Abstract
Cancer and its treatment exert a heavy psychological and physical toll. Of the myriad symptoms which result, pain is common, encountered in between 30% and 60% of cancer survivors. Pain in cancer survivors is a major and growing problem, impeding the recovery and rehabilitation of patients who have beaten cancer and negatively impacting on cancer patients’ quality of life, work prospects and mental health. Persistent pain in cancer survivors remains challenging to treat successfully. Pain can arise both due to the underlying disease and the various treatments the patient has been subjected to. Chemotherapy causes painful chemotherapy-induced peripheral neuropathy (CIPN), radiotherapy can produce late effect radiation toxicity and surgery may lead to the development of persistent post-surgical pain syndromes. This review explores a selection of the common causes of persistent pain in cancer survivors, detailing our current understanding of the pathophysiology and outlining both the clinical manifestations of individual pain states and the treatment options available.
Keywords: Cancer pain, survivors, persistent post-surgical pain, peripheral neuropathy, late effect radiation toxicity, pain management
Introduction
Cancer and its treatment exert a heavy toll on the body, leaving permanent reminders of their presence. The toll can be both physical and psychological, and of the myriad symptoms which may result, chronic pain is commonly encountered with a prevalence of approximately 30%.1,2 Increased survival rates result in increased numbers of patients experiencing persistent pain. This can be due either to the disease process or from treatment. Pain negatively impacts on a survivor’s quality of life, affecting their ability to recover and regain the functional levels possessed prior to their diagnosis. Additionally, persistent pain impedes employment prospects and negatively influences social interactions and emotional well-being.3,4
This review will describe some of the common causes of persistent pain in cancer survivors, detailing our current understanding of the pathophysiology, outlining the clinical manifestations of individual pain states and exploring preventative measures. Persistent pain in cancer survivors represents a major clinical challenge.
Chemotherapy-induced peripheral neuropathy
Peripheral neuropathies represent a major cause of pain in cancer survivors and may arise at any stage of the disease process. Causes of peripheral neuropathy in cancer vary, but it can result from direct effects of the tumour itself as observed in paraneoplastic polyneuropathies,5 or from its treatment with chemotherapeutic agents, termed chemotherapy-induced peripheral neuropathy (CIPN).6 Although chemotherapeutic neurotoxicity may affect the central nervous system, peripheral sensory neuropathy is most prevalent, affecting from 10% to 100% of patients depending on factors such as the presence of co-morbidities, choice of chemotherapeutic agent and cumulative dose.7,8 CIPN represents a major concern in the management of malignancy. Many antineoplastic agents are neurotoxic, and the symptoms which occur with CIPN are often severe enough to make dose adjustment or cessation of treatment necessary, resulting in potentially suboptimal therapy.9
Pathophysiology
The underlying pathophysiology of CIPN is complex and to a certain extent dependent on the causative agent (Table 1). The polyneuropathy encountered in CIPN is predominantly sensory in nature, with both large and small sensory fibres affected, motor nerve fibre involvement being less common and often subclinical.10
Table 1.
Clinical features, putative mechanisms and likely outcome of peripheral neuropathies caused by a variety of chemotherapeutic agents.
| Chemotherapeutic agent | Class of agent | Incidence of CIPN | Features | Onset and coasting | Putative mechanism | Duration |
|---|---|---|---|---|---|---|
| Cisplatin and carboplatin | Platinum | 40–50% | Pain, numbness, paraesthesia, loss of distal reflexes | From 1 month, peak 3 months (++) | ↑ TRPV1, TRPA and TRPM8 | 80% recover with cessation of chemotherapy |
| Activation of P38 MAPK and ERK1/2 | ||||||
| NMDA receptor effects | ||||||
| Mitotoxicity | ||||||
| Oxaliplatin | Platinum | 90% acutely | Sensory neuropathy | Acute onset, 2–3 days | ↑ TRPV1, TRPA and TRPM8 | Median recovery in 3 months |
| 40% chronic | 80% acute cold-induced paraesthesia | Activation of P38 MAPK and ERK1/2 | ||||
| ↓ membrane K+ channels, TREK1 and TRAK | ||||||
| NMDA receptor effects | ||||||
| Mitotoxicity | ||||||
| Vincristine | Vinca alkaloid | 30–40% | Sensory neuropathy, lower>upper limbs, autonomic neuropathy, muscle cramps | Within 3 months (+) | Changes in mitochondrial and cellular Ca2+ flux | 70% full recovery at 2 years |
| NMDA receptor effects | ||||||
| Microtubule disruption | ||||||
| Activate mitochondrial caspases | ||||||
| Paclitaxel Docetaxel | Taxane | 30–50% | Sensory neuropathyMyopathy/muscle spasmsLoss of proprioception | Some onset after first dose, >50% after second dose | Microtubule disruptionNeurotoxicity at DRG | 75% some recovery at 6 months |
| (+) | ||||||
| Bortezomib | Proteasome inhibitor | 30–50% | Painful sensory neuropathy, autonomic neuropathy | Dose related and cumulative | Activate mitochondrial caspases | 60–70% resolve 3 months post cessation |
| Most after second cycle | Demyelination | |||||
| (+) | ||||||
| Thalidomide | Immunomodulator | 20–70% | Sensory neuropathy, muscle cramps | Daily dose–related – not cumulative dose | Not elucidated | Poor recovery from neuropathy observed |
CIPN: chemotherapy-induced peripheral neuropathy; DRG: dorsal root ganglion; ERK1/2: extracellular signal-regulated kinase 1/2; MAPK: mitogen activated protein kinase; NMDA: N-methyl D-aspartate; TRAK: tumour necrosis factor receptor–associated kinase; TRPV: transient receptor potential vanilloid.
Information in this table collated from references 7, 8, 23, 38 and 39.
Sensory nerves are pseudounipolar in structure, with one cytoplasmic extension travelling to the periphery and the other to the spinal cord from the cell body located in the dorsal root ganglion (DRG). This single peripheral axon is of varying length, but it may be over 1.5 m in the limbs.11 Myelinated large diameter Aα and Aβ fibres act as afferents from low-threshold tissue mechanoreceptors. Small calibre myelinated Aδ and unmyelinated C fibres transmit nociceptive information from the peripheries,12 and the skin is richly innervated by a dense plexus of these neurones (Figure 1(a)).13,14 Unmyelinated fibres cross the epidermal–dermal junction (basement membrane) into the epidermis, forming intra-epidermal nerve fibres (IENFs). These nociceptors respond to thermal, mechanical and chemical stimuli.15 A diverse array of receptors and signalling molecules are present in these nociceptor terminals.16
Figure 1.
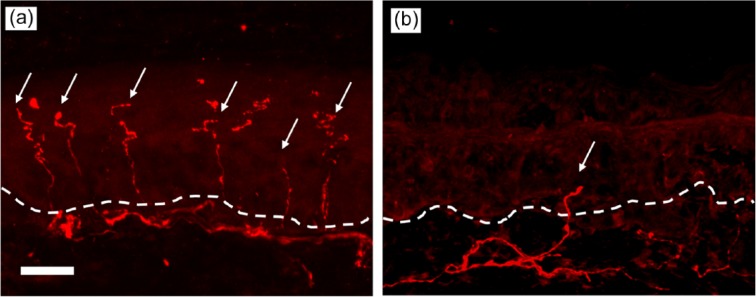
Immunohistochemical staining (primary antibody to PGP 9.5) of sensory nerve fibres in human skin: (a) shows normal intra-epidermal nerve density and (b) shows reduced intra-epidermal nerve density as seen in small fibre neuropathies. Both images 20×objective, scale bar 20 µm, dotted line demarcates the epidermal–dermal junction.
Individual neurones are dependent upon a complex arrangement of anterograde and retrograde axonal transport systems to deliver proteins, lipids and other substrates to the periphery and to return harmful metabolites to the soma to be processed.17 Disruption of this system renders the neurone, whose peripheral segment already functions on a physiological ‘knife-edge’, vulnerable to damage.18
Microtubule disruption
The pathogenesis of CIPN has not been fully elucidated. Chemotherapeutic agents interfere with neuronal functioning via a number of mechanisms, with individual agents differentially affecting specific peripheral nerve structures.19 Predominant among these mechanisms is the disruption of the intracellular microtubule scaffold which facilitates axonal transport, leading to a reduction in peripheral nutrient supply and subsequent neuropathy. Agents which interfere with microtubules include the taxanes, colchicine which inhibits microtubule self-assembly and vinca alkaloids such as vincristine which induce microtubule instability.20–22 Such neuropathies manifest themselves in the form of neuronal ‘die back’ caused by the Wallerian degeneration of the distal segments of nerves, which, due to their distance from the nerve soma are most susceptible.7 This explains the ‘length dependent’ nature of the neuropathy.23 For Aδ and C fibres innervating the skin, die back causes a reduction in the density of unmyelinated fibres crossing into the epidermis with those fibres remaining having abnormal morphology and function (Figure 1(b)).24 Reductions in IENF density or the degeneration of terminal unmyelinated fibres is a common lesion in multiple toxic neuropathies.25,26 However, it is important to recognise that neuronal die back may potentially represent a common final neurodegenerative feature of a multifactorial process.
Several exceptions to the microtubule disruption mechanism of CIPN exist. In some animal models of CIPN, there is little evidence of gross damage to nerves.27 Significant dose-limiting peripheral neuropathy is commonly reported with bortezomib, an agent which does not affect microtubules28 while colchicine, a formidable disruptor of microtubule structure does not cause pain.29 Putative mechanisms have been developed providing alternative neuronal targets for chemotherapy agents. These have focussed on chemotherapy-related mitotoxicity leading to interruptions in neuronal energy supply (the peripheral mitotoxicity theory), on the indirect triggering of immunological mechanisms and the sensitisation of neurones through changes in ion channel function.30
Mitotoxicity
Structural and functional abnormalities in mitochondria are closely associated with painful neuropathies,31,32 with the degree of mitochondrial dysfunction correlating with observed pain behaviour in animal models of CIPN.33,34 High energy demand regions of the neurone, such as the IENFs, are disproportionately affected by any deficiencies in energy supply which leads to malfunction of Na+/K+ pumps, increased spontaneous firing of Aδ and C fibres,35 fibre swelling and distortion and ultimately die back.36 Mitotoxicity occurs through several mechanisms; platinum compounds bind directly to and damage mitochondrial DNA,37 paclitaxel causes swollen, vacuolated and functionally impaired mitochondria,38 while vincristine and bortezomib both activate mitochondrial caspase, a process integral to apoptosis.32,39 Interestingly, compounds that prevent mitochondrial damage, such as acetyl L-carnitine40 and caspase inhibitors, are associated with reductions in neuronal death and neuropathic pain in animal models41,42 raising the possibility of novel therapeutic targets.43
Neuro–immune mechanisms
Neuro–immune interactions in both the central and peripheral nervous system are known to play a major role in the development of neuropathic pain.44–47 In CIPN, neuronal soma and glial cells in the DRG (outside the blood–spinal cord barrier) are exposed to high levels of antineoplastic agents. Glial cell dysfunction48 and an increase in activated macrophages can occur49,50 leading to abnormal cellular signalling and changes in expression of mediators and genes associated with both pain and cell death. These include nerve growth factor (NGF);51 tumour necrosis factor-α (TNF-α); interleukins (ILs) IL-1β, IL-6 and IL-8;52 and activation of pro-apoptotic genes.53 These changes occur prior to the gross anatomical disruption seen in a proportion of more established CIPN and may provide a partial explanation as to why pain precedes functional neuronal changes. Peripherally, Langerhans cells, avid synthesisers of pro-inflammatory mediators,54 have been shown to increase in paclitaxel-evoked painful neuropathy42 as well as in painful peripheral neuropathies associated with other disease states.55,56 The potentially pivotal role played by pro-inflammatory cytokines is further reinforced by the effect blocking these mediators has on the development of CIPN. Administration of an anti TNF-α antibody in a rat model of bortezomib-induced painful neuropathy prevents the development of allodynia57 and vincristine-induced hyperalgesia in rats is abolished by bradykinin B1 and B2 receptor antagonists.58
Neuronal sensitisation
Structural changes in axonopathic sensory neurones are further compounded by alterations in the function, distribution and number of ion channels. Energy deficits due to mitochondrial dysfunction result in membrane depolarisation and spontaneous neuronal discharge.35 Individual chemotherapeutic agents have been demonstrated to directly affect specific ion channels. Oxaliplatin, in a mouse model of CIPN, markedly reduces the expression of membrane K+ channels TREK1 and TRAK and increases the expression of a range of excitatory channels resulting in cold hypersensitivity.59 Additionally, oxaliplatin has been shown to up-regulate spinal N-methyl D-aspartate (NMDA) receptors.60 In the rat, paclitaxol sensitises the polymodal transient receptor potential vanilloid 4 (TRPV4) receptor leading to enhanced nociception.61 The presence of increased levels of reactive oxygen species (ROS, markers of cellular oxidative stress) and NGF in C fibres, a situation not uncommonly encountered in CIPN, contributes to increased expression of TRPV1 thermo-receptors.62
Clinical features
As a predominantly sensory neuropathy, CIPN presents with signs and symptoms resulting from the disturbance of sensory function.63 These include paraesthesia, numbness, impaired vibration, temperature and proprioceptive sensation, dysaesthesia and neuropathic pain.64 The distribution of the sensory symptoms is length dependent, commencing peripherally, normally in either the fingers or toes with gradual proximal spread, leading to a characteristic symmetrical ‘glove and stocking’ pattern.65 Mixed sensory–motor and autonomic neuropathies may occur; autonomic dysfunction commonly occurring in vincristine- and bortezomib-related CIPN which causes paralytic ileus and orthostatic hypotension.66
Development of the symptoms of CIPN is temporally related to the commencement of chemotherapy with peak incidence dependent on agent and dose,8 and is cumulative in nature, with higher doses of drug leading to greater neurotoxicity.39 Cessation of antineoplastic treatment, however, does not guarantee resolution, with symptoms persisting in a large proportion of patients,67 resulting in marked reductions in post-treatment quality of life.68,69 A further complicating factor is the phenomenon of ‘coasting’, whereby the symptoms of peripheral neuropathy may continue to progress or even first appear following termination of treatment.6 Coasting is commonly associated with platinum-derived agents, although it may be seen with other chemotherapeutics such as bortezomib.70
Detection/diagnosis
The presence of CIPN is determined by a combination of clinical history and examination findings, augmented by specific diagnostic tools and investigations. Pre-, peri- and post-chemotherapy neurological assessments are advised as they permit the patient’s baseline status to be established and facilitate early detection of CIPN.71
Clinical examination in patients with CIPN may prove unremarkable as subtle changes in peripheral sensory thresholds are not detected by tests of gross neurological function. A more targeted examination may isolate abnormalities in two-point discrimination (touch), vibration sensation and proprioception in a symmetrical peripheral pattern.7 Localised distal areflexia may also be detected and acts as a surrogate marker for the presence of more advanced CIPN.10 Ramifications of autonomic nerve involvement, such as postural hypotension, may be detected by measuring lying and standing blood pressure.
To aid CIPN diagnosis, a number of clinical tools have been developed which rely upon subjective and objective methods.72 These include the World Health Organisation CIPN grading scale,73 the Eastern Cooperative Oncology Group (ECOG) neuropathy scale and the National Cancer Institute Common Toxicity Criteria (NCI-CTC) neuropathy score.74 The utility of these tools is hampered by high levels of inter-observer variability, the lack of a single universally accepted assessment tool and the omission of pain as an assessment parameter.75 A recent study assessed the validity and reliability of a number of different grading scales used in CIPN,76 including the NCI-CTC, the Total Neuropathy Score Clinical Version (TNSc), the modified Inflammatory Neuropathy Cause and Treatment (INCAT), the modified sensory sum score (mISS), the European Organisation for Research and Treatment of Cancer’s (EORTC) QLQ-C30 and QLQ-CIPN20 quality-of-life measures in 281 patients with stable CIPN. The study demonstrated good validity and reliability scores for the set of selected impairment and quality-of-life outcome measures. Additionally, the group utilised data generated on limitations of activity and participation to create a Rasch-built overall disability scale (R-ODS) for CIPN, which the authors advocate, is used in future clinical studies.77
The measurement of nerve conduction velocities (NCV) in sensory and motor nerves and sensory nerve action potential (SNAP) may indicate axonal loss but are of minimal use in the presence of DRG or small sensory fibre pathology.23 Measurement of SNAPs (combined with clinical scoring tools) may in the future enable patients undergoing chemotherapy to be risk stratified mid-treatment for the risk of developing CIPN. This would potentially abate the need to terminate chemotherapy treatment by permitting early identification of patients at risk of high-grade neuropathy allowing prompt chemotherapy dose reduction before nerve damage occurs.78 Work is currently ongoing to identify and investigate novel biomarkers for CIPN.
Detection of small fibre pathology remains challenging because of the technical difficulties of performing nerve conduction studies on C and Aδ fibres. Quantitative sensory testing (QST) allows the identification of fibre-type involved in CIPN symptoms.79 However, QST findings do not always correlate with clinical symptoms and requires specialist equipment, and time and resources are not always available in clinic. Despite its sensitivity, there is little evidence that QST can provide an earlier diagnosis than patient symptom reporting,80 although some evidence does exist for a correlation between final CIPN severity and attenuation in vibration sensation.81
IENF loss can be assessed using immunohistochemical techniques on skin biopsies,82 quantified to enable a diagnosis of small fibre neuropathy.24 Although invasive, biopsies can provide an accurate evaluation of ‘die back’ associated with peripheral neuropathies. Epidermal innervation does not, however, correlate with the degree of pain experienced by CIPN patients.83 Despite the lack of validation in CIPN, biopsy may have utility when compared to other diagnostic modalities.84
Radiotherapy
Physics and underlying principles
Ionising radiation has been utilised for over 100 years in the treatment of cancer, either as a primary therapy or as an adjunct to surgery or chemotherapy. It remains a common component of cancer management with approximately 50% of patients receiving a form of radiotherapy during their treatment.85 Ionising radiation induces DNA damage in target cells through two distinct mechanisms. First, destruction of chemical bonds by the ionising radiation results in the production of ROS which damage DNA. Second, ionising radiation directly damages DNA and the regulatory proteins which facilitate DNA repair.86
The underlying treatment principle is that the DNA repair capacity of healthy cells is generally greater than that of cancerous cells, and that cancer cells proliferate more rapidly than most normal cells. Damage to DNA results in death of the affected cell through apoptosis or cell senescence.87 Efficacy of radiotherapy is also influenced by the degree of hypoxia of the cell (hypoxic cells are radio-resistant), the ability of the surviving cells to re-populate and the intrinsic radio-resistance of tumour cells.88
Painful side effects
Avoidance of damage to non-cancerous tissues outside the target zone is a major priority in the use of ionising radiation. However, damage not only occurs directly to those cells directly exposed to ionising radiations, but there is a separate indirect mechanism of ‘radiation induced bystander effects’ (RIBEs).89 Although poorly understood, collateral damage is induced in radiation-naïve cells by harmful signals transmitted from neighbouring irradiated cells. RIBE embodies a plethora of deleterious cellular processes including alterations in gene expression, mitochondrial damage, increased intracellular ROS levels and apoptosis.90
Side effects of radiotherapy can be classified as being acute or late, the latter occurring 90 days after treatment and potentially lasting many years, the former manifesting at the time of treatment and resolving following treatment cessation.91 Radiotherapy is conventionally administered in divided doses or fractions, the intensity of this being influenced by the need to limit the number of patients developing late complications to between 5% and 10%.92 Late side effects arise from regional damage to tissues and include radiation-induced fibrosis, atrophy vascular and neural damage.93
Abdominal visceral pain
The mucosa of the gastrointestinal tract, with its rapid cell turnover is particularly susceptible to radiation-induced damage resulting in nausea, vomiting and diarrhoea. Progression to late bowel toxicity following radiotherapy of the abdominal, pelvic and lumbar regions leads to chronic pain. In patients who receive radiotherapy for cancers of the pelvis, chronic abdominal pain is encountered in approximately 10–15% of cases,94,95 leading to marked reductions in survivor’s quality of life.96 Preoperative radiotherapy for bowel cancer is associated with survivors experiencing higher rates of non-specific abdominal pain than radiation-naïve patient’s years after treatment.97 The incidence and severity of late toxicity symptoms encountered in patients is influenced by total radiotherapy dose, dose per fraction, volume of intestine irradiated and previous abdominal surgery.96
In a proportion of patients, acute inflammatory changes in the gut mucosa fail to resolve following cessation of radiotherapy, resulting in pronounced and progressive intestinal fibrosis and ischaemia because of vascular sclerosis.98,99 These changes in turn lead to gut dysmotility, stricture formation and obstruction all of which ultimately manifest as chronic abdominal pain.100
Preventative strategies attempt to ameliorate the degree of gut fibrosis either by interfering with radiation specific mechanisms of injury or by increasing the tolerance of normal tissue to radiation.101 Refinements in dosimetry and beam targeting, the use of anti-inflammatory and antioxidant agents and therapies aimed at increasing tissue vascularity and oxygen supply such as hyperbaric oxygen have been tried.102 Combining agents with differing therapeutic targets (such as pentoxifylline (PTX), which improve perfusion due to vasodilatation and is anti-inflammatory, and Vitamin E, an antioxidant) may be beneficial.103–106 These medications have been recommended by some authors, despite the lack of large randomised-controlled trials (RCTs).107
Neural injury
Late-radiation toxicity may also manifest in the form of neural damage, the classic example being brachial plexus neuropathy (BPN), encountered following radiotherapy in the region of the plexus.108,109 The brachial plexus consists of nerve fibres relaying sensory, autonomic and motor innervation to and from the arm, forearm and hand. Correspondingly, the majority of symptoms of BPN are experienced in the ipsilateral upper extremity and include paraesthesia, motor weakness, pain and oedema.110 The aetiology of radiation-induced neural injury is essentially a progressive process of intra- and extra-neuronal fibrogenesis driven by ROS and pro-inflammatory mediators. This process subsequently results in demyelination, direct axonal injury and nerve ischaemia due to damage to the perfusing microvasculature.111
Radiation-induced neuronal injury is characterised by its clinical heterogeneity and variable onset time; some patients experience symptoms within a year of their radiotherapy, while in others problems may occur a decade later.112 BPN occurrence is influenced by a range of factors including dosimetry (greater dose = faster onset)113 and age of the patient (younger patients develop symptoms more quickly).114 Symptomatology of BPN also exhibits considerable variation with some patients experiencing sensory disturbance as their predominant symptom with minimal pain, while other patients may be afflicted by severe neuropathic pain.115
In assessment of radiation-induced BPN, it is important to consider and exclude the presence of malignant invasion of the brachial plexus, which also causes sensory disturbance and pain leading to the potential for misdiagnosis.116 Differences in the features of the conditions do exist, and these are outlined in Table 2. Investigations such as magnetic resonance imaging can help in reaching a definitive diagnosis.117
Table 2.
Main, distinguishing features of radiation-induced brachial plexopathy and malignant invasion of the brachial plexus.
| Radiation-induced brachial plexopathy | Malignant invasion of the brachial plexus | |
|---|---|---|
| Trunks of brachial plexus predominantly involved | Upper trunks | Lower trunks |
| Timescale of onset | 1–10 years after radiotherapy | Typically a short timescale |
| Pain commonly a major feature | No | Yes |
| Associated signs and symptoms | Cutaneous radiation changes, lymphoedema | Nil |
Persistent post-surgical pain
Surgery remains an important treatment modality for cancer as well as used for diagnosis and palliation. Chronic pain developing after surgery (persistent post-surgical pain (PPSP)) is a relatively new concept, but it is an important condition118 and contributes to the symptom burden of cancer survivors, negatively affecting their quality of life.
PPSP remains poorly defined, but it is broadly recognised as being pain present more than 2–3 months after surgery. To make the diagnosis, surgical and pre-existing causes of the pain should have been excluded.119,120 The condition is common, with estimations of its prevalence ranging from 10% to 30% of all post-surgical patients.121 Certain procedures are associated with a greater risk of developing PPSP, including breast surgery, thoracotomy, cardiac surgery, limb amputation and hernia repair.122 Even relatively limited surgery such as the resection of cutaneous melanoma has been shown to be associated with the development of PPSP.123
Pathophysiology
The acute pain of surgery comprises of a combination of nociceptive, inflammatory and acute neuropathic elements.119 PPSP possesses many of the characteristics and features of neuropathic pain,124,125 although it only fully meets the diagnostic criteria of neuropathic pain in a relatively small proportion of patients.126 The underlying mechanisms which lead to the transition from acute pain to PPSP have not been fully delineated127 but reflect the complex processes which occur when tissues are injured. A constellation of neurone terminal fibres, cells and immunocytes populate the skin and via release of signalling molecules are affected to varying degrees by the noxious insult of surgery.128,129 These processes cause localised neuronal sensitisation,130,131 and the resulting afferent barrages of nociceptive signalling leads to central sensitisation.119 This neuroplastic process is consequent on alterations in gene expression,132 neuro–immune interactions in the spinal cord and DRG,45 and manifests as many of the features encountered clinically in PPSP such as the generation of spontaneous pain, hyperalgesia, hypersensitivity and other abnormal sensations arising at the site of injury.122 Central sensitisation plays a key role in the development and perpetuation of PPSP133 in combination with other peripheral processes.124
Risk factors
Although it is undoubtedly common, some patients undergo surgery and do not develop PPSP, implying certain factors may predispose individuals to the condition. Attempts to identify potential risk factors have highlighted a number of important variables related both to the patient and the surgery performed.
Surgery involving the division or prolonged retraction of nerves such as axillary clearance or thoracotomy are associated with higher rates of PPSP.119 However, robust evidence that damaging specific isolated nerves, such as the intercostobrachial, results in an increased risk of developing PPSP is currently lacking.134 Additional surgical factors which may increase the risk of developing PPSP include extensive tissue disruption and damage (but not in breast procedures135), the use of surgical drains136 and a duration of surgery greater than 3 hours.137 Acute pain over the first 3–4 post-operative days increases the risk of transition to a persistent pain state.138 Multiple studies have shown that severe acute pain accurately predicts the development of PPSP,136,139 likely related to peripheral and central neuronal sensitisation.120 Similar neuroplastic influences are thought to account for the fact that the presence and intensity of preoperative pain strongly predicts the occurrence of persistent pain after a number of different types of surgery.140–142
A range of disparate patient factors have also been shown to contribute to an individual’s risk profile for developing PPSP. Age and sex are important, with younger females at higher risk of developing pain chronicity.143,144 The degree of anxiety or depression present or the propensity to catastrophise renders patients more vulnerable to PPSP,145–147 and it influences independent of the surgery performed.148
Despite the logical assumption that the use of adjuvant chemotherapy or radiotherapy would potentiate the development of PPSP, multiple studies have failed to definitively show an association.134,149 Nevertheless, some chemotherapy is associated with an increased risk of peripheral neuropathy150 and work in animal models of PPSP have demonstrated a role for the TRPV1 channel (whose expression is increased in CIPN) in the development of cutaneous hypersensitivity following surgery.151
Risk prediction
Predicting the risk of the development of PPSP is a nascent field, but it is potentially beneficial if modifiable factors can be identified. Current studies have predominantly focused on identifying those patients at high risk of developing severe acute post-surgical pain either by screening for known risk factors152, or by using defined psychophysical tests such as the patient’s response to painful stimuli.153,154 Work on predicting persistent pain following surgery has been relatively limited in comparison.155–157
Prediction of developing PPSP could identify preventative strategies. A range of interventions have been investigated including pre-emptive analgesia in the form of gabapentinoids and NMDA receptor antagonists,158–160 regional nerve blocks, infiltration of local anaesthetics161 and the use of psychological interventions and education.162 Many of the studies in this area are contradictory, and the jury is out concerning the utility of individual interventions.163 More work in this field is clearly indicated.164
Treatment of pain in cancer survivors
Poorly managed pain significantly contributes to a decreased quality of life in cancer survivors. Treatments should comprise a multidisciplinary biopsychosocial approach which aims to address all aspects and ramifications of the pain and disability.
PPSP and pain due to late radiation toxicity are both similarly benighted by a paucity of research into their effective treatment. Much of the pain experienced by cancer survivors exhibits neuropathic features and is often considered pain of predominantly neuropathic origin, although this is not irrefutable. In PPSP, a series of small studies of anti-neuropathic agents, such as amitriptyline and venlafaxine for different PPSP states165,166 have proved inconclusive, despite the apparent neuropathic nature of PPSP.124 Topically applied 5% lidocaine patches have shown some promise in the treatment of scar pain following cancer surgery, albeit in a small open-label study.167 Extrapolation of clinical guidelines for other neuropathic pain (predominantly not based on data from cancer survivors), such as those recently published by the United Kingdom’s National Institute of Clinical Excellence,168 is an empirical and pragmatic approach in the absence of any suitable alternative.
In severe radiation-induced BPN surgical exploration and subsequent fibrinolysis, revascularisation or omental patching of the plexus is advocated by some, although it is a high-risk approach feasible in only a few subjects.110 For the majority of patients, the emphasis of treatment is supportive allied with anti-neuropathic and opioid drugs and coupled with physiotherapy and other rehabilitative approaches.169 The prognosis for patients with radiation-induced BPN (RIBPN), however, remains poor, with complete resolution of symptoms and the total restoration of limb function being rare.112,114
The abdomino-visceral manifestations of late-radiation toxicity are also difficult to control. Visceral pain states present a challenge, as pain is often intertwined with physiological and functional derangement of the organ system, and many analgesic agents (such as opioids) may further contribute to this dysfunction. Treatment of this condition is also hampered by a lack of understanding and recognition among healthcare professionals.170 Focus must be not just on the control of pain but on optimising visceral functionality (which may in turn also improve pain), ideally by specialist centres.171 The pharmacological management of visceral pain is complex.172 With such a paucity of evidence to guide management, a rational multidisciplinary approach, including ‘opioid sparing’ medications should be taken.173
There are limited drug treatments for CIPN. A single small RCT of duloxetine demonstrated reduced pain intensity in patients with CIPN, although this corresponded to only a reduction of just over 1 on a 0–10 Likert pain scale.174 Despite the limited evidence for the use of other anti-neuropathic agents, recently published guidelines recommend that both amitriptyline and gabapentin may be trialled in CIPN given their proven efficacy in other neuropathic pain states.175 Topical preparations are also used clinically (often off licence) to treat CIPN. Capsaicin 0.025% cream, 8% patches or 5% lidocaine patches have all been shown to be efficacious in a variety of other peripheral neuropathies,176,177 although robust evidence for their use in CIPN is lacking. A number of potential treatments are being evaluated, including topical menthol (an inactivator of nociceptor voltage-gated sodium channels),178 tetrodotoxin (voltage-gated sodium channel inhibitor)179 and the cannabinoid receptor agonists WIN55, 212-2 and AM1710.180
Conclusion
Our continually ageing and expanding population coupled with increases in the number of patients being successfully treated for cancer is resulting in greater numbers of cancer survivors. Many of these survivors experience the after effects of both their malignancy and the treatment they receive for it. Pain represents one of the most common and unpleasant of these after effects, profoundly influencing the quality of life experienced by cancer survivors and detrimentally affecting their recovery and rehabilitation.
Pain in cancer survivors may be caused by a number of disparate mechanisms related to both the underlying disease and the differing modalities used to treat it; surgery, radiotherapy and chemotherapy or a combination of all three. Our understanding of the exact pathophysiological processes which result in pain remains sparse, but ongoing work is likely to lead to improved appreciation and treatment possibilities to reduce the symptom burden for cancer survivors.
Acknowledgments
The authors wish to thank Dr David Bennett, Senior Wellcome Clinical Scientist, Nuffield Department of Clinical Neurosciences, University of Oxford, for allowing the use of images in Figure 1(a) and (b).
Footnotes
Conflict of interest: The authors declare that there are no conflicts of interest. P.F.S. has previously undertaken paid consultancy work for Astellas, Napp, Pfizer and Grunenthal.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1. Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: long-term symptoms in cancer survivors – a systematic review. Int J Psychiatry Med 2010; 40(2): 163–181. [DOI] [PubMed] [Google Scholar]
- 2. Van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels GA, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18(9): 1437–1449. [DOI] [PubMed] [Google Scholar]
- 3. Amir Z, Neary D, Luker K. Cancer survivors’ views of work 3 years post diagnosis: a UK perspective. Eur J Oncol Nurs 2008; 12(3): 190–197. [DOI] [PubMed] [Google Scholar]
- 4. Elliott J, Fallows A, Staetsky L, et al. The health and well-being of cancer survivors in the UK: findings from a population-based survey. Br J Cancer 2011; 105(Suppl. S1): S11–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krarup C, Crone C. Neurophysiological studies in malignant disease with particular reference to involvement of peripheral nerves. J Neurol 2002; 249: 651–661. [DOI] [PubMed] [Google Scholar]
- 6. Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol 2010; 6(12): 657–666. [DOI] [PubMed] [Google Scholar]
- 7. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013; 63(6): 419–437. [DOI] [PubMed] [Google Scholar]
- 8. Cata JP, Weng HR, Lee BN, et al. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol 2006; 72(3): 151–169. [PubMed] [Google Scholar]
- 9. Speck RM, Samuel MD, Farrar JT, et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract 2013; 9(5): e234–e240. [DOI] [PubMed] [Google Scholar]
- 10. Hausheer FH, Schilsky RL, Bain S, et al. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006; 33(1): 15–49. [DOI] [PubMed] [Google Scholar]
- 11. Hogan QH. Labat lecture: the primary sensory neuron: where it is, what it does, and why it matters. Reg Anesth Pain Med 2010; 35(3): 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev 2004; 46(2): 131–145. [DOI] [PubMed] [Google Scholar]
- 13. Shipton EA. Skin matters: identifying pain mechanisms and predicting treatment outcomes. Neurol Res Int 2013; 2013(V): 329364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McArthur JC, Stocks EA, Hauer P, et al. Epidermal nerve fiber density. Arch Neurol 1998; 55(12): 1513–1520. [DOI] [PubMed] [Google Scholar]
- 15. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest 2010; 120(11): 3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413(6852): 203–210. [DOI] [PubMed] [Google Scholar]
- 17. Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol 2003; 160(6): 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Millecamps S, Julien J-P. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 2013; 14(3): 161–176. [DOI] [PubMed] [Google Scholar]
- 19. Balayssac D, Ferrier J, Descoeur J, et al. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin Drug Saf 2011; 10(3): 407–417. [DOI] [PubMed] [Google Scholar]
- 20. LaPointe NE, Morfini G, Brady ST, et al. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology 2013; 37: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ong V, Liem NLM, Schmid MA, et al. A role for altered microtubule polymer levels in vincristine resistance of childhood acute lymphoblastic leukemia. J Pharmacol Exp Ther 2008; 324(2): 434–442. [DOI] [PubMed] [Google Scholar]
- 22. Vandecandelaere A, Martin SR, Engelborghs Y. Response of microtubules to the addition of colchicine and tubulin-colchicine: evaluation of models for the interaction of drugs with microtubules. Biochem J 1997; 323(1): 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol 2012; 14(Suppl. 4): iv45–iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010; 17(7): 903–912, e44–e49. [DOI] [PubMed] [Google Scholar]
- 25. Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol 2012; 238(2): 225–234. [DOI] [PubMed] [Google Scholar]
- 26. Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol 2013; 4: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 2006; 122(3): 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008; 112(5): 1593–1599. [DOI] [PubMed] [Google Scholar]
- 29. Kingery WS, Guo TZ, Poree LR, et al. Colchicine treatment of the sciatic nerve reduces neurogenic extravasation, but does not affect nociceptive thresholds or collateral sprouting in neuropathic or normal rats. Pain 1998; 74(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 30. Ferrier J, Pereira V, Busserolles J, et al. Emerging trends in understanding chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep 2013; 17(10): 364. [DOI] [PubMed] [Google Scholar]
- 31. Bouillot S, Martin-Negrier ML, Vital A, et al. Peripheral neuropathy associated with mitochondrial disorders: 8 cases and review of the literature. J Peripher Nerv Syst 2002; 7(4): 213–220. [DOI] [PubMed] [Google Scholar]
- 32. Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 2009; 10(7): 481–494. [DOI] [PubMed] [Google Scholar]
- 33. Xiao WH, Zheng H, Zheng FY, et al. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience 2011; 199(514): 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol 2011; 232(2): 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain 2008; 135(3): 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Janes K, Doyle T, Bryant L, et al. Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain 2013; 154(11): 2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis 2011; 41(3): 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012; 291(1–3): 1–9. [DOI] [PubMed] [Google Scholar]
- 39. Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care 2011; 5(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 40. Virmani A, Gaetini F, Imam S, et al. Possible mechanism for the neuroprotective effects of L-carnitine on methamphetamine-evoked neurotoxicity. Ann N Y Acad Sci 2003; 993(1): 197–207. [DOI] [PubMed] [Google Scholar]
- 41. Scholz J, Broom DC, Youn D-H, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci 2005; 25(32): 7317–7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin HW, Flatters SJL, Xiao WH, et al. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol 2008; 210(1): 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Benn SC, Woolf CJ. Adult neuron survival strategies – slamming on the brakes. Nat Rev Neurosci 2004; 5(9): 686–700. [DOI] [PubMed] [Google Scholar]
- 44. Calvo M, Dawes JM, Bennett DLH. The role of the immune system in the generation of neuropathic pain. Lancet Neurol 2012; 11(7): 629–642. [DOI] [PubMed] [Google Scholar]
- 45. Calvo M, Bennett DLH. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol 2012; 234(2): 271–282. [DOI] [PubMed] [Google Scholar]
- 46. Calvo M, Zhu N, Tsantoulas C, et al. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci 2010; 30(15): 5437–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci 2005; 6(7): 521–532. [DOI] [PubMed] [Google Scholar]
- 48. Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain 2013; 17(4): 571–580. [DOI] [PubMed] [Google Scholar]
- 49. Peters CM, Jimenez-Andrade JM, Jonas BM, et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 2007; 203(1): 42–54. [DOI] [PubMed] [Google Scholar]
- 50. Peters CM, Jimenez-Andrade JM, Kuskowski MA, et al. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res 2007; 1168(612): 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cavaletti G, Pezzoni G, Pisano C, et al. Cisplatin-induced peripheral neurotoxicity in rats reduces the circulating levels of nerve growth factor. Neurosci Lett 2002; 322(2): 103–106. [DOI] [PubMed] [Google Scholar]
- 52. Wang X-M, Lehky TJ, Brell JM, et al. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine 2012; 59(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alaedini A, Xiang Z, Kim H, et al. Up-regulation of apoptosis and regeneration genes in the dorsal root ganglia during cisplatin treatment. Exp Neurol 2008; 210(2): 368–374. [DOI] [PubMed] [Google Scholar]
- 54. Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol 2012; 132(3 Pt 2): 872–881. [DOI] [PubMed] [Google Scholar]
- 55. Dauch JR, Bender DE, Luna-Wong LA, et al. Neurogenic factor-induced Langerhans cell activation in diabetic mice with mechanical allodynia. J Neuroinflammation 2013; 10: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Casanova-Molla J, Morales M, Planas-Rigol E, et al. Epidermal Langerhans cells in small fiber neuropathies. Pain 2012; 153(5): 982–989. [DOI] [PubMed] [Google Scholar]
- 57. Chiorazzi A, Canta A, Meregalli C, et al. Antibody against tumor necrosis factor-α reduces bortezomib-induced allodynia in a rat model. Anticancer Res 2013; 33(12): 5453–5459. [PubMed] [Google Scholar]
- 58. Bujalska M, Tatarkiewicz J, Gumułka SW. Effect of bradykinin receptor antagonists on vincristine- and streptozotocin-induced hyperalgesia in a rat model of chemotherapy-induced and diabetic neuropathy. Pharmacology 2008; 81(2): 158–163. [DOI] [PubMed] [Google Scholar]
- 59. Descoeur J, Pereira V, Pizzoccaro A, et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med 2011; 3(5): 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mihara Y, Egashira N, Sada H, et al. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol Pain 2011; 7(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alessandri-Haber N, Dina OA, Yeh JJ, et al. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci 2004; 24(18): 4444–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Puntambekar P, Mukherjea D, Jajoo S, et al. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem 2005; 95(6): 1689–1703. [DOI] [PubMed] [Google Scholar]
- 63. Gutiérrez-Gutiérrez G, Sereno M, Miralles A, et al. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol 2010; 12(2): 81–91. [DOI] [PubMed] [Google Scholar]
- 64. Verstappen CCP, Heimans JJ, Hoekman K, et al. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 2003; 63(15): 1549–1563. [DOI] [PubMed] [Google Scholar]
- 65. Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 2008; 44(11): 1507–1515. [DOI] [PubMed] [Google Scholar]
- 66. Van den Bent MJ. Prevention of chemotherapy-induced neuropathy: leukemia inhibitory factor. Clin Cancer Res 2005; 11(5): 1691–1693. [DOI] [PubMed] [Google Scholar]
- 67. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 2002; 249(1): 9–17. [DOI] [PubMed] [Google Scholar]
- 68. Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013; 31(21): 2699–2707. [DOI] [PubMed] [Google Scholar]
- 69. Chaudhary UB, Haldas JR. Long-term complications of chemotherapy for germ cell tumours. Drugs 2003; 63(15): 1565–1577. [DOI] [PubMed] [Google Scholar]
- 70. Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 2006; 7(10): 797–809. [DOI] [PubMed] [Google Scholar]
- 71. Stubblefield MD, McNeely ML, Alfano CM, et al. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer 2012; 118(Suppl. 8): 2250–60. [DOI] [PubMed] [Google Scholar]
- 72. Postma TJ, Heimans JJ. Review: grading of chemotherapy-induced peripheral neuropathy. Ann Oncol 2000; 11: 509–513. [DOI] [PubMed] [Google Scholar]
- 73. Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981; 47(1): 207–214. [DOI] [PubMed] [Google Scholar]
- 74. Cleeland CS, Farrar JT, Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist 2010; 15(Suppl. 2): 13–18. [DOI] [PubMed] [Google Scholar]
- 75. Postma TJ. Original article: pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol 1998; 9: 739–744. [DOI] [PubMed] [Google Scholar]
- 76. Cavaletti G, Cornblath DR, Merkies ISJ, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol 2013; 24(2): 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Binda D, Vanhoutte EK, Cavaletti G, et al. Rasch-built Overall Disability Scale for patients with chemotherapy-induced peripheral neuropathy (CIPN-R-ODS). Eur J Cancer 2013; 49(13): 2910–2918. [DOI] [PubMed] [Google Scholar]
- 78. Velasco R, Bruna J, Briani C, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 2014; 85(4): 392–398. [DOI] [PubMed] [Google Scholar]
- 79. Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011; 152(1): 14–27. [DOI] [PubMed] [Google Scholar]
- 80. Forsyth PA, Balmaceda C, Peterson K, et al. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol 1997; 35(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 81. Cavaletti G, Bogliun G, Marzorati L, et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol 2004; 15(9): 1439–1442. [DOI] [PubMed] [Google Scholar]
- 82. Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol 2007; 3(10): 546–557. [DOI] [PubMed] [Google Scholar]
- 83. Üçeyler N, Zeller D, Kahn A-K, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(Pt 6): 1857–1867. [DOI] [PubMed] [Google Scholar]
- 84. Krøigård T, Schrøder HD, Qvortrup C, et al. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur J Neurol 2014; 21(4): 623–629. [DOI] [PubMed] [Google Scholar]
- 85. Delaney G, Jacob S, Featherstone C, et al. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005; 104(6): 1129–1137. [DOI] [PubMed] [Google Scholar]
- 86. Hubenak JR, Zhang Q, Branch CD, et al. Mechanisms of injury to normal tissue after radiotherapy: a review. Plast Reconstr Surg 2014; 133(1): 49e–56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009; 461(7267): 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11(4): 239–253. [DOI] [PubMed] [Google Scholar]
- 89. Rzeszowska-Wolny J, Przybyszewski WM, Widel M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur J Pharmacol 2009; 625(1–3): 156–164. [DOI] [PubMed] [Google Scholar]
- 90. Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res 2011; 176(2): 139–157. [DOI] [PubMed] [Google Scholar]
- 91. Fogarty GB, Muddle R, Sprung CN, et al. Unexpectedly severe acute radiotherapy side effects are associated with single nucleotide polymorphisms of the melanocortin-1 receptor. Int J Radiat Oncol Biol Phys 2010; 77(5): 1486–1492. [DOI] [PubMed] [Google Scholar]
- 92. Begg AC. Can the severity of normal tissue damage after radiation therapy be predicted? PLoS Med 2006; 3(10): e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006; 6(9): 702–713. [DOI] [PubMed] [Google Scholar]
- 94. Gami B, Harrington K, Blake P, et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2003; 18(10): 987–994. [DOI] [PubMed] [Google Scholar]
- 95. Eifel PJ, Levenback C, Wharton JT, et al. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1995; 32(5): 1289–1300. [DOI] [PubMed] [Google Scholar]
- 96. Bye A, Ha J, Hjermstad M, et al. Health-related quality of life and occurrence of intestinal side effects after pelvic radiotherapy: evaluation of long-term effects of diagnosis and treatment. Acta Oncol 2000; 39(2): 173–180. [DOI] [PubMed] [Google Scholar]
- 97. Birgisson H, Påhlman L, Gunnarsson U, et al. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol 2005; 23(34): 8697–8705. [DOI] [PubMed] [Google Scholar]
- 98. Gervaz P, Morel P, Vozenin-Brotons M-C. Molecular aspects of intestinal radiation-induced fibrosis. Curr Mol Med 2009; 9(3): 273–280. [DOI] [PubMed] [Google Scholar]
- 99. Yarnold J, Brotons MCV. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 2010; 97(1): 149–161. [DOI] [PubMed] [Google Scholar]
- 100. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol 2013; 19(2): 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hauer-Jensen M, Wang J, Denham J. Bowel injury: current and evolving management strategies. Semin Radiat Oncol 2003; 13(3): 358–371. [DOI] [PubMed] [Google Scholar]
- 102. Hamama S, Delanian S, Monceau V, et al. Therapeutic management of intestinal fibrosis induced by radiation therapy: from molecular profiling to new intervention strategies et vice et versa. Fibrogenesis Tissue Repair 2012; 5(Suppl. 1): S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gothard L, Cornes P, Brooker S, et al. Phase II study of vitamin E and pentoxifylline in patients with late side effects of pelvic radiotherapy. Radiother Oncol 2005; 75(3): 334–341. [DOI] [PubMed] [Google Scholar]
- 104. Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol 1999; 17(10): 3283–3290. [DOI] [PubMed] [Google Scholar]
- 105. Hamama S, Gilbert-Sirieix M, Vozenin M-C, et al. Radiation-induced enteropathy: molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-β1 cascade inhibition. Radiother Oncol 2012; 105(3): 305–312. [DOI] [PubMed] [Google Scholar]
- 106. Delanian S, Porcher R, Balla-Mekias S, et al. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol 2003; 21(13): 2545–2550. [DOI] [PubMed] [Google Scholar]
- 107. Chiao TB, Lee AJ. Role of pentoxifylline and vitamin E in attenuation of radiation-induced fibrosis. Ann Pharmacother 2005; 39(3): 516–522. [DOI] [PubMed] [Google Scholar]
- 108. Chen AM, Hall WH, Li J, et al. Brachial plexus-associated neuropathy after high-dose radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012; 84(1): 165–169. [DOI] [PubMed] [Google Scholar]
- 109. Eblan MJ, Corradetti MN, Lukens JN, et al. Brachial plexopathy in apical non-small cell lung cancer treated with definitive radiation: dosimetric analysis and clinical implications. Int J Radiat Oncol Biol Phys 2013; 85(1): 175–181. [DOI] [PubMed] [Google Scholar]
- 110. Gosk J, Rutowski R, Reichert P, et al. Radiation-induced brachial plexus neuropathy – aetiopathogenesis, risk factors, differential diagnostics, symptoms and treatment. Folia Neuropathol 2007; 45(1): 26–30. [PubMed] [Google Scholar]
- 111. Delanian S, Lefaix J, Pradat P. Radiation-induced neuropathy in cancer survivors. Radiother Oncol 2012; 105(3): 273–282. [DOI] [PubMed] [Google Scholar]
- 112. Johansson S. Radiation induced brachial plexopathies. Acta Oncol 2006; 45(3): 253–257. [DOI] [PubMed] [Google Scholar]
- 113. Gałecki J, Hicer-Grzenkowicz J, Grudzień-Kowalska M, et al. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer – a review. Acta Oncol 2006; 45(3): 280–284. [DOI] [PubMed] [Google Scholar]
- 114. Olsen NK, Pfeiffer P, Johannsen L, et al. Radiation-induced brachial plexopathy: neurological follow-up in 161 recurrence-free breast cancer patients. Int J Radiat Oncol Biol Phys 1993; 26(1): 43–49. [DOI] [PubMed] [Google Scholar]
- 115. Fathers E, Thrush D, Huson SM, et al. Radiation-induced brachial plexopathy in women treated for carcinoma of the breast. Clin Rehabil 2002; 16(2): 160–165. [DOI] [PubMed] [Google Scholar]
- 116. Behnke NK, Crosby SN, Stutz CM, et al. Periscapular amputation as treatment for brachial plexopathy secondary to recurrent breast carcinoma: a case series and review of the literature. Eur J Surg Oncol 2013; 39(12): 1325–1331. [DOI] [PubMed] [Google Scholar]
- 117. Qayyum A, MacVicar AD, Padhani AR, et al. Symptomatic brachial plexopathy following treatment for breast cancer: utility of MR imaging with surface-coil techniques1. Radiology 2000; 214(3): 837–842. [DOI] [PubMed] [Google Scholar]
- 118. Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008; 101(1): 77–86. [DOI] [PubMed] [Google Scholar]
- 119. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367(9522): 1618–1625. [DOI] [PubMed] [Google Scholar]
- 120. Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009; 9(5): 723–744. [DOI] [PubMed] [Google Scholar]
- 121. Bruce J, Quinlan J. Chronic post surgical pain. Br J Pain 2011; 5(3): 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care 2013; 7(2): 144–152. [DOI] [PubMed] [Google Scholar]
- 123. Høimyr H, von Sperling ML, Rokkones KA, et al. Persistent pain after surgery for cutaneous melanoma. Clin J Pain 2012; 28(2): 149–156. [DOI] [PubMed] [Google Scholar]
- 124. Haroutiunian S, Nikolajsen L, Finnerup NB, et al. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013; 154(1): 95–102. [DOI] [PubMed] [Google Scholar]
- 125. Høimyr H, Rokkones KA, von Sperling ML, et al. Persistent pain after lymph node excision in patients with malignant melanoma is neuropathic. Pain 2011; 152(12): 2721–2728. [DOI] [PubMed] [Google Scholar]
- 126. Guastella V, Mick G, Soriano C, et al. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain 2011; 152(1): 74–81. [DOI] [PubMed] [Google Scholar]
- 127. Kalso E., IV Persistent post-surgery pain: research agenda for mechanisms, prevention, and treatment. Br J Anaesth 2013; 111(1): 9–12. [DOI] [PubMed] [Google Scholar]
- 128. Asahina a Hosoi J, Grabbe S, Granstein RD. Modulation of Langerhans cell function by epidermal nerves. J Allergy Clin Immunol 1995; 96(6 Pt 2): 1178–1182. [DOI] [PubMed] [Google Scholar]
- 129. Hou Q, Barr T, Gee L, et al. Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms. Pain 2011; 152(9): 2036–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci 1992; 15(3): 96–103. [DOI] [PubMed] [Google Scholar]
- 131. Reichling DB, Green PG, Levine JD. The fundamental unit of pain is the cell. Pain 2013; 154(Suppl. 1): S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 2001; 8(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 133. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152(Suppl. 3): S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 2011; 12(7): 725–746. [DOI] [PubMed] [Google Scholar]
- 135. Vilholm OJ, Cold S, Rasmussen L, et al. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer 2008; 99(4): 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Peng Z, Li H, Zhang C, et al. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One 2014; 9(2): e90014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Peters ML, Sommer M, de Rijke JM, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg 2007; 245(3): 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000; 93(4): 1123–1133. [DOI] [PubMed] [Google Scholar]
- 139. Callesen T, Bech K, Kehlet H. Prospective study of chronic pain after groin hernia repair. Br J Surg 1999; 86(12): 1528–1531. [DOI] [PubMed] [Google Scholar]
- 140. Tsirline VB, Colavita PD, Belyansky I, et al. Preoperative pain is the strongest predictor of postoperative pain and diminished quality of life after ventral hernia repair. Am Surg 2013; 79(8): 829–836. [DOI] [PubMed] [Google Scholar]
- 141. Bruce J, Thornton AJ, Scott NW, et al. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer 2012; 107(6): 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pinto PR, McIntyre T, Ferrero R, et al. Risk factors for moderate and severe persistent pain in patients undergoing total knee and hip arthroplasty: a prospective predictive study. PLoS One 2013; 8(9): e73917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liu SS, Buvanendran A, Rathmell JP, et al. A cross-sectional survey on prevalence and risk factors for persistent postsurgical pain 1 year after total hip and knee replacement. Reg Anesth Pain Med 2012; 37(4): 415–422. [DOI] [PubMed] [Google Scholar]
- 144. Bruce J, Drury N, Poobalan A, et al. The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain 2003; 104(1–2): 265–273. [DOI] [PubMed] [Google Scholar]
- 145. Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain 2013; 14(10): 1185–1195. [DOI] [PubMed] [Google Scholar]
- 146. Theunissen M, Peters ML, Bruce J, et al. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012; 28(9): 819–841. [DOI] [PubMed] [Google Scholar]
- 147. Hinrichs-Rocker A, Schulz K, Järvinen I, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur J Pain 2009; 13(7): 719–730. [DOI] [PubMed] [Google Scholar]
- 148. Masselin-Dubois A, Attal N, Fletcher D, et al. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain 2013; 14(8): 854–864. [DOI] [PubMed] [Google Scholar]
- 149. Mejdahl MK, Andersen KG, Gärtner R, et al. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ 2013; 346: f1865. [DOI] [PubMed] [Google Scholar]
- 150. Andersen KG, Jensen M-B, Kehlet H, et al. Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer: cyclophosphamide, epirubicin and fluorouracil compared with docetaxel + epirubicin and cyclophosphamide. Acta Oncol 2012; 51(8): 1036–1044. [DOI] [PubMed] [Google Scholar]
- 151. Barabas ME, Stucky CL. TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Mol Pain 2013; 9(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu SS, Buvanendran A, Rathmell JP, et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop 2012; 36(11): 2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology 2004; 100(1): 115–119, discussion 5A. [DOI] [PubMed] [Google Scholar]
- 154. Strulov L, Zimmer EZ, Granot M, et al. Pain catastrophizing, response to experimental heat stimuli, and post-cesarean section pain. J Pain 2007; 8(3): 273–279. [DOI] [PubMed] [Google Scholar]
- 155. Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br 2008; 90(2): 166–171. [DOI] [PubMed] [Google Scholar]
- 156. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008; 138(1): 22–28. [DOI] [PubMed] [Google Scholar]
- 157. Werner MU, Mjöbo HN, Nielsen PR, et al. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology 2010; 112(6): 1494–1502. [DOI] [PubMed] [Google Scholar]
- 158. Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010; 110(1): 199–207. [DOI] [PubMed] [Google Scholar]
- 159. Clarke H, Bonin RP, Orser BA, et al. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012; 115(2): 428–442. [DOI] [PubMed] [Google Scholar]
- 160. Chaparro LE, Smith SA, Moore RA, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013; 7(7): CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth 2013; 111(5): 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Riddle DL, Keefe FJ, Nay WT, et al. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil 2011; 92(6): 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Dworkin RH, McDermott MP, Raja SN. Preventing chronic postsurgical pain: how much of a difference makes a difference? Anesthesiology 2010; 112(3): 516–518. [DOI] [PubMed] [Google Scholar]
- 164. Gilron I, Kehlet H. Prevention of chronic pain after surgery: new insights for future research and patient care. Can J Anaesth. 2014;. 61(2): 101–111. [DOI] [PubMed] [Google Scholar]
- 165. Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain 1996; 64(2): 293–302. [DOI] [PubMed] [Google Scholar]
- 166. Tasmuth T, Härtel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain 2002; 6(1): 17–24. [DOI] [PubMed] [Google Scholar]
- 167. Garzón-Rodríguez C, Casals Merchan M, Calsina-Berna A, et al. Lidocaine 5% patches as an effective short-term co-analgesic in cancer pain. Preliminary results. Support Care Cancer 2013; 21(11): 3153–3158. [DOI] [PubMed] [Google Scholar]
- 168. Longson D. Neuropathic pain – pharmacological management: the pharmacological management of neuropathic pain in adults in non-specialist settings. NICE clinical guideline 173, November 2013. London: National Institute for Health and Care Excellence. [PubMed] [Google Scholar]
- 169. Stubblefield MD, Custodio CM. Upper-extremity pain disorders in breast cancer. Arch Phys Med Rehabil 2006; 87(3 Suppl. 1): S96–S99, quiz S100–S101. [DOI] [PubMed] [Google Scholar]
- 170. Denham JW, Hauer-Jensen M. Radiation induced bowel injury: a neglected problem. Lancet 2013; 382(9910): 2046–2047. [DOI] [PubMed] [Google Scholar]
- 171. Andreyev HJN, Benton BE, Lalji A, et al. Algorithm-based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): a randomised controlled trial. Lancet 2013; 382(9910): 2084–2092. [DOI] [PubMed] [Google Scholar]
- 172. Wesselmann U, Baranowski AP, Börjesson M, et al. Emerging therapies and novel approaches to visceral pain. Drug Discov Today Ther Strateg 2009; 6(3): 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Davis MP. Drug management of visceral pain: concepts from basic research. Pain Res Treat 2012; 2012: 265605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309(13): 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol 2014; 32(18): 1941–1967. [DOI] [PubMed] [Google Scholar]
- 176. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 2011; 107(4): 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. De Leon-Casasola O. Multimodal approaches to the management of neuropathic pain: the role of topical analgesia. J Pain Symptom Manage 2007; 33(3): 356–364. [DOI] [PubMed] [Google Scholar]
- 178. Gaudioso C, Hao J, Martin-Eauclaire M-F, et al. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain 2012; 153(2): 473–484. [DOI] [PubMed] [Google Scholar]
- 179. Nieto FR, Entrena JM, Cendán CM, et al. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008; 137(3): 520–531. [DOI] [PubMed] [Google Scholar]
- 180. Rahn EJ, Deng L, Thakur GA, et al. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol Pain 2014; 10(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]


