Abstract
This study investigated the 1-year mortality of patients who underwent brain surgery following traumatic brain injury (TBI) who also had alcoholic and/or nonalcoholic liver cirrhosis (LC) using a nationwide database in Taiwan. A longitudinal cohort study matched by propensity score with age, gender, length of ICU stay, HTN, DM, MI, stroke, HF, renal diseases, and year of TBI diagnosis in TBI patients with alcoholic and/or nonalcoholic LC and TBI patients without LC was conducted using the National Health Insurance Research Database in Taiwan between January 1997 and December 2007. The main outcome studied was 1-year mortality. In total, 7296 subjects (2432 TBI patients with LC and 4864 TBI patients without LC) were enrolled in this study. The main findings were (1) TBI patients with LC had a higher 1-year mortality (52.18% vs 30.61%) and a 1.75-fold increased risk of mortality (95% CI 1.61–1.90) compared with non-LC TBI patients, (2) renal diseases and HF are risk factors, but hypertension could be a protective factor in cirrhotic TBI patients, and (3) TBI patients with non-alcoholic LC and the coexistence of alcoholic and nonalcoholic LC had higher 1-year mortality compared with TBI patients with alcoholic cirrhosis. This study showed that patients with LC who have undergone brain surgery might have higher risk of 1-year mortality than those without LC. In addition, nonalcoholic and the coexistence of alcoholic and nonalcoholic LC show higher 1-year mortality risk than alcoholic in TBI patients with LC, especially in those with comorbidities of hypertension, diabetes mellitus, and stroke.
INTRODUCTION
Liver cirrhosis (LC) is a form of late stage of liver fibrosis that results from non-alcoholic etiologies, such as chronic hepatitis B virus (HBV) and hepatitis C virus (HCV), and alcohol consumption.1,2 Most cases of LC coexist with other comorbidities that could increase the risk of cirrhosis-related death and the need for medical resources.3 A population-based study demonstrated that LC following central nervous system insults is a well-known significant risk factor for morbidity and mortality in patients requiring elective4–7 and emergent surgical intervention.4,5,7–11 Compared with elective surgery, emergency surgery was associated with a significantly higher mortality in LC patients.4,5,8 In Western society, alcohol consumption and hepatitis C are the most important causes of LC;12,13 however, in Taiwan, most LC cases result from nonalcoholic etiologies, particularly hepatitis B.14
Traumatic brain injury (TBI) remains a well-known public health challenge. The worldwide incidence rates were estimated from 103 to 546 per 100,000 people.15–18 Several risk factors may lead to mortality in TBI patients, such as stroke or end-stage renal disease (ESRD). Because LC is a risk factor for spontaneous intracerebral hemorrhage,19 LC could be an important risk factor for TBI patients. A single-center study showed that LC is a poor comorbidity factor for brain surgery and poor outcomes were observed in patients with TBI.20 In a trauma database study, Lustenberger et al found that the risk of in-hospital death after TBI surgery was significantly higher among patients with cirrhosis than in those without.21 However, the impact of LC, which was caused by alcohol or nonalcohol-related factors, on long-term outcomes in TBI patients and the associated risk factors in a population has not been well defined. Therefore, the aim of this study is to investigate the 1-year mortality and associated risk factors among TBI patients with LC.
METHODS
Data Source
The National Health Insurance program of Taiwan began in 1995 and covers 99% of Taiwan's population. To improve medical research, the National Health Research Institutes of Taiwan established the National Health Insurance Research Database (NHIRD) to organize medical claim data. The NHIRD was previously used extensively in various published studies. For each insured subject, detailed information including diagnostic codes for each clinical visit was based on the clinical modification of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9CM) code. Informed consent was originally obtained by the National Health Research Institutes of Taiwan. In addition, the privacy of each individual's information was protected using encrypted personal identification to avoid the potential for ethical violations related to the data. Exemption was obtained from the institutional review board of Chi Mei Medical Center (IRB No. 10208-E02).
Patient Selection and Definition
In this study, the claims data from inpatients hospitalized between 1997 and 2007 was analyzed. Patients receiving TBI surgery were identified from the claiming medical expenditure claim applications, and those patients between 20 and 80 years old were selected for this study. Patients with LC were identified by alcoholic cirrhosis of their liver (ICD-9CM: 571.2) and cirrhosis of the liver without mention of alcohol (ICD-9CM: 571.5). In total, 2432 TBI patients with LC and 4864 TBI patients without LC were matched with the study cohorts according to the propensity score with age, gender, length of ICU stay, and the co-morbidities of hypertension (HTN, ICD-9CM: 362.11, 401–405, 437.2), diabetes mellitus (DM, ICD-9CM: 250, 357.2, 362.0, 366.41), myocardial infarction (MI, ICD-9CM: 410, 412), stroke (ICD-9CM: 430–438), heart failure (HF, ICD-9CM: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 422, 425, 428), and renal diseases (ICD-9CM: 585, 586, 588). In addition, the concerned related liver diseases, such as HBV (ICD-9CM: 070.2, 070.3, and V02.61), HCV (ICD-9CM: 070.41, 070.44, 070.51, 070.54, V02.62, and 070.7), alcohol fatty liver (ICD-9CM:571.0), and nonalcoholic fatty liver (ICD-9CM:571.8), were analyzed in this study. The comorbidities and the concerned related liver diseases were based on the records from 1 year before the date of TBI diagnosis. All the diagnosis of the patients were based on clinical diagnosis and coded by medical professionals.
Measurements
TBI patients with a medical history of LC had reduced survival following surgery. Therefore, the outcome in this study was 1-year mortality. The 1-year survival time was determined from the TBI surgery date to the date of death. Demographic and clinical characteristics, including age, gender, DM, HTN, MI, stroke, HF, renal diseases, HBV, HCV, alcoholic fatty liver, fatty liver, length of ventilation, and length of ICU stay, were also used to estimate the risk of 1-year mortality. The 1-year mortality trends among TBI patients with LC by different comorbidities were also presented in this study.
Statistical Analysis
Pearson's chi square test for categorical variables, such as age group, gender, comorbidities, length of ICU classification, length of ventilation classification, death at 1-year, and related liver diseases, was used to compare the distribution between TBI patients with and without LC. For continuous variables, such as age, length of ventilation and time to death, Student's t test or Wilcoxon rank-sum test was used to examine the distribution difference between TBI patients with and without LC. The proportion of patients who were still alive within 1 year was described by Kaplan–Meier plots, and the difference in the mortality risk was determined by the log-rank test. The Cox proportional regression model was used to estimate the hazard ratio of mortality adjusted by potential confounders. Statistical Analysis System (SAS) statistical software (version 9.3; SAS Institute, Inc, Cary, NC) was used for all statistical analyses, but the Kaplan–Meier curves were plotted using STATA (version 12; Stata Corp., College Station, TX). The level of P value < 0.05 was considered as statistical significance.
RESULTS
Figure 1 showed the 1-year mortality of 7296 TBI patients with and without LC from 1997 to 2007. In Taiwan, the mortality of TBI patients with LC was as low as 6% but as high as 50.8% in 2007. The mortality from 1997 to 2007 between TBI patients with and without LC was significantly different (P < 0.001). The demographic and comorbidities information of TBI patients with LC and those without LC are shown in Table 1. Among TBI patients with LC, 411(16.90%) had alcoholic LC only, 1479(60.81%) had nonalcoholic LC only, and 542(22.29%) had both types of LC. The 1-year mortality rate of the study population was 37.46%, and TBI patients with LC had a significantly higher 1-year mortality rate than those without (52.18% vs 30.61%, P < 0.001). The distribution of related liver diseases such as HBV, HCV, alcoholic fatty liver, and nonalcoholic fatty liver were all significantly higher in LC groups (Table 1). The log-rank test showed a significant difference between TBI patients with LC and the comparison cohort for the 1-year mortality rate (P < 0.001, Figure 2). Table 2 showed the 1-year mortality hazard ratio between TBI patients with LC and those without, and the subtypes of LC were also presented. The hazard ratio of 1-year mortality of TBI patients with LC was 1.75 (95% CI 1.61–1.90) compared with those without LC.
FIGURE 1.
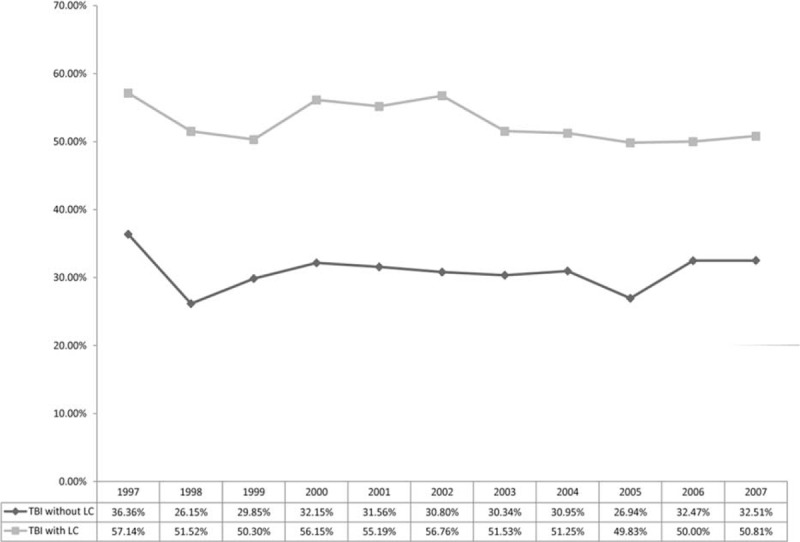
Trend of 1-year mortality rate between TBI with LC and TBI without LC. LC = liver cirrhosis; TBI = traumatic brain injury.
TABLE 1.
Demographic Characteristics and Clinical Information of Traumatic Brain Injury Patients with and without Liver Cirrhosis
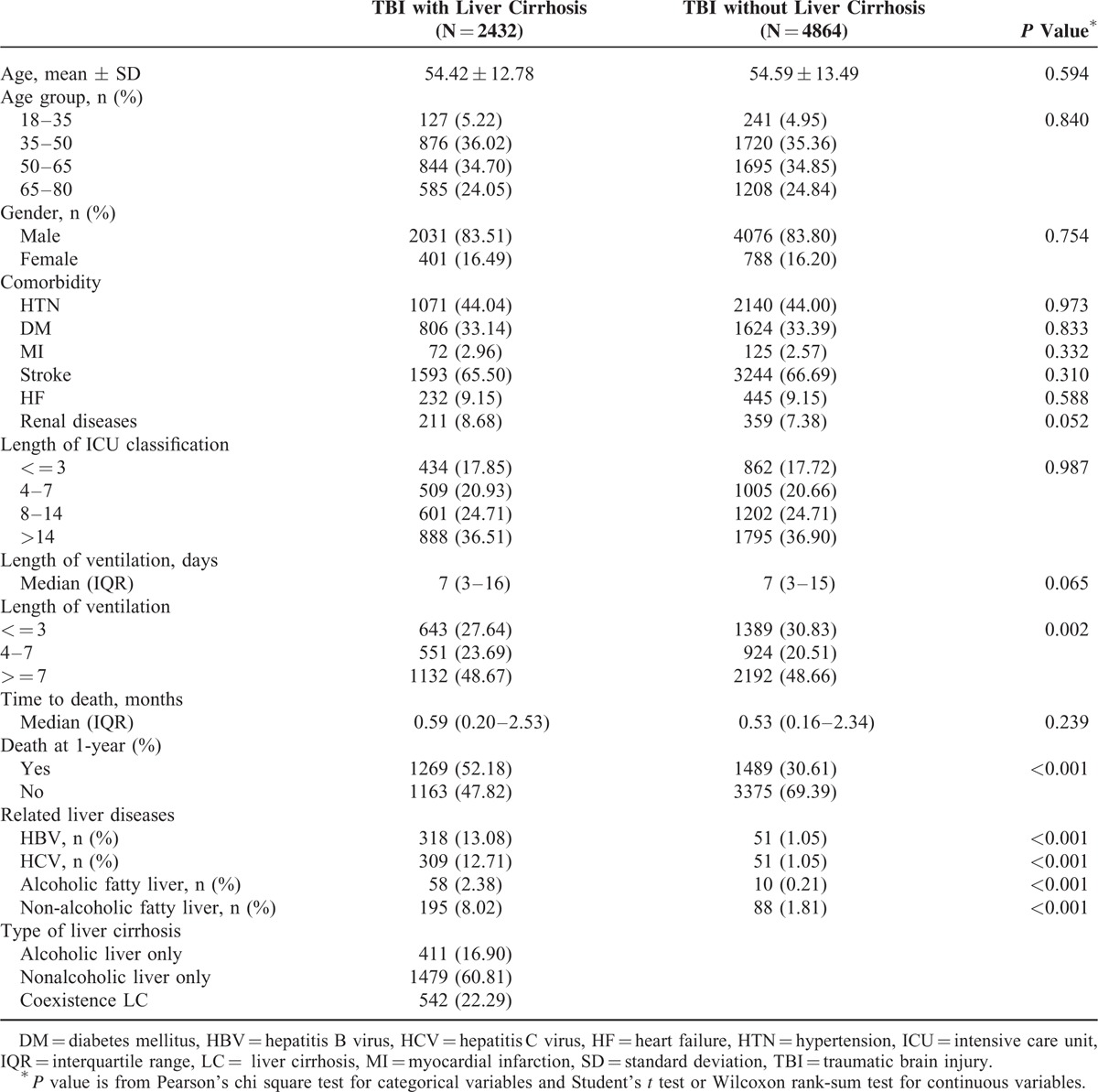
FIGURE 2.
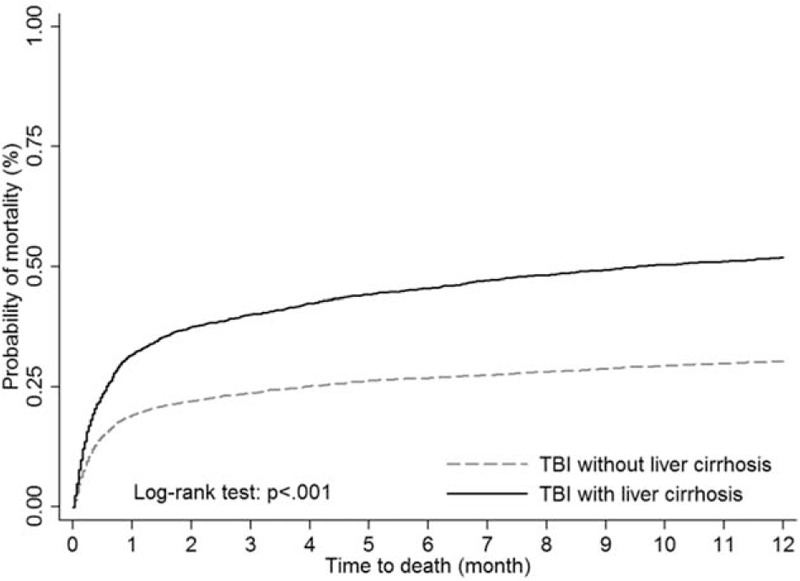
Kaplan–Meier survival curves for mortality of TBI patients stratified by liver cirrhosis. TBI = traumatic brain injury.
TABLE 2.
Mortality Rate Ratios Between Traumatic Brain Injury Patients with Liver Cirrhosis and Those Without
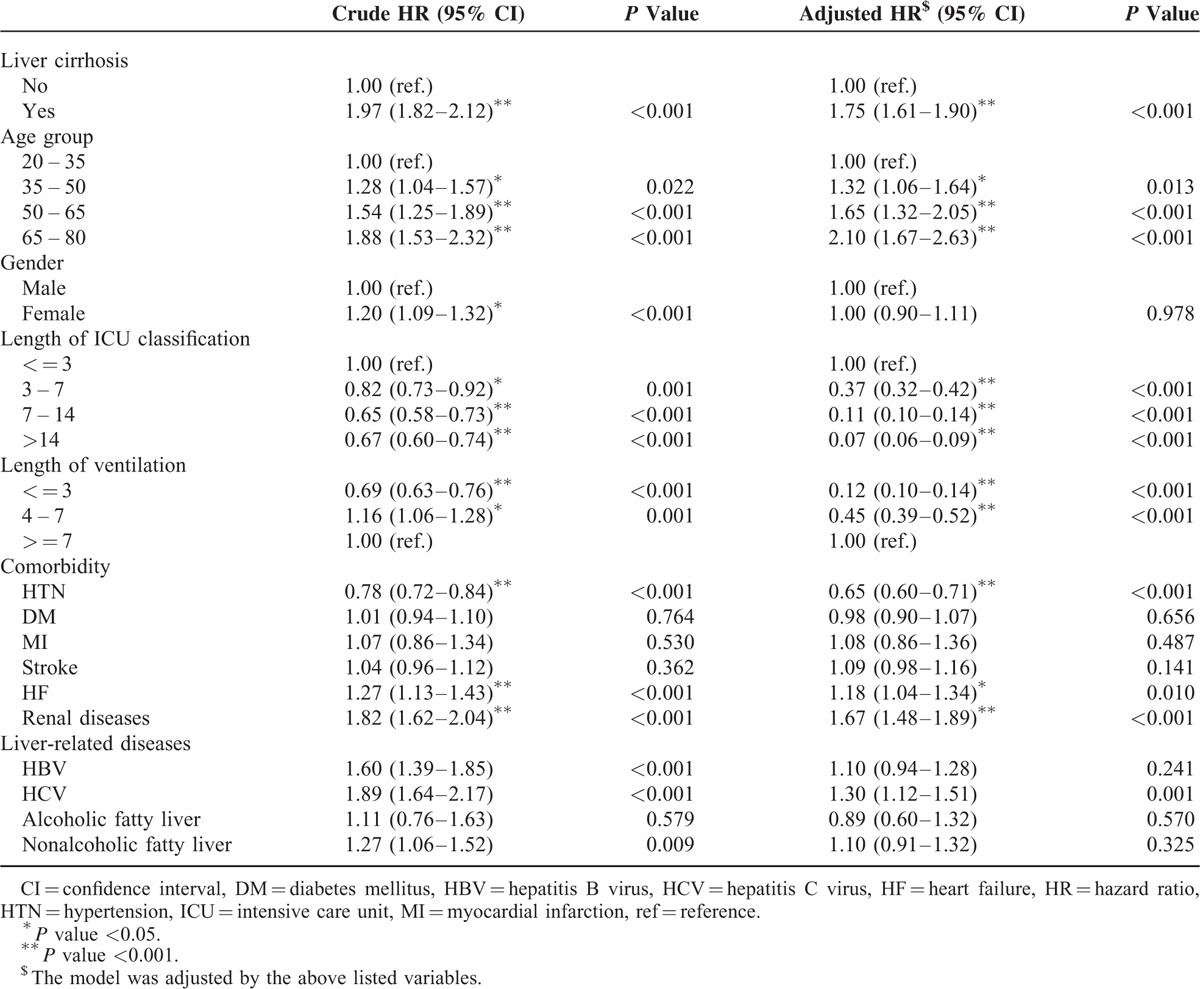
Figure 3 showed Kaplan–Meier plots of the 1-year mortality of TBI patients with LC patients among different comorbidities. For HF, renal diseases, and HTN, the significant difference between TBI patients with these comorbidities and those without was presented. HF and renal diseases presented as the potential risk factors of 1-year mortality for TBI patients with LC, but HTN could be a protective factor.
FIGURE 3.
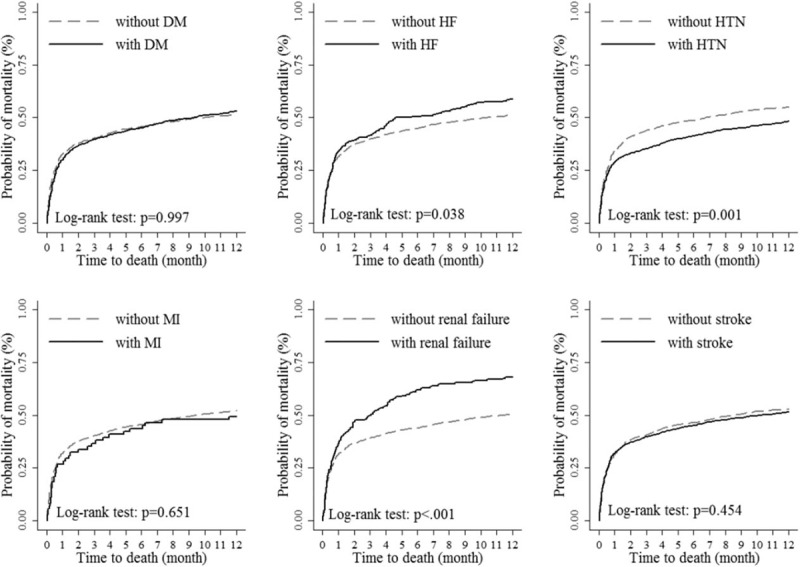
The 1-year mortality of TBI with liver cirrhosis among different comorbidities. TBI = traumatic brain injury.
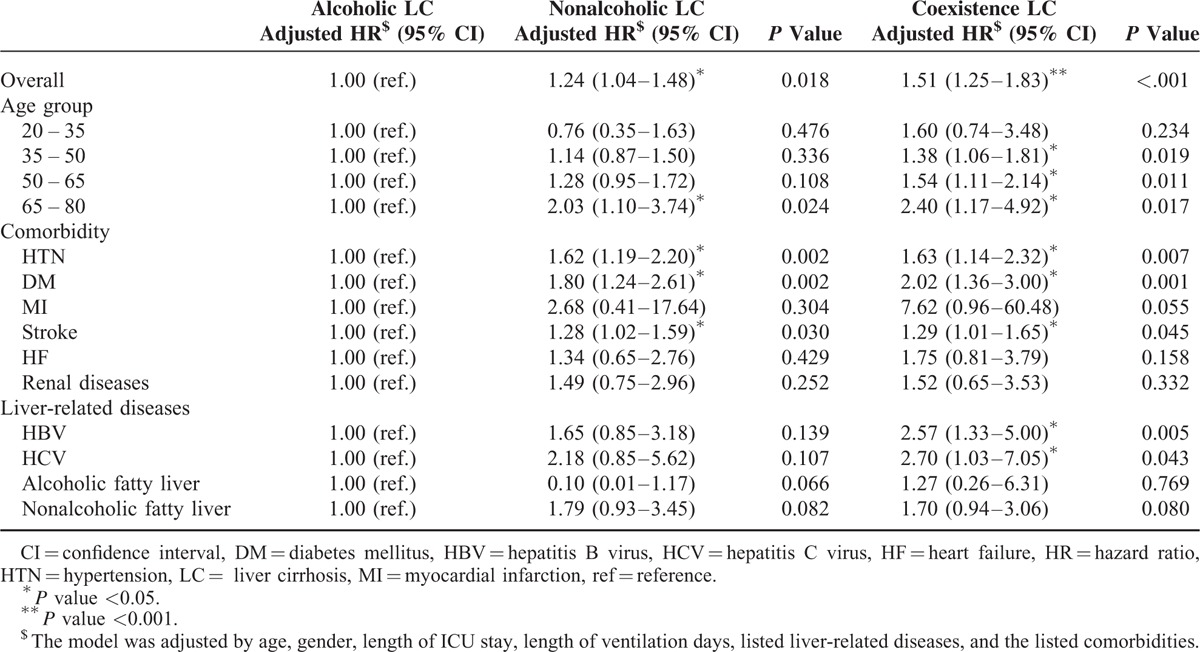
For estimating different types of LC cases among TBI patients, the Kaplan–Meier plot with log-rank test showed a significant difference in the 1-year mortality rate (P < 0.001, Figure 4). The stratified analysis of TBI risk factors for different liver cirrhosis types was presented for TBI patients with LC (Table 3). Compared with alcoholic liver only, the nonalcoholic liver and coexistence LC types show an increased risk of 1.24 and 1.51 times, respectively. Patients >65 years also showed a significant risk of 1-year mortality. Although HTN could be a protective factor of 1-year mortality for TBI patients, patients with nonalcoholic liver and coexistence LC presented with a significant increased risk for 1-year mortality compared with alcoholic LC patients. In addition, compared with alcoholic liver, DM and stroke are both risk factors of 1-year mortality for patients with nonalcoholic LC and coexistence LC. For liver-related diseases, HBV and HCV were associated with higher hazard ratios of mortality risk when comparing nonalcoholic LC and coexistence LC patients with alcoholic LC patients.
FIGURE 4.
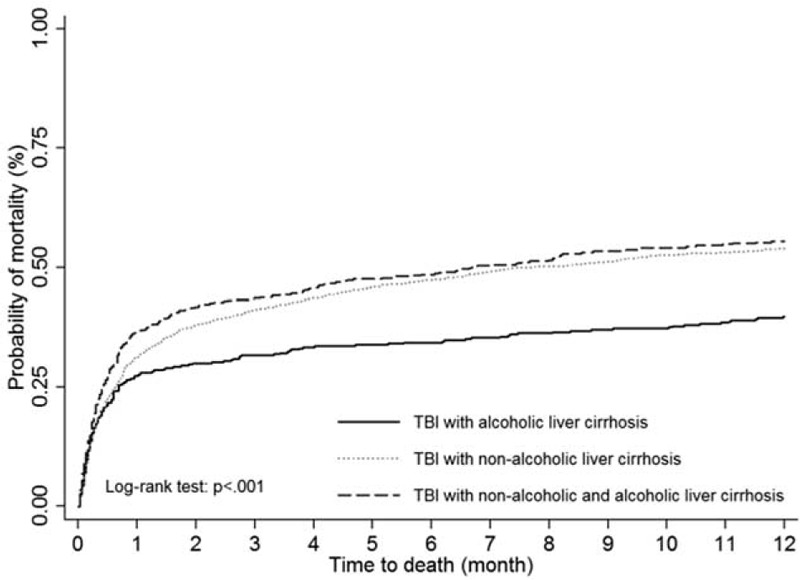
The 1-year mortality of TBI patients with liver cirrhosis by different types. TBI = traumatic brain injury.
TABLE 3.
Mortality Rate Ratios Between Different Liver Cirrhosis Types for Traumatic Brain Injury Patients with Liver Cirrhosis Stratified by each Interested Group
The log-rank test showed a significant difference in the 1-year mortality rate among TBI patients with LC, cryptogenic LC, and the comparison cohort (P < 0.001, Figure 5). TBI patients with cryptogenic LC have higher 1-year mortality trend than those without LC/cryptogenic LC, but lower than TBI patients with LC.
FIGURE 5.
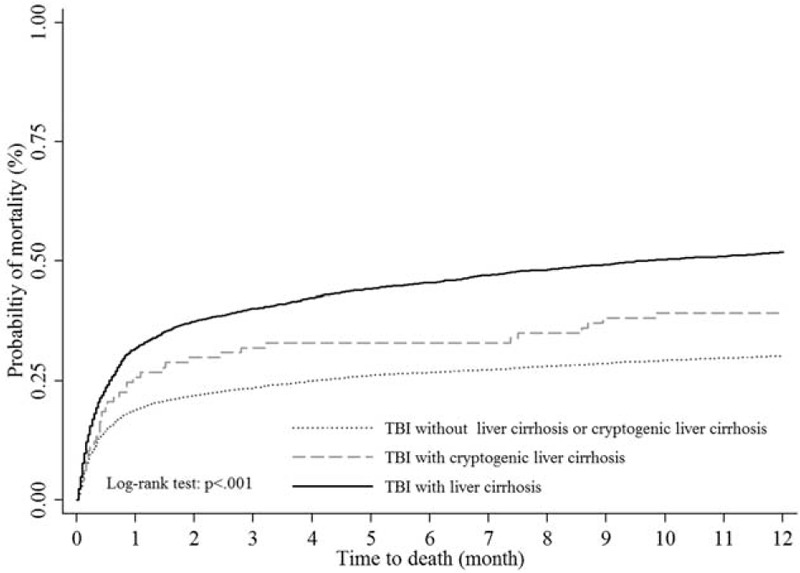
Kaplan–Meier survival curves for mortality of TBI patients stratified by liver cirrhosis, cryptogenic liver cirrhosis, and those without liver cirrhosis. TBI = traumatic brain injury.
DISCUSSION
This study presented the 1-year mortality associations between alcoholic and/or nonalcoholic LC patients after TBI surgery using population-based administrative data. In the present study, LC increased the nearly 2-fold mortality risk among TBI patients compared with non-LC patients. HF and renal diseases were significantly associated with higher mortality for cirrhotic TBI patients; however, HTN could be a protective factor in those patients. In the stratified analysis of comparing different LC types for cirrhotic TBI patients, nonalcoholic and coexistence LC patients have higher mortality risk than alcoholic LC patients, especially in those >65 years, HTN, DM, or stroke. In addition, those with HBV and HCV presented the significant mortality risk for coexistence LC. We hope that this information will help guide treatment recommendations by neurosurgeons, families, and intensive care physicians. These data will hopefully serve as a foundation for future studies on the effects of LC on TBI.
High Mortality Rates are Observed in TBI Patients with LC Who Underwent Brain Surgery Compared with Patients Without LC
In Taiwan, LC remains a major challenge because the prevalence of HBV and HCV is 15–20%22,23 and 1–5%,24 respectively. The yearly incidence rate of TBI was 344 per 100,000 people in Taiwan.15 Thus, neurosurgeons and caregivers may increasingly encounter patients with TBI who also have LC as a comorbidity.
In recent studies, Lin et al demonstrated cirrhotic patients who underwent nonhepatic elective or emergency surgery had nearly twice the risk of 30-day in-hospital mortality compared to those without cirrhosis.5 They also found that patients with LC and alcohol dependence syndrome had more than three times the risk of 30-day mortality. Another study indicated that cirrhotic patients with acute TBI who underwent brain surgery had a hospital mortality rate of 37.5%.20 In addition, LC was associated with a 2-fold increased risk and a higher in-hospital mortality rate (34% vs18.1%) compared with non-LC TBI patients who underwent brain surgery.21
In the present study, we found cirrhotic TBI patients who underwent brain surgery had higher 30-day mortality (32.2% vs 19.4%), which was consistent with previous studies.20,21 We further determined that cirrhotic TBI patients had higher 1-year mortality (52.2% vs 30.6%) and a hazard ratio of 1.75 compared to non-LC TBI patients. Our results support an association between alcohol consumption and mortality in patients with LC.25 We further identify that nonalcoholic LC had a higher hazard ratio than alcoholic LC in 1-year mortality after TBI (HR 1.24, 95% CI 1.04–1.48). Patients with coexistence of alcoholic and nonalcoholic LC had the highest mortality risk (HR 1.51, 95% CI 1.25–1.83), which was consistent with the results of the study by Lin et al5 From the results of stratified analysis, we found that for cirrhotic TBI patients with pre-existing HBV and HCV, coexistence LC was associated with a higher hazard ratio of mortality as HBV and HCV were known risk of LC.26 Thus, the coexistence of alcoholic and non-alcoholic LC enhances disease progression in a synergistic manner.27 However, the mechanisms causing these observations need to be further investigated.
Furthermore, alcoholic LC accounted for 39.2% of all TBI patients with cirrhosis in our study. The relative high ratio may imply that certain factors may cause TBI in these patients. Driving while intoxicated could be a major reason because a previous study indicated a correlation between alcoholic LC and drunk driving.28 In addition, hepatic encephalopathy could be other reasons for body injury resulting from a fall.29,30 The above accidents are both plausible explanations for the observed increased risk for TBI in alcoholic LC patients.
The Relevant Risk Factors for 1-Year Mortality in LC Patients with TBI Who Underwent Brain Surgery
Previous studies have shown that age, hypertension, DM, MI, stroke, HF, renal diseases, and LC are associated with mortality in TBI patients.20,21,31–33 Our study presented that LC, age, stroke, renal diseases, and HF are risk factors, whereas the length of ICU stay and HTN are protectors in TBI patients. Among the observed comorbidities in cirrhotic TBI patients, HF and renal diseases were risk factors, whereas HTN is a protector for 1-year mortality.
Age is a well-known risk factor of 1-year mortality in TBI patients, especially in older TBI patients who tend to have a worse outcome.32,33 In the present study, we further provide new information among older TBI patients with LC that the hazard ratio for 1-year mortality rate was significantly elevated in older, nonalcoholic LC patients, especially those between 65 and 80 years old following TBI surgery. One possible mechanism for this observations is that HCV was prevalent in a large majority of those over the age of 65 and who received blood transfusions before the introduction of HCV screening in Taiwan.34 Given that societal aging may increase the number of older cirrhotic TBI patients who undergo surgical intervention and the older population is at a higher risk for mortality, neurosurgeons should be cautious when performing brain surgery on older cirrhotic TBI patients.
Patients with advanced cirrhosis have an increased heart rate and stroke volume, which leads to a higher cardiac output, and thus, HF may develop in the late stages of LC.35,36 Therefore, HF maybe a sign of late stage of LC. Despite the rate of HF in cirrhotic TBI patients being relatively low at 9.2% (232/2432), HF is still a risk factor for 1-year mortality in cirrhotic TBI patients. This explains the increase in mortality to 59.5% (138/232) in patients with LC and HF. Thus, pre-existing HF exacerbates the risk for mortality in cirrhotic patients under conditions of TBI. The causes of this observations may be that HF occurring in late-stage LC may represent a more serious condition,35,36 and cardiac dysfunction may manifest when TBI activates a systemic hyper adrenergic state to increase both cardiac and cerebral oxygen demands.37 Consequently, cirrhotic TBI with HF may represent a critical status, and the etiologies of LC do not affect the 1-year mortality as shown in Table 3.
Furthermore, RF is a frequent complication of advanced cirrhosis related to disturbances in circulatory function.38 Patients with cirrhosis and RF have a 7-fold increased risk of mortality within 1 year (HR 7.6, 95% CI 5.4–10.8) compared with those without RF.39 In our previous study, we found that ESRD patients have a higher 1-year mortality (76.7% vs 29.8%) than non-ESRD TBI patients.32 In the present study, we further identified that RF increases the 1-year mortality in TBI patients by 1.67-fold. Although the comorbidity of renal diseases in cirrhotic TBI patients is 9.15% (211/2432), these patients have a very high mortality of 68.3% (144/211). Our results support that RF occurring in cirrhosis patients represents a critical condition.38 Therefore, we conclude that 1-year mortality rates are unaffected by whether LC occurs from alcohol, hepatitis, or coexistence.
In addition, TBI and stroke are closely related. TBI was associated with an increased risk for stroke and post-stroke mortality.40 Patients that have had a stroke have an increased risk of TBI and mortality after TBI.31,33 Our study further provided new information that stroke is a risk factor for 1-year mortality in nonalcoholic/coexistence cirrhotic TBI patients who underwent brain surgery.
It is well-known that HTN (essential, secondary) may disappear after the development of cirrhosis in humans;41,42 nevertheless, arterial hypertension manifests in ∼10–15% patients with cirrhosis.43–45 Our study found that the prevalence of HTN in cirrhotic TBI patients was 44% (1071/2432). This relatively high clinical presentation of HTN may be due to the coexistence of LC and essential HTN in our patients because HTN has a high prevalence in mid to late life (94.8% patients age between 35 and 80 y/o).46 Additionally, alcoholic liver and renal involvement in nonalcoholic LC patients may be accompanied by arterial hypertension.41 Meanwhile, some of our patients with early stages of cirrhosis indicated that their high blood pressure levels remained unaffected by the onset of cirrhosis.46 Additionally, HTN could be a protective factor in our study, and this finding has been reported for ESRD47 or ERRD in TBI patients.32 HTN may be a protective factor because normal-to-low BP levels may reflect the fact that organ failure or other events leading to hypotension had occurred before death. Despite of this, cirrhotic TBI patients with HTN have higher mortality risk in those with nonalcohol and coexistence LC compared with alcohol-induced LC. Similar to age, DM, and stroke, we identify that hepatitis-induced/coexistence LC has a higher mortality than alcohol-induced LC in cirrhotic TBI patients.
Nonalcoholic fatty liver disease (NAFLD) is now viewed as the hepatic manifestation of metabolic syndrome.48 In addition, NAFLD is thought as the major cause of cryptogenic cirrhosis, which is a common cause of liver-related mortality and morbidity in the United States.49 In our study, we found the mortality was higher in TBI patients with cryptogenic LC than those without LC, but still less than those with LC (Figure 5). This finding gave us an important information that cryptogenic LC may be a potential risk of mortality for TBI patients, especially cryptogenic LC may progress to LC.50 It is worth to examine the role of cryptogenic LC in the future research.
This study has some limitations. First, all diagnoses relied on claims data and ICD-9-CM diagnosis codes; thus, there may be some disease misclassifications, missing data, and reporting discrepancies, especially in large databases such as the NHIRD. However, the previous study has validated the accuracy of major diagnosis codes in NHIRD,51 and all the diagnosis of the patients based on clinical diagnosis and coded by medical professionals. Second, we were unable to take into account the illness severity scores of TBI and LC because the data, such as Glasgow outcome scales and child classification, were unavailable, which may also interfere with our conclusions regarding the outcomes after TBI. In addition, as this study only used the inpatient claims data, liver-related diseases may be underestimated because the outpatient claims data are not available. Nevertheless, this nationwide population-based study still presented important information regarding the outcomes in TBI patients with or without LC.
In conclusion, TBI patients with LC, especially non-alcoholic/coexistence cirrhotic livers, have an increased 1-year mortality of 2-fold, even after controlling for other demographic and clinical variables. In cirrhotic TBI patients, those aged 65 and over or those with comorbidity of HTN, DM, and stroke have higher 1-year mortality risk in nonalcoholic/coexistence LC patients. These results will provide valuable information for neurosurgeons and intensive care physicians that will result in them paying more attention to these high-risk groups during the decision-making process for surgical intervention and treatment protocol planning.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health, and Welfare and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, the Ministry of Health and Welfare or the National Health Research Institutes. In addition, this work was supported by Chi-Mei Medical Center grant number CMFHR10337.
Footnotes
Abbreviations: CI = confidence interval, DM = diabetes mellitus, ESRD = end-stage-renal-disease, HBV = hepatitis B virus, HCV = hepatitis C virus, HF = heart failure, HTN = hypertension, ICD-9CM = International Classification of Diseases - Ninth Revision Clinical Modification, ICU = intensive care unit, LC = liver cirrhosis, MI = myocardial infarction, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, RF = renal failure, TBI = traumatic brain injury.
C-YC and C-H H equally contributed to this study. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mendenhall CL, Seeff L, Diehl AM, et al. Antibodies to hepatitis B virus and hepatitis C virus in alcoholic hepatitis and cirrhosis: their prevalence and clinical relevance. Hepatology 1991; 14:581–589. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi PH, Di Bisceglie AM. The progression of hepatitis B- and C-infections to chronic liver disease and hepatocellular carcinoma: epidemiology and pathogenesis. Medical Clin N Am 2005; 89:371–389. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen P, Vilstrup H, Andersen PK, et al. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology 2008; 48:214–220. [DOI] [PubMed] [Google Scholar]
- 4.de Goede B, Klitsie PJ, Lange JF, et al. Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis: a systematic review. Best practice & research. Clin. Gastroenterol 2012; 26:47–59. [DOI] [PubMed] [Google Scholar]
- 5.Lin CS, Lin SY, Chang CC, et al. Postoperative adverse outcomes after non-hepatic surgery in patients with liver cirrhosis. British J Surg 2013; 100:1784–1790. [DOI] [PubMed] [Google Scholar]
- 6.Lin TY, Liao JC, Chen WJ, et al. Surgical risks and perioperative complications of instrumented lumbar surgery in patients with liver cirrhosis. Biomed J 2014; 37:18–23. [DOI] [PubMed] [Google Scholar]
- 7.Neeff HP, Streule GC, Drognitz O, et al. Early mortality and long-term survival after abdominal surgery in patients with liver cirrhosis. Surgery 2014; 155:623–632. [DOI] [PubMed] [Google Scholar]
- 8.Christmas AB, Wilson AK, Franklin GA, et al. Cirrhosis and trauma: a deadly duo. Am Surg 2005; 71:996–1000. [PubMed] [Google Scholar]
- 9.Georgiou C, Inaba K, Teixeira PG, et al. Cirrhosis and trauma are a lethal combination. World J Surg 2009; 33:1087–1092. [DOI] [PubMed] [Google Scholar]
- 10.Tinkoff G, Rhodes M, Diamond D, et al. Cirrhosis in the trauma victim. Effect on mortality rates. Ann Surg 1990; 211:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahlstrom K, Ney AL, Jacobson S, et al. Trauma in cirrhotics: survival and hospital sequelae in patients requiring abdominal exploration. Am Surg 2000; 66:1071–1076. [PubMed] [Google Scholar]
- 12.Corrao G, Aricò S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998; 27:914–919. [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z, Zheng L, Stepanova M, et al. Moderate, excessive or heavy alcohol consumption: each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther 2013; 37:703–709. [DOI] [PubMed] [Google Scholar]
- 14.Liaw YF, Tai DI, Chu CM, et al. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988; 8:493–496. [DOI] [PubMed] [Google Scholar]
- 15.Chiu WT, Yeh KH, Li YC, et al. Traumatic brain injury registry in Taiwan. Neurol Res 1997; 19:261–264. [DOI] [PubMed] [Google Scholar]
- 16.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehab 2005; 20:229–238. [DOI] [PubMed] [Google Scholar]
- 17.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008; 7:728–741. [DOI] [PubMed] [Google Scholar]
- 18.Tagliaferri F, Compagnone C, Korsic M, et al. A systematic review of brain injury epidemiology in Europe. Acta Neurochir 2006; 148:255–268. [DOI] [PubMed] [Google Scholar]
- 19.Lai CH, Cheng PY, Chen YY. Liver cirrhosis and risk of intracerebral hemorrhage: a 9-year follow-up study. Stroke—J Cereb Circulation 2011; 42:2615–2617. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Hsu PW, Lee ST, et al. Brain surgery in patients with liver cirrhosis. J Neurosurg 2012; 117:348–353. [DOI] [PubMed] [Google Scholar]
- 21.Lustenberger T, Talving P, Lam L, et al. Liver cirrhosis and traumatic brain injury: a fatal combination based on National Trauma Databank analysis. Am Surg 2011; 77:311–314. [PubMed] [Google Scholar]
- 22.Chen DS, Sung JL. Hepatitis B virus infection on Taiwan. N Engl J Med 1977; 297:668–669. [DOI] [PubMed] [Google Scholar]
- 23.Stevens CE, Beasley RP, Tsui J, et al. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975; 292:771–774. [DOI] [PubMed] [Google Scholar]
- 24.Chen D-S, Kuo GC, Sung J-L, et al. Hepatitis C virus infection in an area hyperendemic for hepatitis B and chronic liver disease: the Taiwan experience. J Infect Dis 1990; 162:817–822. [DOI] [PubMed] [Google Scholar]
- 25.Kerr WC, Fillmore KM, Marvy P. Beverage-specific alcohol consumption and cirrhosis mortality in a group of English-speaking beer-drinking countries. Addiction 2000; 95:339–346. [DOI] [PubMed] [Google Scholar]
- 26.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45:529–538. [DOI] [PubMed] [Google Scholar]
- 27.Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol 2009; 15:3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vingilis E. Drinking drivers and alcoholics are they from the same population. Research Advances in Alcohol and Drug Problems. Springer. 1983; 7:299–342 [Google Scholar]
- 29.Riggio O, Ridola L, Pasquale C. Hepatic encephalopathy therapy: an overview. World J Gastrointest Pharmacol Ther 2010; 1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Román E, Córdoba J, Torrens M, et al. Minimal hepatic encephalopathy is associated with falls. Am J Gastroenterol 2010. [DOI] [PubMed] [Google Scholar]
- 31.Chou YC, Yeh CC, Hu CJ, et al. Risk and mortality of yraumatic brain injury in stroke patients: two nationwide cohort studies. J Head Trauma Rehab 2013. [DOI] [PubMed] [Google Scholar]
- 32.Liao JC, Ho CH, Liang FW, et al. One-year mortality associations in hemodialysis patients after traumatic brain injury—an eight-year population-based study. PloS One 2014; 9:e93956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson HJ, Rivara FP, Nathens A, et al. Development and validation of the mortality risk for trauma comorbidity index. Ann Surg 2010; 252:370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YW, Wu CJ, Wang TE, et al. The mortality survey of older patients with cirrhosis in Taiwan—a single-center experience. J Am Geriat Soc 2010; 58:2230–2232. [DOI] [PubMed] [Google Scholar]
- 35.Moller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart 2002; 87:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiese S, Hove JD, Bendtsen F, et al. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2014; 11:177–186. [DOI] [PubMed] [Google Scholar]
- 37.Alali AS, McCredie VA, Golan E, et al. Beta blockers for acute traumatic brain injury: a systematic review and meta-analysis. Neurocrit Care 2014; 20:514–523. [DOI] [PubMed] [Google Scholar]
- 38.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290. [DOI] [PubMed] [Google Scholar]
- 39.Fede G, D’Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol 2012; 56:810–818. [DOI] [PubMed] [Google Scholar]
- 40.Liao CC, Chou YC, Yeh CC, et al. Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clinic Proc 2014; 89:163–172. [DOI] [PubMed] [Google Scholar]
- 41.Henriksen JH, Moller S. Liver cirrhosis and arterial hypertension. World J Gastroenterol 2006; 12:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loyke HF. Reduction of hypertension after liver disease. Arch Intern Med 1962; 110:45–49. [DOI] [PubMed] [Google Scholar]
- 43.Andersen UO, Henriksen JH, Jensen G. Copenhagen City Heart Study G Sources of measurement variation in blood pressure in large-scale epidemiological surveys with follow-up. Blood Press 2002; 11:357–365. [DOI] [PubMed] [Google Scholar]
- 44.Krousel-Wood MA, Muntner P, He J, et al. Primary prevention of essential hypertension. Med Clin North Am 2004; 88:223–238. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz DT. The relation of cirrhosis of the liver to renal hypertension. A review of 639 autopsied cases. Ann Int Med 1967; 66:862–869. [DOI] [PubMed] [Google Scholar]
- 46.Veglio F, Pinna G, Melchio R, et al. Hormonal aspects of the relation of liver cirrhosis to essential hypertension. Clin Exp Hypertens 1992; 14:889–903. [DOI] [PubMed] [Google Scholar]
- 47.Chien CC, Yen CS, Wang JJ, et al. Reverse epidemiology of hypertension-mortality associations in hemodialysis patients: a long-term population-based study. Am J Hypertens 2012; 25:900–906. [DOI] [PubMed] [Google Scholar]
- 48.Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol 2013; 19:3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 2003; 289:3000–3004. [DOI] [PubMed] [Google Scholar]
- 50.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346:1221–1231. [DOI] [PubMed] [Google Scholar]
- 51.Cheng CL, Kao YHY, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]


