Abstract
In a recent genome-wide association study, the zinc finger, C3HC-type containing 1 (ZC3HC1) polymorphism was strongly associated with coronary artery disease (CAD) by an unknown mechanism. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is related with CAD through low-density lipoprotein (LDL) metabolism. The association of both of the above genetic variants with hypertension was studied in a Finnish 50-year-old cohort.
A total of 325 hypertensive cases and 444 nonhypertensive controls were obtained from the Tampere adult population cardiovascular risk study. Samples were genotyped for ZC3HC1 rs11556924 and PCSK9 rs11206510 polymorphisms using Competitive Allele Specific PCR technique. A subpopulation that had available follow-up data from ages of 40, 45, and 50 years was also analyzed.
ZC3HC1 rs11556924 (C > T) genotype CC was associated with hypertension compared with the T-allele carriers (P = 0.013). PCSK9 rs11206510 (T > C) genotype was not associated with hypertension. Its major TT-genotype was associated with higher total cholesterol (P = 0.044) and LDL (P = 0.029) compared with the C-allele.
We report for the first time that ZC3HC1 rs11556924 was associated with essential hypertension in 50-year-old patients. Although PCSK9 rs11206510 was not associated with hypertension, our study confirms its association with serum cholesterol levels.
INTRODUCTION
Genome-wide association studies (GWAS) performed in recent years have shown which genes are most strongly associated with cardiovascular disease. GWAS in patients with coronary artery disease (CAD) have revealed about 30 genes that are likely to represent the most important genetic risk factors.1 Although many of these genes have been identified previously, most of them are involved in pathways that are not known to be associated with metabolic disturbances contributing to atherogenesis.2 The known pathways include elevated blood pressure and increase of low-density lipoprotein (LDL).
According to the largest GWAS for CAD, the CARDIoGRAM study,1 several well-known lipid genes stand out, such as those encoding the LDL receptor and the associated proprotein convertase subtilisin/kexin type 9 (PCSK9),3 supporting the importance of LDL in atherosclerosis. However, most of the loci had no effects on known risk factors and were not involved in pathways previously associated with atherosclerosis.1,2 These include the zinc finger, C3HC-type containing 1 (ZC3HC1) rs11556924 polymorphism.1,4 ZC3HC1 also called nuclear interaction partner of ALK. ZC3HC1 is a mammalian E3 ligase4 that regulates mitotic entry and may contribute to the development of carcinogenesis together with constitutively active oncogenic proteins.5 To our knowledge, after the GWAS findings for CAD,1 there is only one study that addressed a further association of ZC3HC1 rs11556924 with carotid intima-media thickness (CIMT), a marker of CAD risk.6 Genetic–epidemiologic studies have shown that the genetic component of atherosclerosis tends to diminish with increasing age of the population.2 Therefore, we wanted to assess the roles of PCSK9 rs11206510 and ZC3HC1 rs11556924 variants in a Finnish 50-year-old population, by analyzing cohorts from the Tampere adult population cardiovascular risk study (TAMRISK).7
METHODS
Participants
The TAMRISK study is based on periodic health examinations (PHE) done for local 50-year-old patients in the city of Tampere, Finland, as previously described.7,8 The basic 60-minute interview by a public health nurse was done on the basis of a self-administered questionnaire. Questions included current and previous diseases as diagnosed by a physician, including hypertension. Blood pressure was registered (mm of mercury) using a calibrated mercury sphygmomanometer. Total cholesterol (mmol/L) and glucose (mmol/L) were analyzed from serum samples after an overnight fast by standard techniques. Participants gave informed consent to use PHE data, and the study was approved by Ethics Committees of the Tampere University Hospital and the City of Tampere. Buccal swabs for DNA extraction were collected by mail during years 2006 to 2010.
For the cases who had a diagnosis of hypertension by the age of 50 years (n = 325), at least 1 matching normotensive control (n = 444) was chosen on the basis of sex and smoking habit in the order of admission from the PHE cohort (n = 6000). The present study population at the age of 50 years thus included 769 patients. Of these same individuals, we also analyzed the subpopulation of men and women who had available previous PHE data from the ages of 45 years (n = 675) and 40 years (n = 592).
DNA Genotyping
A commercial kit was used to extract DNA from the collected buccal swabs (Qiagen Inc, Valencia, CA). After transferring the samples into 96-well plates, they were genotyped for ZC3HC1 rs11556924 and PCSK9 rs11206510 polymorphisms at the KBioscience Institute (UK) using Competitive Allele Specific PCR technique.
Statistical Analysis
Sample size was calculated by Quanto 1.2.4 (Copyright© 2000–2009, University of Southern California), with a choice of gene-only model. According to the World Health Organization, a prevalence ratio of raised blood pressure in adults aged >25 is about 40%. The T-allele frequency of ZC3HC1 rs11556924 was 0.1558 reported by National Center for Biotechnology Information. Using 80% power, a type I error rate of 0.05 and a 2-sided statistical test, the required sample size per group was calculated to be 101. The C-allele frequency of PCSK9 rs11206510 was 0.1018 reported by National Center for Biotechnology Information. Using 80% power, a type I error rate of 0.05 and a 2-sided statistical test, the required sample size per group was calculated to be 136. A total of 325 cases and 444 controls with successful genotyping were included in the final study population.
Statistical analyses were run by SPSS 20.0 (SPSS Inc, Chicago, IL). Group differences for continuous variables were tested by t test or analysis of variance (ANOVA). For categorical variables the χ2 test was used. Binary logistic regression was applied to assess risk factors for hypertension. P values were considered significant if <0.05. The ANOVA for repeated measures was used to assess the differences in mean blood pressures between genotypes at the age of 40, 45, and 50 years.
RESULTS
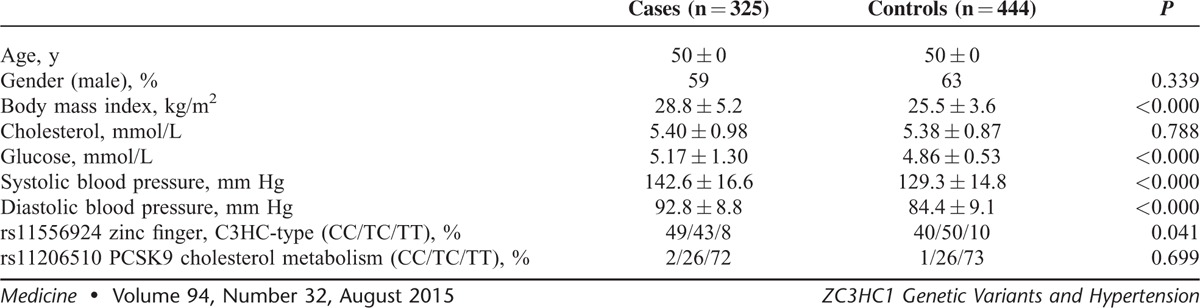
Table 1 shows the background characteristics of the 50-year-old cases with hypertension (325) and their controls (444). Significant differences between the groups were found in body mass index (BMI), glucose, and blood pressure, although cholesterol levels were similar. Genotype frequencies for ZC3HC1 rs11556924 were 0.43 for CC (n = 333), 0.47 for TC (n = 361), and 0.10 (n = 75) for TT. These frequencies were in Hardy–Weinberg equilibrium (P = 0.108). There was a significant difference between the variant frequencies of the hypertension group and normotensive group (P = 0.041). For PCSK9 rs11206510, the genotype frequencies were 0.02 for CC (n = 14), 0.26 for TC (n = 201), and 0.72 (n = 554) for TT. These frequencies were in Hardy–Weinberg equilibrium (P = 0.386), and no difference between the groups was found.
TABLE 1.
Clinical Characteristics (Means ± SD) of the Study Population at the Age of 50 Years
When ZC3HC1 rs11556924 (genotype CC vs T-allele), BMI, and glucose were all included in logistic regression, ZC3HC1 genotype CC (P = 0.007, odds ratio [OR]: 1.42, 95% confidence interval [CI]: 1.10–1.84), BMI (P < 0.001, OR: 1.19, 95% CI: 1.14–1.24), and glucose (P = 0.002, OR: 1.46, 95% CI: 1.15–1.78) were associated with hypertension.
The statistical analysis of clinical characteristics between different genotype groups for ZC3HC1 rs11556924 is shown in Table 2. Genotype CC was associated with hypertension at the age of 50 years compared with the T-allele carriers (P = 0.013). Patients with genotype CC had significantly higher diastolic blood pressure at the age of 45 years than T-allele carriers (P = 0.045). In addition, at the age of 40, patients with genotype TT had significantly lower diastolic blood pressure than C-allele carriers (P = 0.004). There were no differences between the genotype groups in BMI, systolic blood pressure, serum cholesterol, or prevalence of coronary heart disease.
TABLE 2.
Clinical Characteristics (Means ± SD) of the Study Population Stratified According to ZC3HC1 rs11556924 Polymorphism
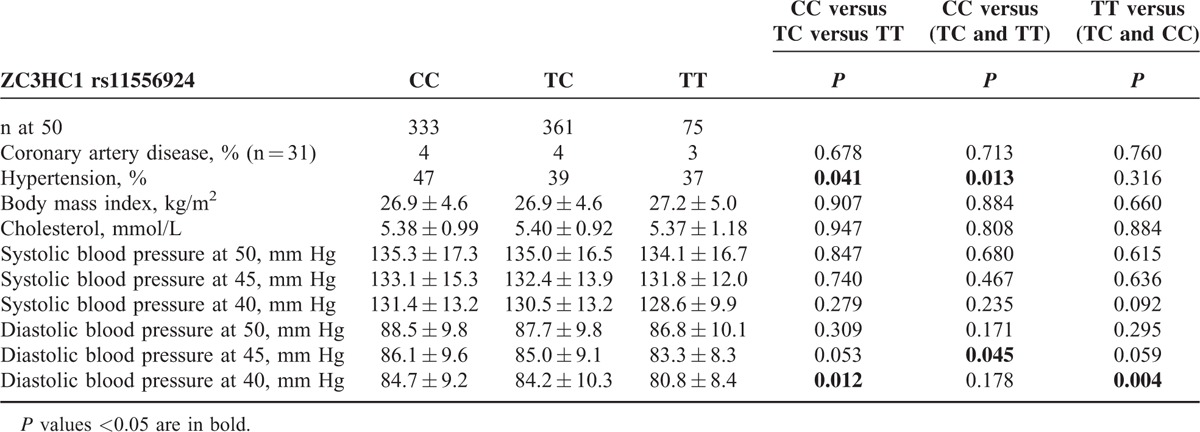
When the 40-, 45-, and 50-year follow-up data of blood pressure measurements in Table 2 were analyzed by ANOVA for repeated measures, it was apparent that diastolic blood pressure was lowest in genotype TT, and higher in the order of CT and CC (P = 0.019). By post hoc analysis, the difference was between TT and CT (P = 0.006), as well as TT and CC (0.049). The difference between genotypes TT and CC in diastolic blood pressure was 3.9 mm Hg at its highest at the age of 40 years. There was no time by genotype interaction; blood pressure increased in all genotype groups (P = 0.275). The trend was similar between genotypes for systolic blood pressure, but not statistically significant (P = 0.349). However, each registration of blood pressure was made at one examination visit only, and most of the patients with hypertension were already on medication by the age of 50 years.7
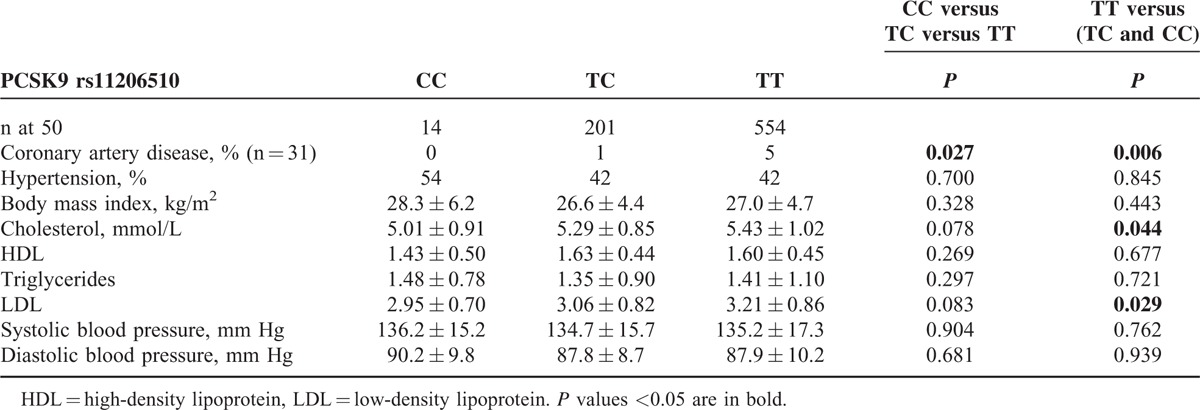
The statistical analysis of clinical characteristics between different genotype groups for PCSK9 rs11206510 is shown in Table 3. The minor C-allele was associated with lower serum cholesterol (P = 0.044) and LDL (P = 0.029) levels compared with patients with TT-genotype. The TT-genotype was also associated with early CAD, when compared with the C-allele carriers (P = 0.006).
TABLE 3.
Clinical Characteristics (Means ± SD) of the Study Population Stratified According to PCSK9 rs11206510 Polymorphism
DISCUSSION
The genetic variant of ZC3HC1 rs11556924 (C > T) is located at 7q32.2 and encodes a nonsynonymous substitution (R363H) in the ZC3HC1 gene. The ZC3HC1 rs11556924 C-allele was strongly associated with CAD in European patients by a so far unknown mechanism.1 In accordance with these findings, we report for the first time that the genotype CC is also associated with hypertension in a well-documented cohort follow-up population, the TAMRISK study. The present study was conducted in Finland, where >48% of 25- to 64-year-old adults have mean systolic pressure readings of ≥140 mm Hg, among the highest levels in Europe.9 The hereditability of hypertension has been estimated to cause 30% to 50% of the disease.10 On the contrary, only a few percent of the genes behind blood pressure have been explained by candidate-gene and GWAS studies.11 They have so far not included variations in ZC3HC1.12
ZC3HC1 is a protein that monitors the timing of mitotic entry and is thought to contribute to the development of carcinogenesis together with oncogenic proteins.5 A recent investigation by López-Mejías et al6 has shown that rheumatoid arthritis patients carrying the ZC3HC1 rs11556924 TT-genotype had significantly higher CIMT values than those homozygous for the CC-genotype.6 CIMT is marker of subclinical atherosclerosis. Their hypothesis was that changes in the stability and functional properties of ZC3HC1 protein may play a role in endothelial dysfunction and, in the long run, in the development of atherosclerosis. These results are somewhat in contrast to our findings of an association of the CC-genotype with the risk of hypertension and the findings by Schunkert et al1 that report an association of the C-allele with CAD.
Hypertension is promoted by arterial stiffness with vascular smooth muscle cell (VSMC) hypertrophy, which is strongly associated with VSMC polyploidy. Despite extensive investigation of growth factors in VSMC polyploidy,13 little is known of molecular mechanisms underlying this phenomenon in hypertensive individuals. Because ZC3HC1 is a contributor to control of mitotic phase,4 its dysfunction could theoretically lead to such cell cycle reentry.
We also sought for associations to the phenotypes observed in our population with variations in PCSK9, a protease involved in cholesterol metabolism by controlling LDL receptor (LDLR) protein levels, and consequently blood LDL levels. PCSK9 accelerates the degradation of hepatic LDLR,14 and inactivation of PCSK9 would theoretically decrease LDL levels. Several PCSK9 loss-of-function mutations associated with low LDL cholesterol plasma levels have been described and variations in this gene decrease the risk of CAD.15 Accordingly, it has been observed that African Americans who had a loss-of-function PCSK9 mutation leading to 40 mg/dL lower LDL-C had a 90% reduction in coronary events in their middle years.16 The association of total and LDL cholesterol has been replicated with the SNP rs11206510, where the minor C-allele was significantly associated with lower concentration of both LDL and total cholesterol in an Italian population.17 We have replicated this result and also demonstrate an association of the TT-genotype with early CAD.
The study group was restricted to residents of a large city in Finland, which poses a challenge to how broadly one can apply the findings. Because the study patients are from a restricted genetic pool (Finnish Caucasian), the findings might not extrapolate to different genetic populations.
In conclusion, our results indicate that ZC3HC1 rs11556924 polymorphism is associated with hypertension in the TAMRISK study. Our results also confirm the results of others that PCSK9 SNP rs11206510 is associated with increased serum levels of cholesterol.
ACKNOWLEDGMENTS
The authors thank all the participants of the TAMRISK study. The expert technical assistance by Mirka Pietiläinen is gratefully acknowledged.
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, CAD = coronary artery disease, CIMT = carotid intima-media thickness, GWAS = genome-wide association studies, LDL = low-density lipoprotein, LDLR = LDL receptor, PCSK9 = proprotein convertase subtilisin/kexin type 9, PHE = periodic health examinations, TAMRISK = Tampere adult population cardiovascular risk study, VSMC = vascular smooth muscle cell, ZC3HC1 = zinc finger, C3HC-type containing 1.
This work was supported by grants from Competitive research funding of the Pirkanmaa Hospital District.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Schunkert H, König IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011; 43:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lusis AJ. Genetics of atherosclerosis. Trends Genet 2012; 28:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JC, Boerwinkle E, Mosley TH, Jr, et al. Sequence variations inPCSK9, lowLDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 4.Bassermann F, von Klitzing C, Münch S, et al. NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry. Cell 2005; 122:45–57. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Morris SW. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev 2008; 3:372–412. [DOI] [PubMed] [Google Scholar]
- 6.López-Mejías R, Genre F, García-Bermúdez M, et al. The ZC3HC1 rs11556924 polymorphism is associated with increased carotid intima-media thickness in patients with rheumatoid arthritis. Arthritis Res Ther 2013; 15:R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunnas T, Määttä K, Palmroos P, et al. Periodic cohort health examinations in the TAMRISK study show untoward increases in body mass index and blood pressure during 15 years of follow-up. BMC Public Health 2012; 12:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunnas T, Nikkari ST. Contribution of syndecan-4 genetic variants to hypertension, the TAMRISK study. BMC Res Notes 2014; 7:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geleijnse JM, Kok FJ, Grobbee DE. Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. Eur J Public Health 2004; 14:235–239. [DOI] [PubMed] [Google Scholar]
- 10.Kupper N, Willemsen G, Riese H, et al. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 2005; 45:80–85. [DOI] [PubMed] [Google Scholar]
- 11.Munroe PB, Johnson T, Caulfield M. The genetic architecture of blood pressure variation. Curr Cardiovasc Risk Rep 2009; 3:418–425. [Google Scholar]
- 12.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hixon ML, Gualberto A. Vascular smooth muscle polyploidization—from mitotic checkpoints to hypertension. Cell Cycle 2003; 2:105–110. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice. Proc Natl Acad Sci U S A 2004; 101:7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benn M, Nordestgaard BG, Grande P, et al. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol 2010; 55:2833–2842. [DOI] [PubMed] [Google Scholar]
- 16.Cohen JC, Boerwinkle E, Mosley TH, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 17.Guella I, Asselta R, Ardissino D, et al. Effects of PCSK9 genetic variants on plasma LDL cholesterol levels and risk of premature myocardial infarction in the Italian population. J Lipid Res 2010; 51:3342–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]


