Abstract
Adropin is a recently identified bioactive protein that promotes energy homeostasis by affecting glucose and lipid metabolism. Recently, adropin has also been reported to be associated with endothelial dysfunction. Also, ET-1, as a biomarker for endothelial dysfunction, is a key regulator in hypertension. Accordingly, the aim of the present study was to detect the relationship between plasma adropin and ET-1 levels in hypertension.
A total of 123 participants, diagnosed with primary hypertension on the basis of World Health Organization criteria (systolic blood pressure [SBP] ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg), and 58 normotensive subjects were enrolled in the cross-sectional study from October 2011 to December 2013. All study participants were older than 18 years of age. Adropin and ET-1 levels were measured by enzyme-linked immunosorbent assay (ELISA).
We found that plasma adropin levels were significantly lower in hypertensives compared with controls (3.18 ± 1.00 vs 4.21 ± 1.14 ng/mL, P < 0.001). Plasma ET-1 levels were higher in hypertensives than controls (2.60 ± 1.14 vs 1.54 ± 0.66 pg/mL, P < 0.001). Adropin had a negative correlation with DBP (r = −0.40, P < 0.001), SBP (r = −0.49, P < 0.001), and adjusted for age, body mass index, SBP, DBP, glucose, TC, TG, LDL, and Cr, there was a negative correlation between ET-1 and adropin (r = −0.20, P = 0.04). In multivariate logistic regression analysis of the variables, ET-1 (odds ratio [OR], 3.84; 95% CI, 2.16–6.81; P < 0.001) and adropin (OR, 0.99; 95% CI, 0.99 −1.0; P < 0.001) were found to be independent predictors for hypertension.
In conclusion, decreased plasma adropin levels are associated with increased blood pressure in hypertension. Adropin is an independent predictor for hypertension, and may influence blood pressure by protecting endothelial function.
INTRODUCTION
Hypertension contributes greatly to the global burden of disease and in many patients current treatments are often inadequate. Endothelial dysfunction plays an important role in the progression of hypertension.1 Endothelin-1 (ET-1) is elevated in hypertensive subjects and is a potential marker of endothelial dysfunction.2 ET-1 is a potent vasoconstrictor that is implicated in the pathogenesis of hypertension,3,4 and reduced ET-1 levels have been shown to decrease systolic blood pressure (SBP).5 In the context of the strong association between hypertension and chronic kidney disease (CKD),6 ET-1 was also found to have a close relationship with renal dysfunction induced by hypertension.7 Thus ET-1, as a biomarker for endothelial dysfunction, is a key regulator in hypertension.
Endothelial dysfunction is an important manifestation of diabetes. Increased production of ET-1 mediates many pathophysiological events contributing to the development of vascular complications in diabetes mellitus.8 Adropin, encoded by the energy homeostasis-associated (ENHO) gene, participates in the maintenance of energy homeostasis and the response to insulin.9 Recently, adropin has also been reported to be associated with endothelial dysfunction.10 Low adropin concentrations correlate with endothelial dysfunction in diabetes mellitus type 211 and the protective role of adropin has been ascribed to the upregulation of endothelial NO synthase.10 However, whether adropin can mediate progression of pathophysiological events by regulating ET-1 is still unclear.
In studies using obese pediatric patients, adropin was found to have no relationship with blood pressure, whether similar results would be found in adult hypertenisives is still open to debate.12,13 However, another study indicated that adropin likely has ability to regulate blood pressure.14 Therefore, our study was designed to investigate plasma adropin levels in essential hypertension, and to investigate the correlation between adropin and ET-1 in these patients.
METHODS
Subjects
A total of 123 participants, in total, were diagnosed with primary hypertension in accordance with the World Health Organization criteria (SBP ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg), and were enrolled in the cross-sectional study. These patients were assessed, either, in the clinic or the in-patient department of the Second Affiliated Hospital of Soochow University (Suzhou, China) from October 2011 to December 2013. Exclusion criteria were established on the presence of any one of the following: secondary hypertension or antihypertensive medication; diabetes; hyperlipidemia; kidney dysfunction; liver disease; lung disease; and cancer. Secondary hypertension was barred from the study on the basis of clinical and biochemical assessments. In parallel, 58 normotensive subjects were assessed in the same hospital and enrolled as a control group, using the same exclusion criteria. All study participants were older than 18 years.
Ethical and Legal Issues
The experimental protocol was in accordance with the Declaration of Helsinki (2008) of the World Medical Association, was approved by the ethics committee of the Second Affiliated Hospital of Soochow University. All subjects provided written informed consent before participating in the study.
Clinical and Biochemical Assessment
The electronic medical records were reviewed to obtain demographic and clinical data. The patients’ body mass index (BMI) was calculated by dividing their weight in kilograms by their height in meters squared. Blood pressure was measured, using an automatic BP monitor, in the sitting position, using the right upper arm and an appropriately sized cuff, measurements were performed three times in the participant's right arm with a 2-minute interval, after a rest period of at least 5 minutes. Hyperlipidemia was categorized as a total cholesterol (TC) concentration >5.69 mmol/L, triglyceride (TG) concentration >1.7 mmol/L, low-density lipoprotein (LDL) concentration >3.1 mmol/L, and/or the presence of cholesterol-lowering medication. All venous blood samples were collected after an overnight fasting routine. Blood biochemical parameters (fasting plasma glucose, total cholesterol, TG, and plasma creatinine [Cr] concentration) were performed using an AU5400 automated chemistry analyzer (Beckman Coulter, Fullerton, CA) on the same day of blood collection. LDL cholesterol was indirectly measured using the Friedewald formula. To separate plasma, other venous blood samples were collected into tubes with EDTA2Na (1 mg/mL), aprotinin (500 U/mL) (Becton Dickinson, Franklin Lakes, NJ), and then centrifuged at 1500g at 4°C for 15 min. The prepared plasma samples stored at −80°C until use. Plasma adropin concentrations were measured using the adropin ELISA kit (catalog no. EK-032-35; Phoenix pharmaceuticals Inc., Burlingame, CA), according to the manufacturer's instructions. The detection range of this kit is 0.01 to 100 ng/mL and the sensitivity is 0.3 ng/mL. Circulating ET-1 concentrations were determined using the ET-1 (human) ELISA kit (catalog no. QET00B; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The detection range for this kit is 0.34 to 250 pg/mL and its sensitivity is 0.102 pg/mL.
Statistical Analysis
Data were analyzed using SPSS software (version 22.0; SPSS, Inc., Chicago, IL). Continuous data are expressed as the mean ± SD, categorical variables are expressed as a percentage, and values of P < 0.05 are considered statistically significant. A 2-group student t test with a 0.05 2-sided significance level will have 80% power to detect an effect size of 0.45 when the sample sizes in the 2 groups are 58 and 123. The difference between the 2 continuous variables was studied using the Student t test, and the difference between categorical variables was applied using the χ2 test. Correlation relationships were performed using Pearson coefficient of correlation. Multivariable logistic regression analyses were used to access the association of various determinants with hypertension, the final multivariable model was selected using backward selection, all variables in the final model are significant P < 0.05.
RESULTS
Clinical and Laboratory Characteristics of the Study Participants
A total of 123 patients with newly established hypertension and 58 health controls were included in the study. The demographic characteristics are described in Table 1. Patients with hypertension had a higher DBP and SBP than healthy controls (DBP: 85.3 ± 15.5 vs 70.8 ± 8.3 mmHg, p < 0.001; SBP: 164.2 ± 16.7 vs 112.5 ± 12.1 mmHg, P < 0.001). There were no significant differences in terms of age, sex, smoking status, glucose, TG, TC, and LDL between hypertensive subjects and healthy controls. Plasma ET-1 was significantly higher in hypertensive subjects than in controls (2.60 ± 1.14 vs 1.54 ± 0.66 pg/mL, P < 0.001), whereas adropin levels were lower in hypertensive subjects compared with controls (3.18 ± 1 vs 4.21 ± 1.14 ng/mL, P < 0.001). Even excluding patients with kidney dysfunction, hypertensive subjects had higher Cr than controls (65.98 ± 11.64 vs 61.60 ± 13.43 μmol/L, P < 0.05).
TABLE 1.
Clinical and Biochemical Characteristics of the 123 Hypertensive Patients and 58 Healthy Controls
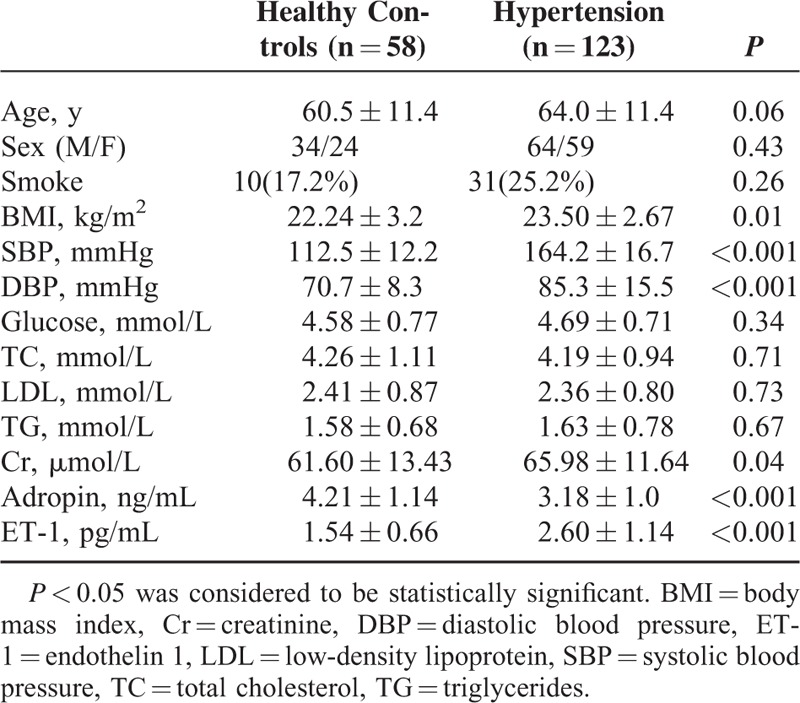
Correlation Between Adropin and Other Clinical Characteristics in Hypertension
As demonstrated in Table 2, plasma adropin levels were negatively correlated with DBP (r = −0.40, P < 0.001), SBP (r = −0.49, P < 0.001), and BMI (r = −0.27, P < 0.001) in 123 hypertensive patients. However, plasma adropin levels did not correlate with glucose (r = 0.14, P = 0.06) or Cr levels (r = −0.11, P = 0.13). ET-1 was positively correlated with DBP (r = 0.40, P < 0.001) and SBP (r = 0.51, P < 0.001). When adjusted for age, BMI, SBP, DBP, Glucose, TC, TG, LDL, and Cr, there was a negative correlation between ET-1 and adropin (r = −0.20, P = 0.04) (Figure 1).
TABLE 2.
Pearson Analysis of ET-1 and Adropin With Other Parameters in Hypertension
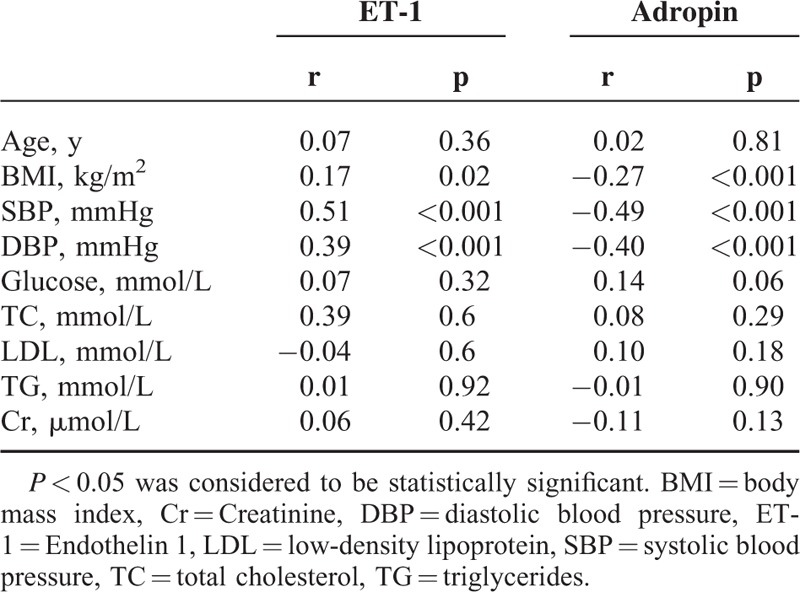
FIGURE 1.
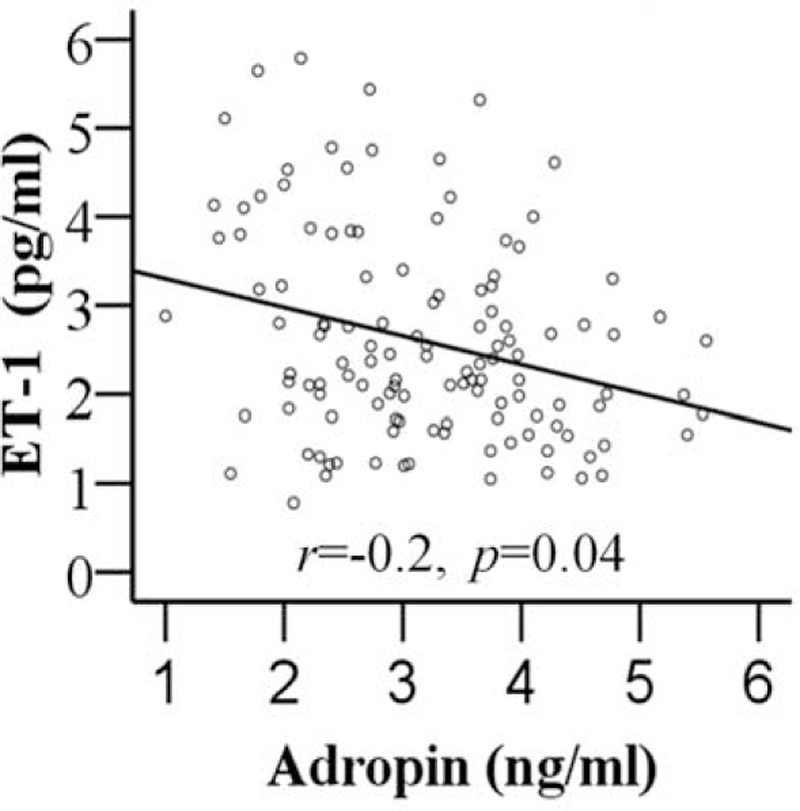
The relationship between adropin and endothelin 1 (ET-1) in hypertension subjects. Adropin showed significant correlation with ET-1 (r = −0.20, P = 0.04).
Multivariate Regression Analysis
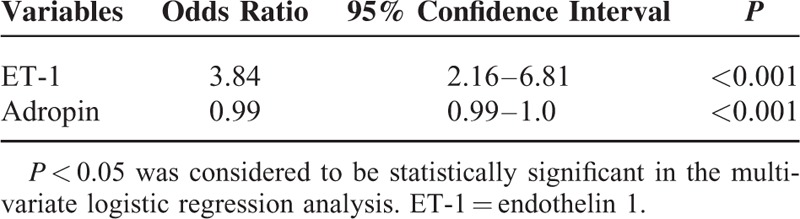
Multivariate logistic regression analysis was run with the variables (age, sex, smoking, glucose, TC, TG, LDL, Cr) in all 181 participants. The final model included only ET-1 and adropin. ET-1 (odds ratio [OR], 3.84; 95% confidence interval [CI], 2.16–6.81; P < 0.001) and adropin (OR, 0.99; 95% CI, 0.99–1.0; P < 0.001) were independent predictors of hypertension (Table 3). Controlling for adropin, for every 1-pg/mL increase in ET-1, the odds of having hypertension increase by 3.84. Likewise, controlling for ET-1, for every 1ng/ml increase in adropin the odds of having hypertension decrease by 0.99.
TABLE 3.
Multivariable Logistic Regression Analysis of Factors Correlated With Hypertension in the 58 Healthy Participants and the 123 Hypertensive Patients
DISCUSSION
In this paper, we first show that in the hypertension group, plasma adropin levels are lower than that of healthy controls. Secondly, decreasing adropin is negatively correlated with blood pressure in hypertensive patients. After adjusting for age, BMI, DBP, SBP, glucose, TC, TG, LDL and Cr, decreasing plasma adropin levels continued to be associated with increasing ET-1 levels. Lastly, we report that adropin and ET-1 are independent predictors of hypertension. Thus, the current study suggests an independent link between adropin and endothelial dysfunction in hypertension.
Given the significant impact hypertension has on the global burden of disease, investigating the risk factors and underlying mechanisms of hypertension will shed light on new methods of therapy. ET-1 is a potential vasoconstrictor that is involved not only in the pathogenesis of essential hypertension, but also in progression of chronic kidney disease secondary to hypertension.15–17 ET-1 is a peptide originally isolated from the supernatants of cultured endothelial cells and it exerts a wide variety of biological effects on different tissues, including the vascular wall. Hypertensive patients have a higher ET-1 than healthy controls.18 Circulating levels of ET-1 and endogenous ET(A)-mediated constriction are increased in human aging.19 ET-1 is also involved in progression of renal hypertension. In CKD, activation of the ET system seems to contribute not only to increased blood pressure but also to loss of nocturnal blood pressure dipping.7 Clinical trials have addressed the use of ET receptor antagonists as monotherapy in primary hypertension, and as an add-on therapy in resistant hypertension and CKD.6 Our study also shows ET-1 to be elevated in the hypertension group and with ET-1 having a positive relationship with blood pressure. We show that ET-1 is an independent risk factor for hypertension.
Previous studies show that certain energy adipokine regulators such as adiponectin and leptin have an effect on blood pressure by regulating endothelial function and the central nervous system.20,21 As a new adipokine energy regulator, whether adropin has the same kind of effect is a question that warrants further investigation. In our study, we find that adropin is negatively correlated with blood pressure and is an independent risk factor for hypertension. Because adropin can be influenced by BMI, glucose, and TGs,22 we adjusted for these parameters, and continued to find a negative correlation between adropin and ET-1. Out results suggest that adropin may influence blood pressure by promoting endothelial dysfunction. Findings from several articles support our results. Lovren et a10 have reported that one of the endothelial protective actions of adropin is likely mediated via upregulation of endothelial NO synthase and that in patients with obstructive sleep apnea, a significant risk factor for hypertension, adropin concentration is a reliable indicator of endothelial dysfunction.23 Lower serum adropin levels are also associated with cardiac syndrome X (CSX), and endothelial dysfunction is involved in the pathophysiology of CSX.24 In individuals with type 2 diabetes, adropin is an independent risk factor for endothelial dysfunction.11 In the context of these studies, we conclude from our results that adropin influences blood pressure by protecting endothelial function.
It is possible that there are other mechanisms by which adropin affects blood pressure. Our study does not show the relationship between adropin and the autonomic nervous system. However, it has been demonstrated that adropin has increased expression in the brain in diabetic rats, and that adropin knockout mice exhibited decreased locomotor activity and impaired motor coordination coupled with defective synapse formation.25,26 Thus adropin may be a regulator of nervous system activity. Whether adropin can influence blood pressure by regulating the central automatic nervous system is a question remains to be studied.
In our study, the hypertension group had higher Cr and lower adropin levels compared with the control group. Because adropin has a low molecular weight of about 5 Kda,27 and the normal urine adropin concentration were found to be approximately 4 times higher than that of corresponding serum adropin concentration.14 Moreover, glomerular perfusion pressure and molecular weight are 2 important determinants for protein to pass through the glomerular filtration barrier. Thus, in hypertensive patients, it is possible that adropin is more easily filtered and may therefore be lower in plasma. In our protocol, we excluded patients with kidney dysfunction, but we continued to observe an appreciable difference in plasma Cr between the hypertensive and control group. It is well known that the kidney has a large functional reserve, with rises in Cr virtually undetectable in early renal dysfunction. Increased protein filtration and decreased protein reabsorption are 2 characteristics of renal dysfunction. Thus, decreased adropin reabsorption may also account for our results. Kuloglu and Aydin28 reported that adropin and iNOS immunoreactivity were co-localized to the glomeruli, peritubular interstitial cells and peritubular capillary endothelium of the cortex. Because adropin is highly expressed in the kidney,25 it may play a role in compensatory mechanisms against renal damage inflicted by diabetes. Whether adropin has a true role in hypertension or is an artifact of renal dysfunction remains to be fully elucidated.
Our study has several limitations. First, our study sample is relatively small, the survey was mainly carried out at a single site, and selection bias might be present, which may restrict the application of the results to broader populations. Second, although our study shows that adropin has a relationship with blood pressure and is an independent risk factor for hypertension, whether the correlation beween blood pressure and adropin represents a true cause-and-effect relationship or merely reflects parallel changes because of a common underlying etiology in this population needs to be further clarified. Confirmation that alteration of adropin is a major risk factor for hypertension may require investigation using adropin transgenic overexpression mice models, or systemic treatment using recombinant adropin.
In conclusion, adropin is a risk factor for hypertension; it has a negative relationship with blood pressure and regulates blood pressure by possibly influencing endothelial function.
Acknowledgment
The authors thank Charlotte Burmeister, a biostatistician at Henry Ford Hospital, for statistical advice.
Footnotes
Abbreviations: BMI = body mass index, CKD = chronic kidney disease, Cr = Creatinine, DBP = diastolic blood pressure, ET-1 = Endothelin 1, LDL = low-density lipoprotein, OR = odds ratio, SBP = systolic blood pressure, TC = total cholesterol, TG = triglycerides.
This study is supported in part by Soochow University Young Teachers Foundation (SDY2012A38); Suzhou Science and Technology Bureau foundation (KJXW2011011); and Second Affiliated Hospital of Soochow University Foundation (SDFEYGJ1302). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors declare no conflicts of interest or disclosures.
REFERENCES
- 1.Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing-Implications in hypertension. J Mol Cell Cardiol 2015; 83:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akter S, Jesmin S, Iwashima Y, et al. Higher circulatory level of endothelin-1 in hypertensive subjects screened through a cross-sectional study of rural Bangladeshi women. Hypertens Res 2015; 38:208–212. [DOI] [PubMed] [Google Scholar]
- 3.Lin YJ, Kwok CF, Juan CC, et al. Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor. Biochem Biophys Res Commun 2014; 451:263–269. [DOI] [PubMed] [Google Scholar]
- 4.Zarzuelo MJ, Gómez-Guzmán M, Jiménez R, et al. Effects of peroxisome proliferator-activated receptor-( activation in endothelin-dependent hypertension. Cardiovasc Res 2013; 99:622–631. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Li J, Weng X, et al. Farnesoid X receptor agonist CDCA reduces blood pressure and regulates vascular tone in spontaneously hypertensive rats. J Am Soc Hypertens 2015; 9:507–516. [DOI] [PubMed] [Google Scholar]
- 6.Moorhouse RC, Webb DJ, Kluth DC, et al. Endothelin antagonism and its role in the treatment of hypertension. Curr Hypertens Rep 2013; 15:489–496. [DOI] [PubMed] [Google Scholar]
- 7.Dhaun N, Moorhouse R, MacIntyre IM, et al. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension 2014; 64:296–304. [DOI] [PubMed] [Google Scholar]
- 8.Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci 2012; 91:507–516. [DOI] [PubMed] [Google Scholar]
- 9.Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 2008; 8:468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovren F, Pan Y, Quan A, et al. Adropin is a novel regulator of endothelial function. Circulation 2010; 122:S185–192. [DOI] [PubMed] [Google Scholar]
- 11.Topuz M, Celik A, Aslantas T, et al. Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med 2013; 61:1161–1164. [DOI] [PubMed] [Google Scholar]
- 12.Altincik A, Sayin O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J Pediatr Endocrinol Metab 2015; 28:1095–1100. [DOI] [PubMed] [Google Scholar]
- 13.Kocaoglu C, Buyukinan M, Erdem SS, et al. Are obesity and metabolic syndrome associated with plasma adropin levels in children? J Pediatr Endocrinol Metab 2015; 2015: in press. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Ouyang F, Zhou S. Adropin as a novel energy factor likely has the ability to regulate blood pressure. Med Hypotheses 2015; 85:234. [DOI] [PubMed] [Google Scholar]
- 15.Wei D, He WY, Lv QZ. Effect of nisoldipine and olmesartan on endothelium-dependent vasodilation in essential hypertensive patients. CNS Neurosci Ther 2012; 18:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisanuki YY, Emoto N, Ohuchi T, et al. Low blood pressure in endothelial cell-specific endothelin 1 knockout mice. Hypertension 2010; 56:121–128. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Wang W, Yu H, et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012; 59:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, Zhao L, Zhu J, et al. Serum mimecan is associated with arterial stiffness in hypertensive patients. J Am Heart Assoc 2015; 4:e002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel A, Su B, Flavahan S, et al. Increased endothelial exocytosis and generation of endothelin-1 contributes to constriction of aged arteries. Circ Res 2010; 107:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Head GA, Lim K, Barzel B, et al. Central nervous system dysfunction in obesity-induced hypertension. Curr Hypertens Rep 2014; 16:466. [DOI] [PubMed] [Google Scholar]
- 21.Tesauro M, Mascali A, Franzese O, et al. Chronic kidney disease, obesity, and hypertension: the role of leptin and adiponectin. Int J Hypertens 2012; 2012:943605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu HY, Zhao P, Wu MC, et al. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul Pept 2014; 190–191:46–49. [DOI] [PubMed] [Google Scholar]
- 23.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, et al. Circulating adropin concentrations in pediatric obstructive sleep apnea: potential relevance to endothelial function. J Pediatr 2013; 163:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celik A, Balin M, Kobat MA, et al. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther 2013; 31:174–178. [DOI] [PubMed] [Google Scholar]
- 25.Aydin S, Kuloglu T, Aydin S, et al. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol Cell Biochem 2013; 380:73–81. [DOI] [PubMed] [Google Scholar]
- 26.Wong CM, Wang Y, Lee JT, et al. Adropin is a brain membrane-bound protein regulating physical activity via the NB-3/Notch signaling pathway in mice. J Biol Chem 2014; 289:25976–25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydin S. Three new players in energy regulation: preptin, adropin and irisin. Peptides 2014; 56:94–110. [DOI] [PubMed] [Google Scholar]
- 28.Kuloglu T, Aydin S. Immunohistochemical expressions of adropin and ınducible nitric oxide synthase in renal tissues of rats with streptozotocin-ınduced experimental diabetes. Biotech Histochem 2014; 89:104–110. [DOI] [PubMed] [Google Scholar]


