Abstract
Intestinal necrosis is a life-threatening disease, and its prompt and accurate diagnosis is very important. This study aimed to evaluate the value of d-dimer as a marker for early diagnosis of bowel necrosis.
From 2009 to 2013, patients undergoing operation due to acute intestinal obstruction were retrospectively analyzed. Clinicopathologic characteristics were compared among no ischemia group, reversible ischemia group, and bowel necrosis group.
There were totally 274 patients being included for analyses. Patients with bowel necrosis had a significant highest level of d-dimer compared with other 2 groups (P = 0.007) when FEU unit was applied. The optimal cutoff value of d-dimer levels as an indicator in diagnosing bowel necrosis was projected to be 1.965 mg/L, which yielded a sensitivity of 84.0%, a specificity of 45.6%, a positive predictive value of 60.7%, and a negative predictive value of 74.0%. And the sensitivity of 84.0% and specificity of 70.0% were detected, when 1.65 mg/L of d-dimer was set as the cutoff value to distinguish the reversible ischemia and bowel necrosis. The corresponding results in patients with no or slight peritoneal irritation signs were 85.2%, 44.7%, 35.4% and 89.5% respectively. The sensitivity and negative predictive value were 96.0% and 91.7%, respectively, when d-dimer and peritoneal irritation signs were combined to perform the parallel analysis.
The combination of d-dimer and peritoneal irritation signs could generate a reliable negative predictive value, which is helpful to exclude the diagnosis of intestinal necrosis. However, it should also be proved in well-designed large-scale prospective study.
INTRODUCTION
Acute intestinal obstruction is one of the most common surgical emergencies, which involves a partial or complete blockage of the bowel preventing contents from passing through the intestine.1 Over 3% of all emergency surgical admissions were due to acute intestinal obstruction.2
Strangulated obstruction, which is highly associated with intestinal necrosis, accounts for about 9% to 38% in all the intestinal obstruction cases and may induce higher risk of morbidity or mortality,3–5 compared with those of obstruction without intestinal necrosis. The early diagnosis and treatment of patients with reversible intestinal ischemia or bowel necrosis are essential for a successful management of this disease. It is important to intervene at the early stage of strangulated bowel obstruction to avoid the occurrence of intestinal necrosis. In addition, delaying in the resection of the necrotic segment of intestine may be associated with severe outcomes. Furthermore, conservative therapy is still considered as an optional treatment approach of some selective patients for which operation is not necessary, such as incomplete obstruction without intestinal necrosis to be suspected.1 Therefore, it is important to determine whether intestinal ischemia or necrosis exists in early stage.
Several examinations or markers are used for the diagnosis of intestinal ischemia or necrosis, such as contrasted computed tomography (CT) scan, intestinal fatty acid-binding protein (IFABP), phosphate, lactate dehydrogenase (LDH), C-reactive protein (CRP), etc.6–9 However, these examinations or markers are not highly sensitive or specific either as expected. Kim et al10 reported the sensitivity and specificity of CT in diagnosis of bowel ischemia were 71% and 83%, respectively. van Noord et al9 have showed that IFABP, LDH, and CRP levels did not differ between patients with and without gastrointestinal ischemia. Block et al11 also proved that IFABP and LDH had insufficient accuracy for patients with intestinal ischemia. The accuracy of LDH was only 69%. Therefore, it would be useful to find more sensitive or specific methods and markers for diagnosis.
d-dimer is one of the terminal fibrin decomposition products and has been proved to be positively associated with coronary heart disease,12 acute ischemic stroke,13 acute pulmonary embolism,14 or deep vein thromboembolism, etc.15 Recent researches found the measurements of the plasma d-dimer levels might be a useful tool for the early diagnosis of acute mesenteric obstruction.16–19 However, the application of D-dimer on the diagnosis of strangulated intestinal obstruction is still controversial. Some researchers reported that D-dimer test was neither sensitive nor specific in diagnosing strangulated obstruction.20 And whether it is predictive or preventive for resection in strangulated intestinal hernia patients remains a question.21 Therefore, further studies are needed to confirm the diagnostic validity of d-dimer in cases with intestinal necrosis.
In the present study, we aimed to evaluate the value of d-dimer as a marker for early diagnosis of bowel necrosis.
METHODS
Patients
From June 2009 to October 2013, patients with complete preoperative D-dimer data who were admitted to the Department of Gastrointestinal Surgery at West China Hospital of Sichuan University and underwent operations due to acute intestinal obstruction were included for analyses retrospectively. The diagnosis of intestinal obstruction depended on symptoms, physical examinations, and radiological findings of distended intestinal loops with air-fluid levels. Patients with dynamic ileus, such as paralytic ileus, or acute mesenteric vascular disease were excluded from the study. Patients diagnosed with any previous or synchronous thrombogenic diseases, such as brain stroke, pulmonary embolism, or deep vein thrombosis, etc were excluded. The West China Hospital research ethics committee approved retrospective analysis of anonymous data. Signed patient informed consent was waived per the committee approval since it was a retrospective analysis.
Clinicopathologic Variables
The clinicopathological features (such as sex, age, laparotomy history, duration of symptoms, characteristics of pain, peritoneal irritation signs, body temperature, pulse, white blood cell count, shock, preoperative d-dimer level, results of radiological tests, pathological results of resected specimens, etc) and operative data (such as reason of obstruction, operative methods, intestinal vitality, etc) were collected and analyzed.
Treatment of Intestinal Obstruction
All patients were treated by fasting, gastrointestinal decompression, and intravenous fluids rehydration. Monitoring of abdominal signs was lasted until operation. Radiological tests such as CT scan were taken repeatedly if necessary. All the patients had undergone operations finally.
Determination of Intestinal Reversible Ischemia and Intestinal Necrosis
Bowel necrosis was determined by intraoperative findings and postoperative pathological examinations. Bowel necrosis was considered intraoperatively when signs of hypoxic discoloration, disappearance of terminal arteriola pulses, loss of tension and peristalsis, or nonresponse of stimulations were present. Postoperative pathological examinations revealing the presence of transmural necrosis of the muscularis indicated bowel necrosis.20,22 Reversible ischemia was considered, if the color of bowel was improved and the intestinal segment eventually fully recovered after the release of the obstruction was followed by improvement in color and eventually by full recovery of the intestinal segment.
Measurements of d-Dimer
Venous blood samples were taken upon the establishment of diagnosis of intestinal obstruction to measure the d-dimer levels. The d-dimer levels were measured with the calibrated SYSMEX7000 analyzer (Sysmex Corporation, Hyogo, Japan). However, 2 kits and units were adopted to measure the levels of d-dimer at different periods in our hospital. d-dimer PLUS kit was used before November 2011. The DDU unit was applied for reporting the levels of d-dimer and the normal range of plasma d-dimer concentration was <246.4 μg/L. The Innovance d-dimer kit with FEU unit was used after November 2011. The normal range of plasma d-dimer concentration was <0.55 mg/L. The concentrations of d-dimer up to 246.4 μg/L or 0.55 mg/L were considered normal.
For all measurements, levels that were not detectable were considered to have a value equal to the limit of detection of the assay.
Statistical Analysis
SPSS 19.0 software (SPSS Inc, Chicago, IL) was used for statistical analyses. Variables of normality were tested and if conforming to the normal distribution, data were expressed as means ± standard deviation. Two independent t tests for quantitative data, and χ2 test or Fisher exact test for categorical data were performed; otherwise, data were expressed as medians with a range taking the Spearman test into consideration. The receiver-operating characteristics (ROC) curve was obtained to determine the cutoff value and was utilized to evaluate the accuracy. Sensitivity, specificity, positive predictive value, and the negative predictive value were also calculated. Area under the curve (AUC) was calculated as measurement of the accuracy of the test. A P value <0.05 (2-sided) was considered statistically significant.
RESULTS
Characteristics of Patients
There were totally 274 patients being included for analyses, among which 17 patients experienced reversible intestinal ischemia and 99 patients suffered from intestinal necrosis. The general clinicopathologic characteristics are summarized in Table 1. There were no significant differences in age and laparotomy history among the 3 groups (Table 1). The durations from symptoms to admission were significantly longest in group without ischemia. However, there was no statistical difference between reversible ischemia group and bowel necrosis group (P = 0.688). Although the durations from d-dimer test to operation were significant different among the 3 groups, there was also no statistical difference between reversible group and bowel necrosis group (P = 0.587). The reasons of obstruction included adhesion, tumor, volvulus, foreign body, intussusception, and hernia. However, the spectrums of diseases were significant different among the 3 groups (P = 0.000). Also, the operative methods are listed in Table 1. Continuous pain, peritoneal irritation signs, high temperature, tachycardia, increased white blood cell count, and shock were significantly more frequent in group with bowel necrosis.
TABLE 1.
General Clinicopathologic Characteristics of Patients
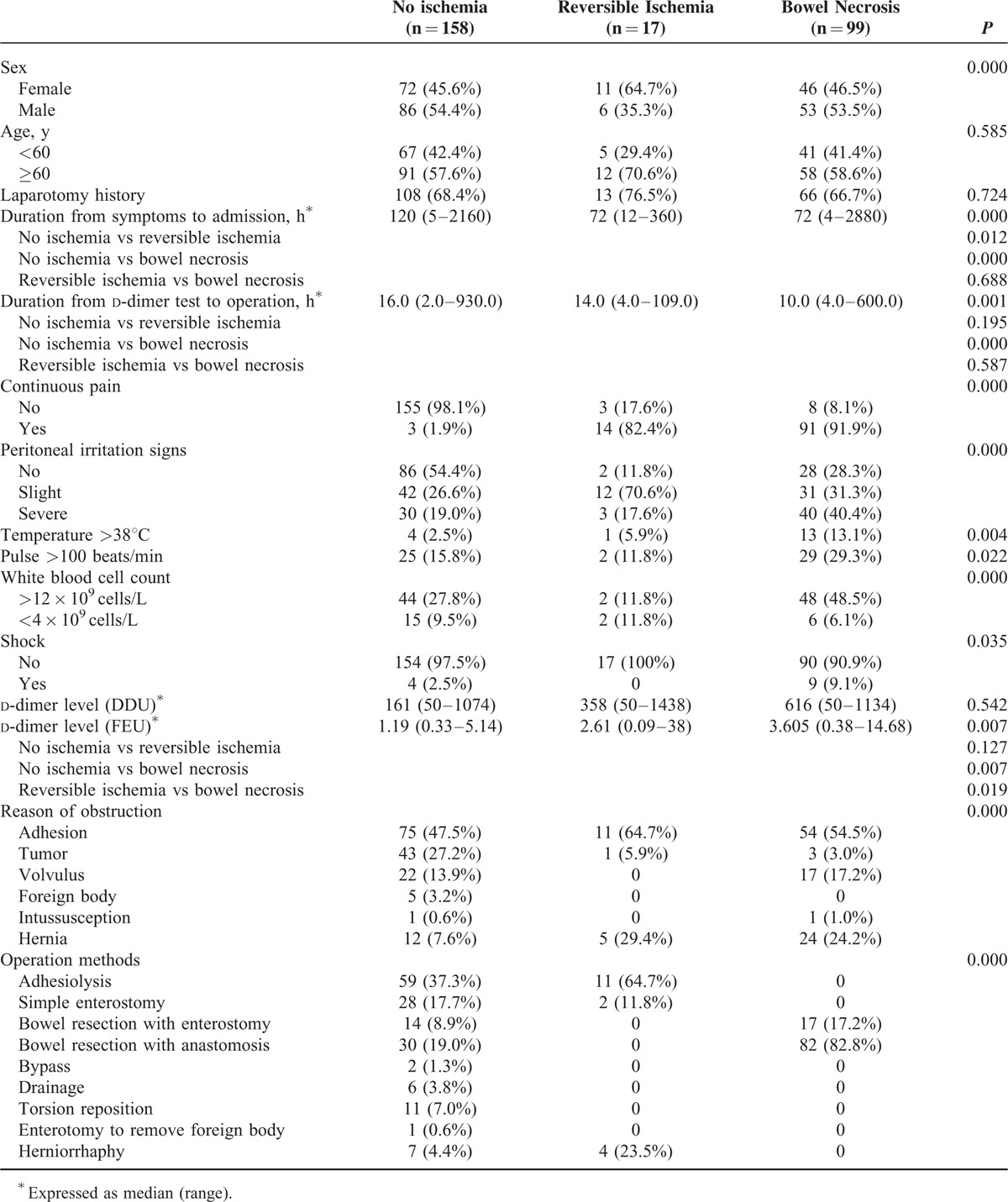
We compared the d-dimer levels among the 3 groups according to the different units and test methods, and found that patients with bowel necrosis had a significant highest level of d-dimer compared with patients who had reversible ischemia or those who had no ischemia (P = 0.007), when FEU unit was applied for report. Instead of comparison between no ischemia group and reversible ischemia group, the d-dimer level of group with reversible ischemia was also significantly different from that of group with bowel necrosis. Nevertheless, these observations could not be found when DDU unit was used (Table 1).
Correlation Between the d-Dimer Level and the Length of the Necrotic Bowel
The correlation between the d-dimer level and the length of the necrotic bowel was investigated by Spearman rank correlation. The results demonstrated that the correlation coefficient between the level of d-dimer and the length of the necrotic bowel was 0.320 (P = 0.005).
The Cutoff Value, Sensitivity and Specificity of d-Dimer for Bowel Necrosis
Only when FEU unit rather than DDU unit was applied, patients with bowel necrosis had a significant increased level of d-dimer compared with other 2 groups. And actually, the Innovance D-dimer kit with FEU unit is more sensitive, more reasonable, more stable, and used more widely than d-dimer PLUS kit with DDU unit. So we investigated the role of d-dimer in determining the bowel necrosis further by analyzing the data of patients with FEU unit applied. Based on the ROC curve, the optimal cutoff value of plasma d-dimer level as an indicator in diagnosing bowel necrosis was projected to be 1.965 mg/L, which yielded a sensitivity of 84.0% and a specificity of 45.6%. The AUC was found to be 0.639 (95% confidence interval [CI] 0.546–0.732) (P = 0.006) (Figure 1). The positive predictive value was 60.7%, and the negative predictive value was 74.0%. We also evaluated the role of d-dimer to distinguish the reversible ischemia and bowel necrosis. The optimal cutoff value was projected to be 1.65 mg/L, which yielded a sensitivity of 84.0% and a specificity of 70.0%. The AUC was found to be 0.773 (95% CI 0.613–0.933) (P = 0.007) (Figure 2). The positive predictive value was 73.7%, and the negative predictive value was 81.4%.
FIGURE 1.
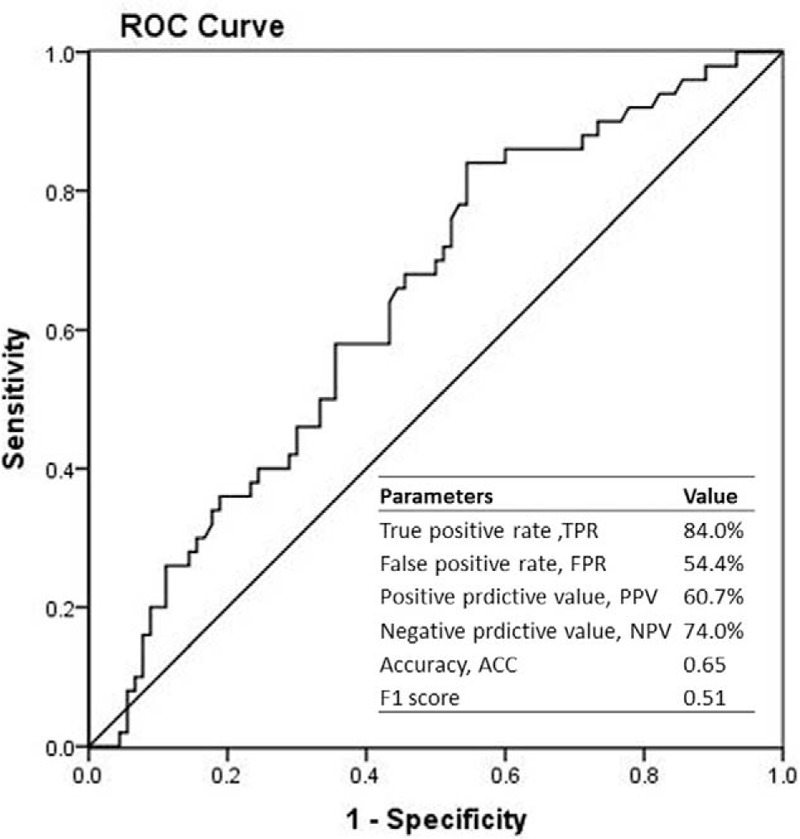
Receiver-operating characteristic (ROC) curves were utilized to evaluate the sensitivity and specificity of d-dimer levels to diagnose intestinal necrosis.
FIGURE 2.
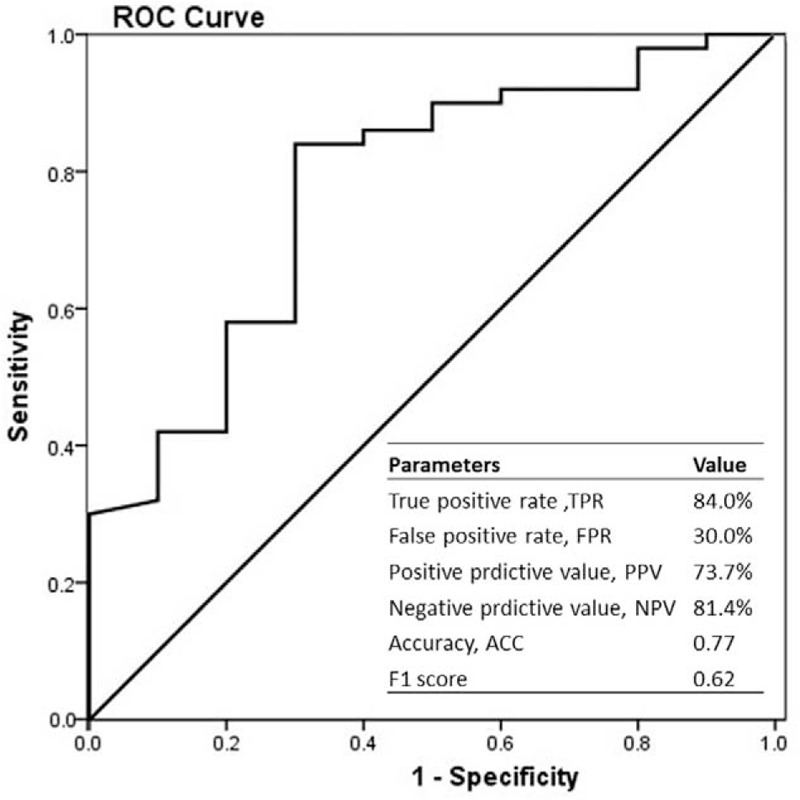
Receiver-operating characteristic (ROC) curves were utilized to evaluate the sensitivity and specificity of d-dimer levels to distinguish the reversible ischemia and bowel necrosis.
The Role of d-Dimer in Diagnosing Bowel Necrosis When There Was No or Slight Peritoneal Irritation Signs
In patients with no or slight peritoneal irritation signs, the sensitivity and specificity of d-dimer in diagnosing bowel necrosis were 85.2% and 44.7% respectively, when the cutoff value was set as 1.965 mg/L, and the positive predictive value and the negative predictive value were 35.4% and 89.5%, respectively.
Parallel Combined Diagnostic Tests of d-Dimer and Peritoneal Irritation Signs in Diagnosing Bowel Necrosis
If d-dimer (the cutoff value: 1.965 mg/L) and peritoneal irritation signs were used to perform the parallel combined diagnostic tests. The sensitivity and specificity were 96.0% and 27.5%, and the positive predictive value and the negative predictive value were 41.4% and 91.7% respectively.
DISCUSSION
Intestinal necrosis is a life-threatening disease, and its prompt and accurate diagnosis is very important. Some clinical signs, such as continuous abdominal pain, fever, tachycardia, peritoneal irritation signs, increased white blood cells, isolated abdominal mass, disappearance of bowel sounds, or hematochezia, may conduce to the diagnosis of bowel necrosis.23 In this study, we also found continuous pain, peritoneal irritation signs, high temperature, tachycardia, increased white blood cell count and shock were significantly more frequent in group with bowel necrosis.
However, if the diagnosis were determined according to the clinical signs mentioned above, that would be too late and indicate severe and too advanced disease.20 So, the clinical difficulty for doctors is to intervene at the early stage of strangulated bowel obstruction to avoid the occurrence of intestinal necrosis, or identify and treat bowel necrosis early when it happens inevitably. Unfortunately, there are no satisfied tests that can predict or diagnose intestinal necrosis accurately yet.
d-dimer is one of the terminal products of the destruction of fibrin, which constitutes the basic elements of the fibrinolytic system.24 In clinical practice, d-dimer level can be detected easily whenever and dynamically. The level of d-dimer in blood was found to be increased in some thrombotic diseases, such as acute ischemic stroke, acute pulmonary embolism, or disseminated intravenous coagulation, etc,12–15 and some nonspecific stressed condition, such as trauma, etc.25 Some researchers have proved that the plasma d-dimer levels would increase when acute bowel ischemia happened.16–19 That may be because when intestinal necrosis occurs, the mesenteric artery is occluded and the thrombosis is formed in the involved mesenteric vessels, which may initiate the fibrinolytic system to thrombolysis and therefore generate d-dimer.18,20 At the same time, the stress pressure and systemic reaction to this disease would lead to the increase of the d-dimer.20,25 In our study, we found that patients with bowel necrosis had a significant increased level of d-dimer compared with other 2 groups when FEU unit was applied for report. And we also found that there was a correlation between the d-dimer level and the length of the necrotic bowel. So it might be reasonable to use d-dimer as a marker for early diagnosis of bowel necrosis, although the correlation coefficient could not indicate a strong relationship, which may be caused by the nonspecific stressed systemic reactions of d-dimer.
In the present study, we used the ROC curve to obtain the optimal cutoff value of plasma d-dimer levels as an indicator in diagnosing bowel necrosis, which yielded a sensitivity of 84.0%, a specificity of 45.6%, a positive predictive value of 60.7%, and a negative predictive value of 74.0%. The results may be similar to others. Icoz G et al21 found that elevated d-dimer level had a high sensitivity but low specificity for identifying patients with intestinal ischemia. Compared with the patients without intestinal resection, d-dimer levels in patients requiring resection were not significantly higher, which generated sensitivity of 85%, specificity of 36%, and negative predictive value of 88%.21 However, Bogusevicius et al reported that the d-dimer test had a sensitivity of 60%, specificity of 68%, positive predictive value of 43%, and negative predictive value of 81% in diagnosing strangulated intestinal obstruction and they considered that d-dimer test was neither sensitive nor specific in diagnosing strangulation.20 In our study, we could see although the specificity was not very high, the relative high sensitivity may attract our attention to notice the potential presence of intestinal necrosis if the level of d-dimer increases. And the relative low specificity may be caused by the nonspecific stressed systemic reactions.
It is more meaningful to prevent intestinal necrosis at the stage of intestinal ischemia. In animal experiments, the plasma d-dimer level increased along with the prolongation of intestinal ischemia duration.21 Some patients with intestinal ischemia who had consistently atypical or mild symptoms and signs but gradually increased plasma d-dimer during the conservative therapy, were also finally proved of intestinal necrosis.26 Our results showed that the d-dimer levels were also significantly different between reversible ischemia group and bowel necrosis group, and the sensitivity of 84.0% and specificity of 70.0% were detected, when 1.65 mg/L of d-dimer was set as the cutoff value to distinguish the reversible ischemia and bowel necrosis. Although our results could not be considered to be highly sensitive or highly specific in distinguishing the reversible ischemia and bowel necrosis, what is a vexing problem in clinic, it may clue us to perform the operation before the dynamical increment of d-dimer level up to 1.65 mg/L based on a continuous monitoring.
When the classical clinical signs are present, there is often no difficulty for the diagnosis of intestinal necrosis. However, that would be too late and indicate severe and too advanced disease.20 And sometimes even intestinal necrosis happened, the clinical signs were absent or the physical signs were slight. So tests that can identify intestinal necrosis earlier or in patients with no or slight physical signs are more useful in clinic. In the study, we investigated the effectiveness of d-dimer in diagnosing bowel necrosis for patients with no or slight peritoneal irritation signs. We found the sensitivity and specificity of d-dimer test were 85.2% and 44.7% respectively, when the cutoff value was set as 1.965 mg/L. And the positive predictive value and the negative predictive value were 35.4% and 89.5% respectively. These results were similar to those of the total population, which meant that the d-dimer test was relatively stable even in patients with no or slight physical signs. Furthermore, with respect to the relative high negative predictive value of 89.5%, bowel necrosis in patients with no or slight physical signs could be less frequently considered if the value of d-dimer is not high.
Considering to the severe outcome caused by the missed diagnosis of intestinal necrosis, parallel combined diagnostic tests are often used to ensure the high sensitivity of the diagnosis, and it is most common to synthesize the peritoneal irritation signs and some accessory examinations in clinic.5 In the present study, we analyzed the role of parallel combined diagnostic tests including d-dimer and peritoneal irritation signs. The extreme high sensitivity and negative predictive value were demonstrated, which meant that the presence of negative parallel combined diagnostic tests may suggest other diagnoses. Zeybek et al18 also found the similar results in the early diagnosis of strangulated hernia of rat. However, even if the initial results of d-dimer are not high, we still need monitoring the d-dimer dynamically. Because prolonged conservative therapy may increase the rate of strangulation, the risk of intestinal necrosis and the mortality.1
In our study, the specificity is low for d-dimer to diagnose bowel necrosis, although the sensitivity is relatively high. However, we do not think that the “positive” d-dimer will help to determine a diagnosis. The value is in the absence of an elevated d-dimer. As a matter of fact, 2 aspects for the value of d-dimer in the diagnosis of bowel necrosis were emphasized in this study. One was that the relatively high sensitivity may attract our attention to notice the potential presence of intestinal necrosis if the level of d-dimer increased, which was just like a kind of warning, although the specificity was not high enough to diagnose the bowel necrosis definitely. Another but the most important aspect was applying the d-dimer to exclude the diagnosis of bowel necrosis, which could provide us the attemptable opportunity to treat the patients conservatively sometimes. That is because the extremely high negative predictive value of parallel combined diagnostic tests means bowel necrosis could be less frequently considered if the result of tests is negative. And the dynamical increment of d-dimer level from the original negative value may clue us that there is a possibility of condition deterioration and the intervention should be given early based on a continuous monitoring. Furthermore, because we just emphasize the value of a negative d-dimer rather than an increased positive d-dimer, the results remain meaningful constantly even if the prevalence of intestinal ischemia varies greatly in different clinical settings. Of course, d-dimer should be used in conjunction with other appropriate diagnostic criteria of bowel necrosis and must be interpreted in combination with clinical probability.
As in any other retrospective study, limitation of the current analysis includes possible selection bias, detection bias, and performance of analysis bias,27 and the sample size of reversible ischemia group was relatively small. Another limitation of this study is that the results are only applied to the d-dimer assay with Innovance D-dimer kit in which FEU unit was used.
CONCLUSIONS
In conclusion, we considered that the combination of d-dimer and peritoneal irritation signs could generate a reliable negative predictive value, which is helpful to exclude the diagnosis of intestinal necrosis. However, the real role of d-dimer as a marker for early diagnosis of bowel necrosis should also be proved in well-designed large-scale prospective study.
Acknowledgments
This work was also internal supported by Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, P.R.China.
Footnotes
Abbreviations: AUC = Area under the curve, CRP = C-reactive protein, CT = Computed tomography, IFABP = Intestinal fatty acid-binding protein, LDH = Lactate dehydrogenase, ROC = Receiver operating characteristics, VOLTGA = Volunteer Team of Gastric Cancer Surgery.
KY and WW contributed equally to this study.
Domestic support from National Natural Science Foundation of China (No. 81301867 and No. 81372344); National High-Technology Research and Development Program (“863” Program) of China (2015AA020306); Sichuan University Scholarship Fund. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The manuscript has been presented at the 70th General Meeting of the Japanese Society of Gastroenterological Surgery, Hamamatsu, Japan, July 2015.
The authors report no conflicts of interest.
REFERENCES
- 1.Chen XZ, Wei T, Jiang K, et al. Etiological factors and mortality of acute intestinal obstruction: a review of 705 cases. Zhong Xi Yi Jie He Xue Bao 2008; 6:1010–1016. [DOI] [PubMed] [Google Scholar]
- 2.Miller G, Boman J, Shrier I, et al. Natural history of patients with adhesive small bowel obstruction. Br J Surg 2000; 87:1240–1247. [DOI] [PubMed] [Google Scholar]
- 3.Paladino NC, Inviati A, Di Paola V, et al. Predictive factors of mortality in patients with acute mesenteric ischemia. A retrospective study. Ann Ital Chir 2014; 85:265–270. [PubMed] [Google Scholar]
- 4.Ameh EA, Nmadu PT. Intestinal volvulus: aetiology, morbidity, and mortality in Nigerian children. Pediatr Surg Int 2000; 16:50–52. [DOI] [PubMed] [Google Scholar]
- 5.Jancelewicz T, Vu LT, Shawo AE, et al. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg 2009; 13:93–99. [DOI] [PubMed] [Google Scholar]
- 6.Mallo RD, Salem L, Lalani T, et al. Computed tomography diagnosis of ischemia and complete obstruction in small bowel obstruction: a systematic review. J Gastrointest Surg 2005; 9:690–694. [DOI] [PubMed] [Google Scholar]
- 7.Kanda T, Fujii H, Fujita M, et al. Intestinal fatty acid binding protein is available for diagnosis of intestinal ischaemia: immunochemical analysis of two patients with ischaemic intestinal diseases. Gut 1995; 36:788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feretis CB, Koborozos BA, Vyssoulis GP, et al. Serum phosphate levels in acute bowel ischemia. An aid to early diagnosis. Am Surg 1985; 51:242–244. [PubMed] [Google Scholar]
- 9.van Noord D, Mensink PB, de Knegt RJ, et al. Serum markers and intestinal mucosal injury in chronic gastrointestinal ischemia. Dig Dis Sci 2011; 56:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Ha HK, Kim JK, et al. Usefulness of known computed tomography and clinical criteria for diagnosing strangulation in small-bowel obstruction: analysis of true and false interpretation groups in computed tomography. World J Surg 2004; 28:63–68. [DOI] [PubMed] [Google Scholar]
- 11.Block T, Nilsson TK, Björck M, et al. Diagnostic accuracy of plasma biomarkers for intestinal ischaemia. Scand J Clin Lab Invest 2008; 68:242–248. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Filippozzi L, Montagnana M, et al. Diagnostic value of D-dimer measurement in patients referred to the emergency department with suspected myocardial ischemia. J Thromb Thrombolysis 2008; 25:247–250. [DOI] [PubMed] [Google Scholar]
- 13.Zi WJ, Shuai J. Plasma D-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS One 2014; 9:e86465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Akpinar EE, Hoşgün D, Doğanay B, et al. Should the cut-off value of D-dimer be elevated to exclude pulmonary embolism in acute exacerbation of COPD? J Thorac Dis 2013; 5:430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linkins LA, Bates SM, Lang E, et al. Selective D-dimer testing for diagnosis of a first suspected episode of deep venous thrombosis: a randomized trial. Ann Intern Med 2013; 158:93–100. [DOI] [PubMed] [Google Scholar]
- 16.Altinyollar H, Boyabatli M, Berberoğlu U. D-dimer as a marker for early diagnosis of acute mesenteric ischemia. Thromb Res 2006; 117:463–467. [DOI] [PubMed] [Google Scholar]
- 17.Acosta S, Nilsson TK, Björck M. D-dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br J Surg 2004; 91:991–994. [DOI] [PubMed] [Google Scholar]
- 18.Zeybek N, Yildiz F, Kenar L, et al. D-dimer levels in the prediction of the degree of intestinal necrosis of etrangulated hernias in rats. Dig Dis Sci 2008; 53:1832–1836. [DOI] [PubMed] [Google Scholar]
- 19.Cesarini C, Monreal L, Armengou L, et al. Association of admission plasma D-dimer concentration with diagnosis and outcome in horses with colic. J Vet Intern Med 2010; 24:1490–1497. [DOI] [PubMed] [Google Scholar]
- 20.Bogusevicius A, Grinkevicius A, Maleckas A, et al. The role of D-dimer in the diagnosis of strangulated small-bowel obstruction. Medicina (Kaunas) 2007; 43:850–854. [PubMed] [Google Scholar]
- 21.Icoz G, Makay O, Sozbilen M, et al. Is D-dimer a predictor of strangulated intestinal hernia? World J Surg 2006; 30:2165–2169. [DOI] [PubMed] [Google Scholar]
- 22.Sarr MG, Bulkley GB, Zuidema GD. Preoperative recognition of intestinal strangulation obstruction. Prospective evaluation of diagnostic capability. Am J Surg 1983; 145:176–182. [DOI] [PubMed] [Google Scholar]
- 23.Bass KN, Jones B, Bulkley GB. Current management of small bowel obstruction. Adv Surg 1997; 31:1–34. [PubMed] [Google Scholar]
- 24.Wilde JT, Kitchen S, Kinsey S, et al. Plasma D-dimer levels and their relationship to serum fibrinogen/fibrin degradation products in hypercoagulable states. Br J Haematol 1989; 71:65–70. [DOI] [PubMed] [Google Scholar]
- 25.Owings JT, Gosselin RC, Anderson JT, et al. Practical utility of the D-dimer assay for excluding thromboembolism in severely injured trauma patients. J Trauma 2001; 51:425–430. [DOI] [PubMed] [Google Scholar]
- 26.Chen XZ, Hu JK. D-dimer test may contribute to detect acute mesenteric ischemia and intestinal necrosis. World J Surg 2015; 39:1584–1585. [DOI] [PubMed] [Google Scholar]
- 27.Yang K, Zhang WH, Chen XZ, et al. Survival benefit and safety of no. 10 lymphadenectomy for gastric cancer patients with total gastrectomy. Medicine (Baltimore) 2014; 93:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]


