The variable “SEER cause-specific death classification” from PEDSF was used to discern if death was prostate cancer-specific.
Explanatory Variables
Treatment
Treatments for prostate cancer were grouped as follows: Surgery alone; surgery with radiation only, or with hormone therapy, and/or chemotherapy; radiation alone; radiation with hormone therapy, and/or chemotherapy; hormone therapy and/or chemotherapy; none. (treatment codes are reported in the Appendix).
Demographic and clinical information: we obtained demographic data on age and marital status (married vs. other) from the PEDSF file. Data on grade (moderately differentiated, poorly differentiated, undifferentiated, and unknown), and stage (regional vs. distant) were also obtained from the PEDSF file. We adjusted for disease severity using prostate cancer grade and comorbidity score. Charlson comorbidity score was calculated to assess medical comorbidity using inpatient, and outpatient claims in the 1-year prediagnosis period.15–18
Statistical Analysis
To begin with, we tested for underlying difference in demographic and clinical characteristics of non-Hispanic black, Asian, Hispanic, and non-Hispanic white patients from our cohort. We used 3 different approaches to analyze the cost of care. First, we used the 2-part model approach to study the disparity in cost of care across different racial/ethnic groups. Part 1 of the 2-part model is a logistic regression to model the odds of incurring cost as a function of race/ethnicity, after adjusting for demographic attributes, clinical attributes, stage, grade, treatment, and propensity score. Part 2 models the cost of care using a log-link and gamma distribution variance function for those who have incurred any cost.19–22 The second approach to analyze cost of care is using quantile regression. In 2-part models, the disparities in racial/ethnic group were measured at the population's mean conditional on socio-demographic variables. Mean differences may not capture difference occurring along different parts of the distribution. Therefore, we first compared the unadjusted cost distributions across 4 racial/ethnic groups and then applied quantile regression to study the magnitude and direction of disparity in cost of care at lower, middle, and higher percentiles of cost distribution.20 These analyses were conducted separately for treatment phase, follow-up phase, and terminal phase of care. Finally, in our third approach to analyze the association between race/ethnicity and cost of care, we used 3 sequential sets of generalized linear model (GLM) with a log-link and gamma distribution variance function [17–20]. In the first set, race/ethnicity, marital status, comorbidity, grade, stage, and geographic area (urban/rural) were the independent variables. Coefficient of race/ethnicity was the statistic of interest. In the second set of models, we included prostate cancer treatments and in the third set of models, we introduced propensity score. To develop propensity score, using multinomial logistic regression we first estimated, for each prostate cancer patient, the probability of receiving surgery, external beam radiation therapy, hormone therapy, chemotherapy, or no treatment based on age, race, grade, stage, geographic location, marital status, and comorbidity score.20 To study the extent of treatment group matching, we compared the t-statistics for these covariates before and after adjusting with propensity score. Cox regression was used to study the association between race/ethnicity and mortality (prostate cancer-specific and all-cause), with the help of 3 sets of sequential models.
RESULTS
Sample Characteristics
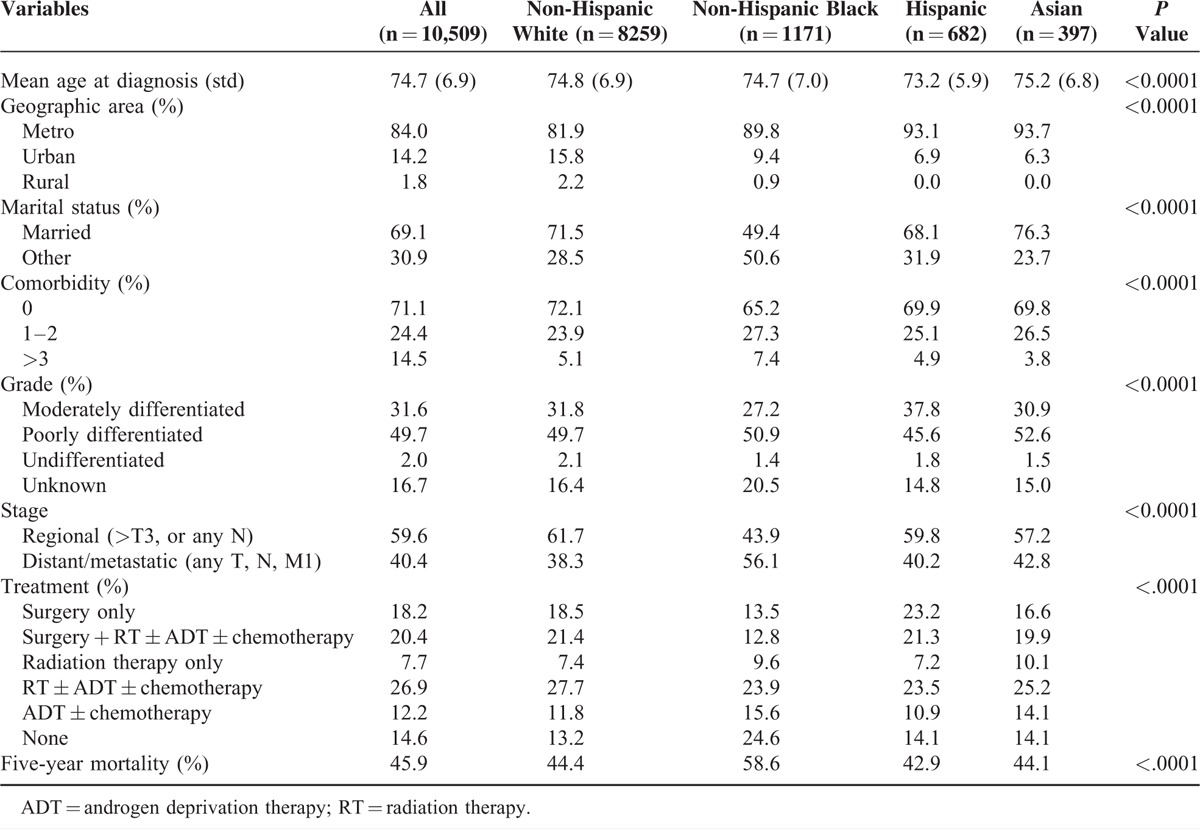
Our cohort consisted of 10,509 fee-for-service elderly (aged ≥66 years) Medicare beneficiaries diagnosed with advanced stage prostate cancer between 2001 and 2004. We observed significant differences in the demographic and clinical characteristics between racial/ethnic groups (Table 1). Hispanic patients were slightly younger than other racial/ethnic groups. Non-Hispanic black patients were less likely to be married and had higher comorbidity, compared with other groups. Treatment differed between racial/ethnic groups. A higher proportion of non-Hispanic black patients did not receive treatment compared with other racial/ethnic groups.
TABLE 1.
Baseline Demographic and Clinical Characteristics (n = 10,509)
Cost of Care Outcome
In Table 2, we present the results of 2-part models for costs during treatment phase, follow-up phase, and terminal phase.
TABLE 2.
Two-Part Model for Cost Across Phases of Care
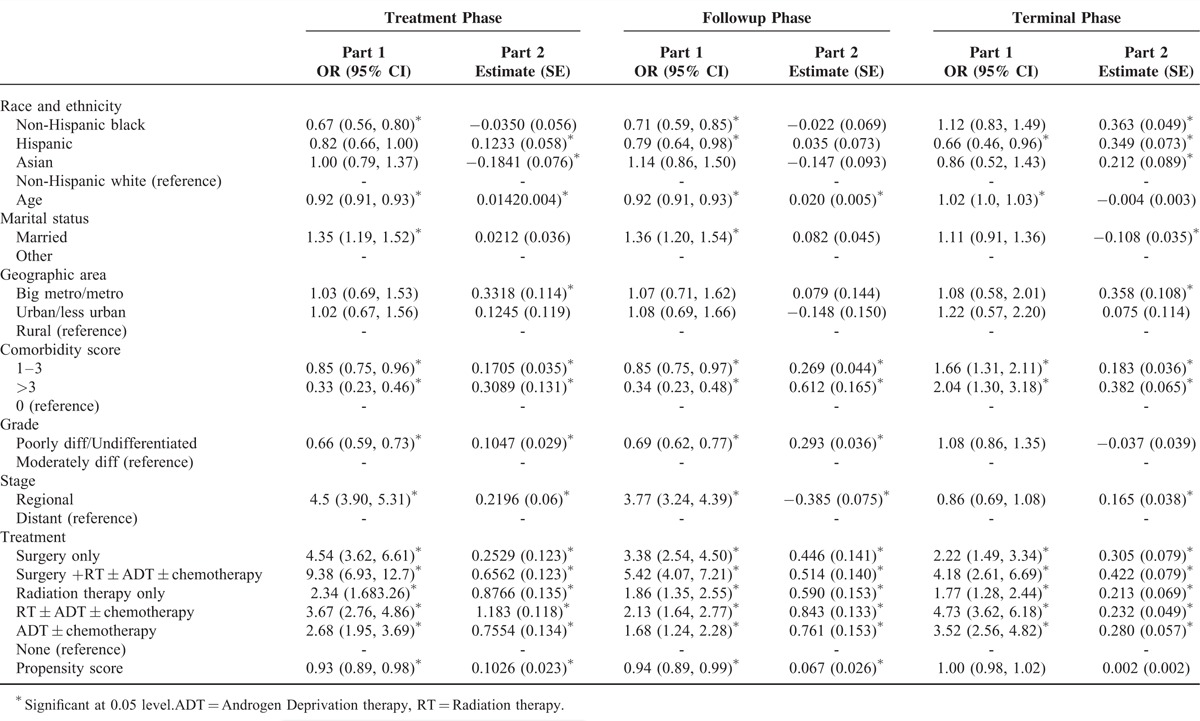
Treatment Phase
Part 1 of the 2-part model indicates that the odds of incurring costs in treatment phase were significantly lower for non-Hispanic black elderly men (odds ratio (OR) = 0.67; 95% confidence interval (CI) = 0.56, 0.80). However, Part 2 of the 2-part model shows that among those who incurred costs, Asians had significantly lower cost of care in the treatment phase (estimate = −0.1841, standard error (SE) = 0.076). On the other hand, Hispanic patients had higher cost (estimate = 0.1233, SE = 0.058), compared with non-Hispanic whites.
Follow-Up Phase
For this phase, we observed results somewhat similar to those for the treatment phase. Non-Hispanic blacks and Hispanics had lower odds of incurring costs (OR = 0.71, CI = 0.59, 0.85; and OR = 0.79, CI = 0.64, 0.98, respectively).
Terminal Phase
In this phase, Hispanic patients had significantly lower odds of incurring any cost (OR = 0.66, CI = 0.46, 0.96), compared with non-Hispanic white patients. Among those who incurred any cost, compared with non-Hispanic white group, all other racial/ethnic groups had higher cost, the magnitude being highest for non-Hispanic black patients.
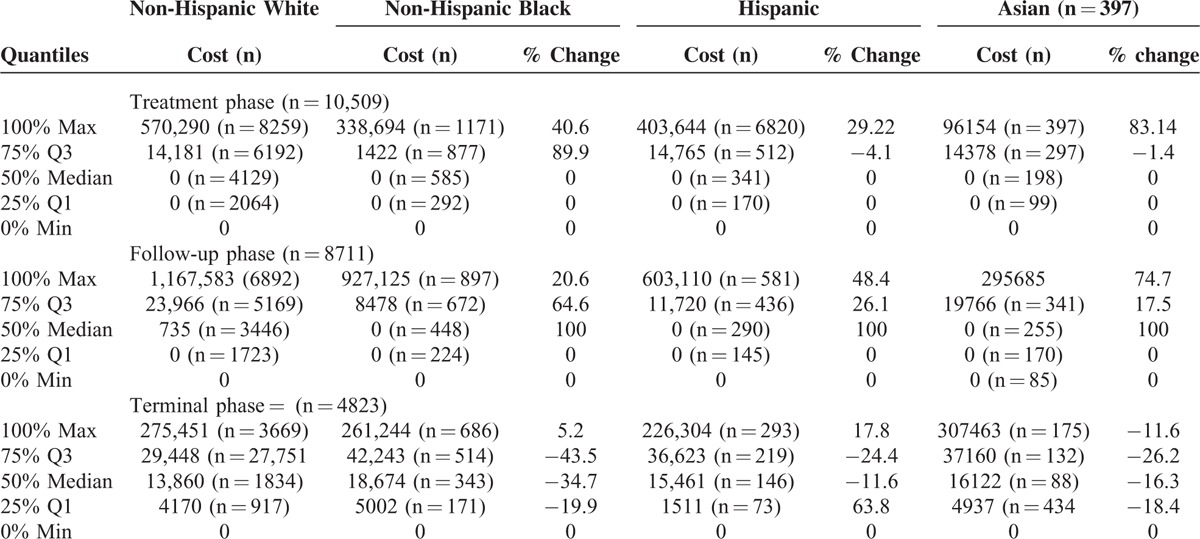
In Table 3, we present cost distribution for each racial/ethnic group during the 3 phases of care using quantile regression. For treatment phase, all racial/ethnic groups had zero costs over the lower quarters of the distribution. Non-Hispanic black patients had lower costs at 75th and 100th percentiles, and Hispanic and Asian patients had lower costs at 100th percentile, compared with non-Hispanic white patients. For the follow-up period, the difference in cost was highest at the median. Compared with non-Hispanic white group, all other racial/ethnic groups had lower costs across the distribution. However, the magnitude of the differences varied. The pattern of cost distribution also varied for the terminal phase. In this phase, compared with non-Hispanic white patients, Asian patients had higher cost over the entire distribution, whereas non-Hispanic black patients had higher costs at the 25th, 50th, and 75th percentiles. Hispanic patients had higher cost at the 50th and 75th percentiles only.
TABLE 3.
Distribution of Cost Across Phases ($)
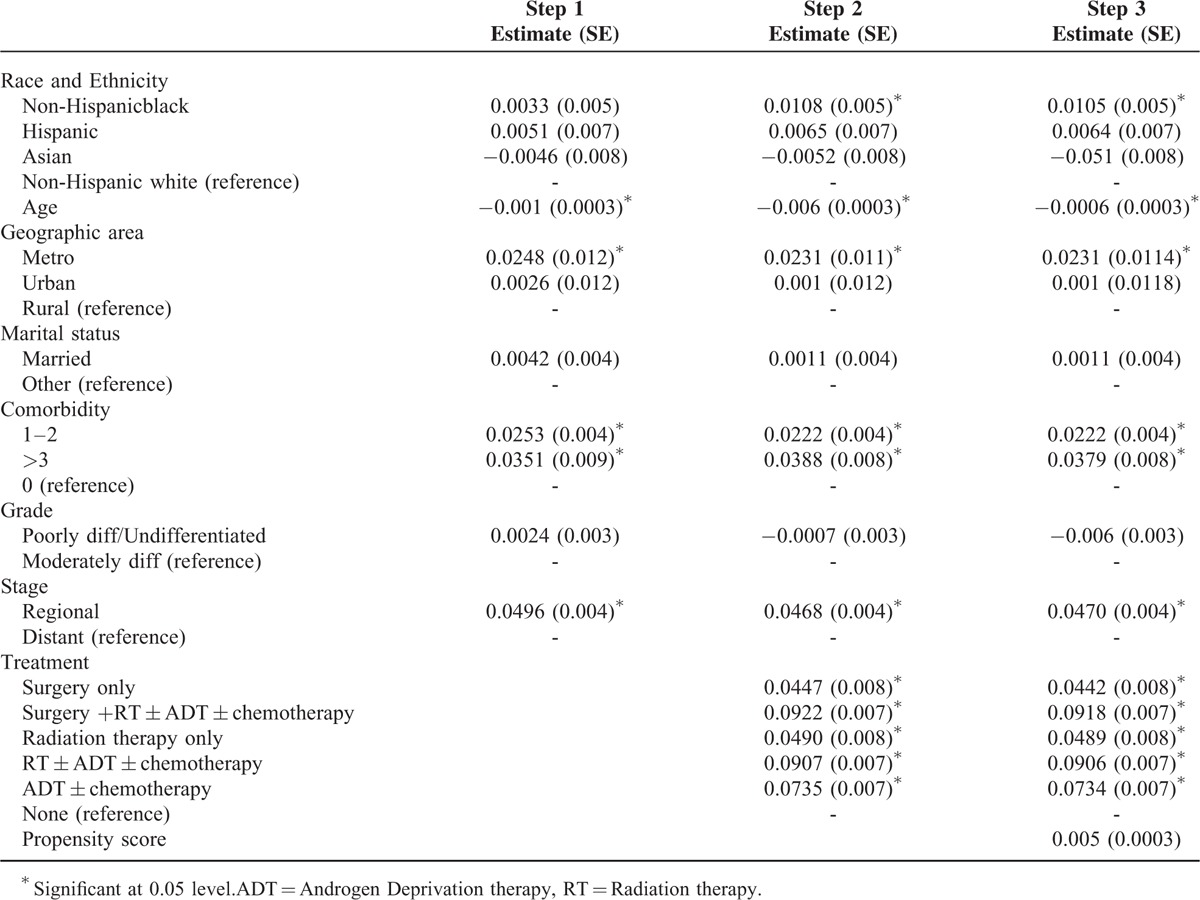
The results of quantile regression are presented in Table 4. The dependent variable was log of cost and we adjusted for socio-demographic and clinical variables, grade, stage, treatment type, and propensity score. For the treatment phase, no disparities were observed at the lower 3 quantiles. At 95th percentile, non-Hispanic black patients had significantly lower cost of care and Hispanic patients had higher cost of care, compared with non-Hispanic white patients. During the follow-up phase, compared with non-Hispanic white patients, all others had lower cost of care at 25th, 50th, and 75th percentiles. For terminal phase, non-Hispanic blacks had higher cost of care at all quantiles of the cost distribution, and Hispanics had higher cost of care at 75th and 95th percentiles, compared with non-Hispanic white patients. Results of our third approach to analyze cost of care are presented in Table 5. As seen from the last column, the overall cost of care was significantly higher for non-Hispanic black group compared with non-Hispanic white group.
TABLE 4.
Quantile Regression
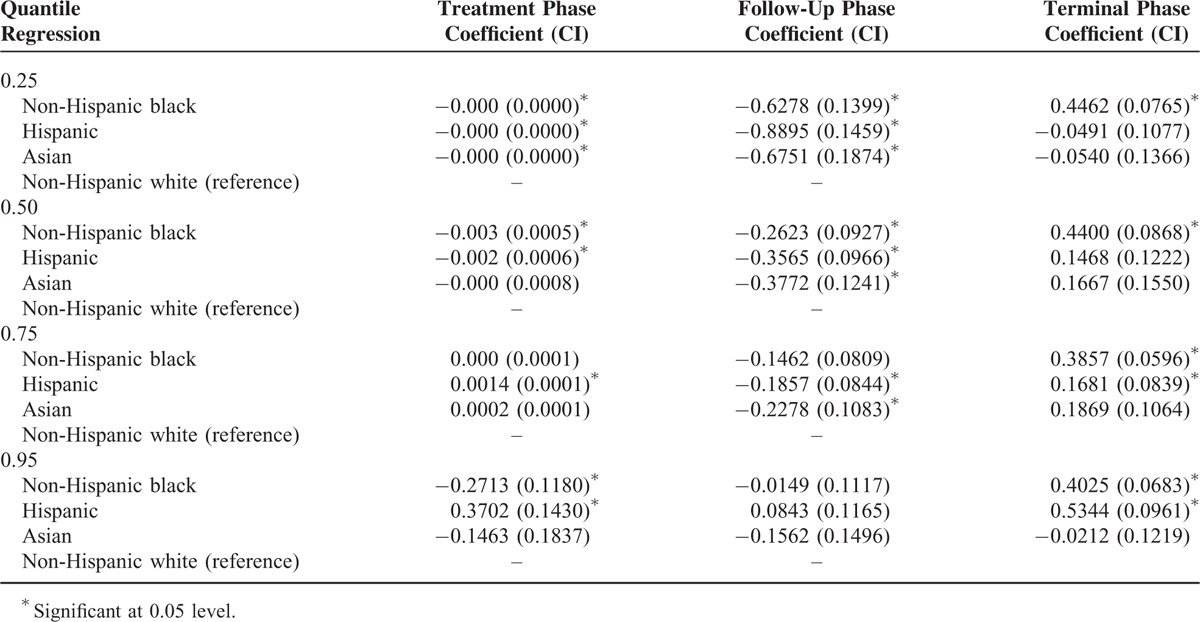
TABLE 5.
Race and Ethnicity and Five Years Total Cost [Step Wise GLM-Log Link Model
Mortality
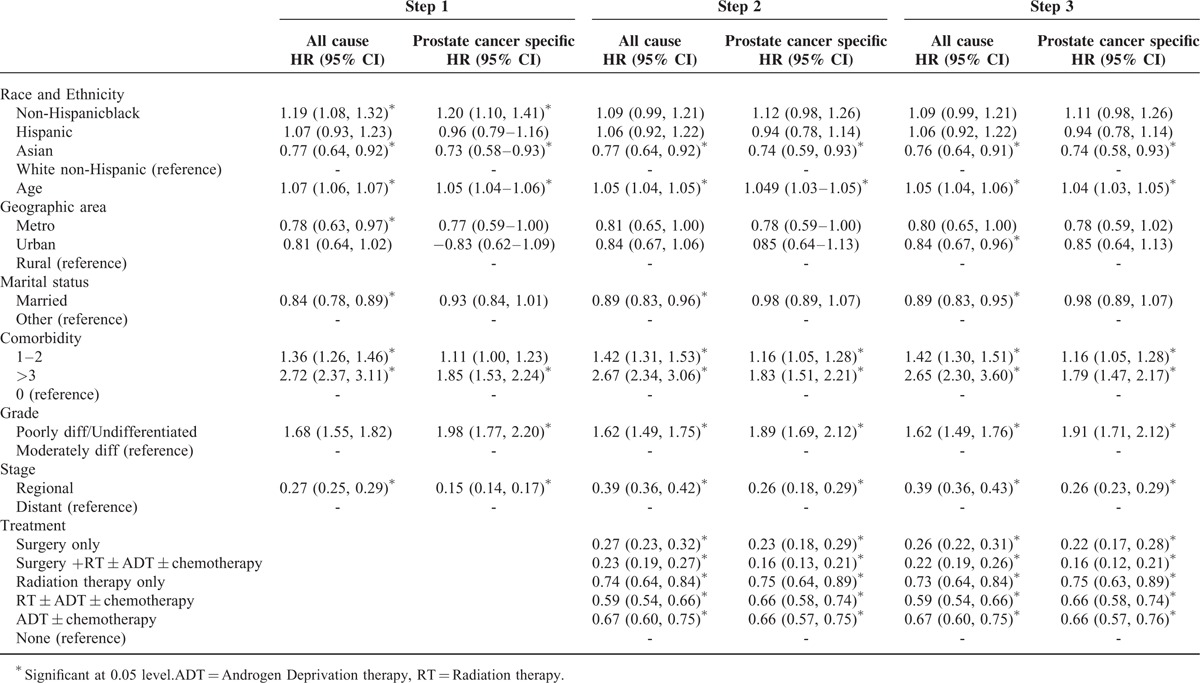
Table 6 depicts sequentially adjusted models for all-cause and prostate cancer-specific mortality. In step 1, non-Hispanic black patients had higher hazard of all-cause mortality (HR = 1.19, CI = 1.08, 1.32), whereas Asians had lower hazard of all-cause mortality (HR = 0.77, CI = 0.64, 0.92), compared with non-Hispanic whites. Similarly, non-Hispanic black men had higher hazard of prostate cancer-specific mortality (HR = 1.20, CI = 1.10, 1.41) and Asian men had lower hazard of prostate cancer-specific mortality (HR = 0.73, CI = 0.58, 0.93) compared with non-Hispanic white men. However, after adjusting for prostate cancer treatment and propensity score (Step 2 and Step 3), the hazard of all-cause mortality and prostate cancer-specific mortality was not significantly higher for non-Hispanic black patients compared with non-Hispanic white patients. On the other hand, for Asians, the hazard of all-cause mortality (HR = 0.76, CI = 0.64, 0.91) and prostate cancer-specific mortality (HR = 0.74, CI = 0.58, 0.93) remained lower compared with non-Hispanic white patients.
TABLE 6.
Race/Ethnicity and Five Year Mortality-Step Wise Cox Regression Models
DISCUSSION
In this population-based study, we found significant racial/ethnic disparity in outcomes among Medicare fee-for-service elderly with advanced stage prostate cancer. Important findings of the study are as follows: first, pattern of cost and mortality varies between racial/ethnic groups; second, prostate cancer treatment may explain some of the racial/ethnic disparity in mortality and cost; third, lower all-cause mortality and prostate cancer-specific mortality was observed among Asian men; and fourth, non-Hispanic black and Hispanic elderly with advanced prostate cancer have higher costs in terminal phase. Some studies have shown that treatments for a given stage of prostate cancer vary by age and ethnicity,4,5,23 while others have addressed the variations in cost and disease-specific mortality.2,24 However, phase-specific costs and mortality in elderly patients with advanced prostate cancer remain unexplored. Our study fulfills some of these gaps in knowledge.
Racial/ethnic disparities in healthcare are defined as “racial/ethnic differences in the quality of healthcare that are not due to access-related factors or clinical needs”6 and are viewed as a major quality problem.6,23 Disparities are multifaceted issues and involve patient, provider, healthcare system, community, and environmental factors.25 Understanding the disparity in mortality and the pattern of variation along different areas of cost distribution is crucial for development of policies aimed at improving quality of care for all racial/ethnic groups. Quality of healthcare is defined as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.”25 Quality of care for advanced prostate cancer care comes in 3 distinct forms: overuse, underuse, and misuse. Services provided in circumstances where patients cannot benefit lead to overuse. Underuse occurs when a provider fails to provide proven effective care, and misuse occurs when a provider delivers a service that could be beneficial, but performs it with inadequate skill and exposes the patient to a preventable complication.
In this study, we assessed the racial/ethnic disparities in cost and mortality in Medicare elderly with advanced prostate cancer. We observed that disparities fluctuated along different parts of the cost distribution, both between and within racial/ethnic groups. After adjusting for socio-demographic attributes, clinical attributes, treatment, and propensity score, these disparities persisted along higher end of cost distribution for Hispanic and non-Hispanic black men in the treatment phase, and for Hispanic and Asian men in the follow-up phase. For the terminal phase of care, after adjusting for socio-demographic attributes, clinical attributes, treatment, and propensity score, disparities in costs persisted for non-Hispanic black and Hispanic men, compared with non-Hispanic white men.
These differences may reflect the differences in intensity of services received by each racial/ethnic group. Differences at the lower end of cost distribution indicate disparity in basic services. On the other hand, disparities at the higher end of cost distribution may imply that intensity of services differs even among those who are sicker and thus have greater need for the services. Mortality is an important measure of quality of care. Our results indicate that the hazard of mortality varies between racial/ethnic groups. Asian elderly men with advanced stage prostate cancer had lower hazard of mortality, as well as lower cost of care in treatment and follow-up phases compared with non-Hispanic white men. For non-Hispanic black men, the hazard of all-cause and prostate cancer-specific mortality was not significant after adjusting for treatment; however, the cost of care in this group remained elevated after adjusting for treatment, compared with non-Hispanic white men. These results have clear implications for quality of prostate cancer care. It is observed that addressing disparity in treatment can help in reducing mortality (prostate cancer-specific and all-cause) among non-Hispanic black men. Additionally, a higher proportion of non-Hispanic blacks in our cohort did not receive any treatment, indicating under-treatment, which may have direct implications for mortality. Thus, it is clear that opportunities exist to improve the quality of care without increasing cost, via effective management of overuse, underuse, and misuse of treatment and health services.
We note some limitations to our study. Our study cohort included non-Hispanic black, Hispanic, non-Hispanic white, and Asian men aged 66 or older, living in an SEER area and not enrolled in an HMO. Although SEER data are a representative sample of the US, only a relatively small Hispanic population is included. Our findings may not be generalizable to men younger than 66 years of age or to men enrolled in HMOs. The age and sex distribution for individuals 66 years and older in the SEER areas is comparable with that of the US elderly population. However, the SEER area distribution differs from that in the US elderly population in that SEER areas have a lower portion of whites and a higher concentration of persons of other races. Another imitation of claims data is potential for misclassification of codes. It is possible that some biases remain unaccounted for and we plan to address those in our future studies. Additionally, at the time of the study, SEER data were available for up to year 2007 and linked Medicare claims were available for up to year 2009. We used cases diagnosed between 2001 and 2004 so that at least 5 years of follow-up Medicare data will be available.
A major concern of policymakers in the US is the escalating cost of care and disparities in care and outcomes.6,8 New treatment options for advanced stage prostate cancer translate into new opportunities to improve outcomes, and thus careful consideration and management of new therapies is needed.26,27 Our results show that the relationship between race/ethnicity, cost of care, and mortality along different phases of care is complex. Future research can address treatment-specific attributes and other factors that contribute to the observed racial/ethnic variation in advanced stage prostate cancer care and outcomes. Thus, addressing the issues of underuse, overuse, and misuse of treatment and health services may be an appropriate strategy to alleviate disparities and improve quality of care for all racial/ethnic groups.
Footnotes
Abbreviations: GLM = generalized linear model, PEDSF = patient entitlement and diagnosis summary, SEER = surveillance epidemiology and end results.
SC and RJ planned the study, secured the data, conducted the data analysis, and wrote the paper.
JSS and SBM contributed to writing and revising the paper. This work was supported by the National Institute on Aging at the National Institutes of Health (grant # R21AG034870-01A1), the Department of Defense (grant # W81XWH-12-1-0089-PC110707), and Agency for Healthcare and Research Quality 1R01HS024106-01.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel R, Ma J, Zoy Z, et al. Cancer Statistics 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Instit 2008; 100:630–641. [DOI] [PubMed] [Google Scholar]
- 3.Fung C, Dale W, Mohile SG. Prostate cancer in the elderly patient. J Clin Oncol 2014; 55:1531–1539. [DOI] [PubMed] [Google Scholar]
- 4.Gross CP, Smith BD, Wolf E, et al. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer 2008; 112:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polednak AP. Prostate cancer treatment in black and white men: the need to consider both stage at diagnosis and socioeconomic status. J Natl Med Assoc 1998; 90:101–104. [PMC free article] [PubMed] [Google Scholar]
- 6.Smedley BD, Stith AY, Nelson AR. (Editors). Unequal Treatment-Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academic Press; 2002. [PubMed] [Google Scholar]
- 7.Yannurajalingam S, Atkinson B, Masterson J, et al. The impact of an outpatient palliative care consultation on symptom burden in advanced prostate cancer patients. J Palliat Med 2012; 15:20–24. [DOI] [PubMed] [Google Scholar]
- 8.Penson DF, Chan JM. Urologic Diseases in America. Washington, DC2007. US Government Printing Office, 2007; NIH Pub. No. 07-5512 [pp. 71–123]. [Google Scholar]
- 9.Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer 2007; 109:518–527. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunder CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research application and generalizability to the United States elderly population. Med Care 2002; 40 (supp): IV-3-18. [DOI] [PubMed] [Google Scholar]
- 11.Adamo MB, Johnson CH, Ruhl JL, Dickie Le. 2013 SEER Program Coding and Staging Manual. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 12.Brown ML, Riley GF, Schussler N, et al. Estimating care costs related to cancer treatment from SEER-Medicare data. Med Care 2002; 40 Suppl: IV-104-117. [DOI] [PubMed] [Google Scholar]
- 13.Riley GF, Potosky AL, Lubitz JD, et al. Medicare payments from diagnosis to death for elderly cancer patients by stage and diagnosis. Med Care 1995; 33:828–841. [DOI] [PubMed] [Google Scholar]
- 14.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst 2008; 100:888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 17.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993; 46:1075–1079. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 19.Zhou XH, Melfi CA, Hui SL. Methods of comparison of cost data. Ann Intern Med 1997; 127:752–756. [DOI] [PubMed] [Google Scholar]
- 20.Green WH. Econometric Analysis. Upper Saddle River, NJ: Prentice-Hall; 2007. [Google Scholar]
- 21.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001; 20:461–494. [DOI] [PubMed] [Google Scholar]
- 22.Allison PD. Logistic Regression Using the SAS System: Theory and Application. Cairy, NC: SAS Institute Inc; 1999. [Google Scholar]
- 23.Marlow NM, Halpern MT, Pavluck AI, et al. Disparities associated with advanced prostate cancer stage at diagnosis. J Health Care Poor Underserved 2010; 21:112–131. [DOI] [PubMed] [Google Scholar]
- 24.Hu Yue-Yung, Kwok AC, Jiang W, et al. High cost imaging in elderly patients with stage IV cancer. J Natl Cancer Inst 2012; 104:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on quality of health care in America-Institute of Medicine. Cross the Quality Chasm: a new health care system for the 21st century. Washington, DC, 2005. [Google Scholar]
- 26.Payne H, Bahl A, Mason M, et al. Optimizing the care of patients with advanced prostate cancer in the UK: current challenges and future opportunities. BJUI 2012; 110:658–667. [DOI] [PubMed] [Google Scholar]
- 27.Polednak AP. Black-white differences in survival from late-stage prostate cancer. Ethn Dis 2003; 13:220–225. [PubMed] [Google Scholar]


