Abstract
Biomarker assessment is based on quantifying several proteins and metabolites. Recent developments in proteomics and metabolomics have enabled detection of these small molecules in biological samples and exploration of the underlying disease mechanisms in obstructive sleep apnea (OSA). This systemic review was performed to identify biomarkers, which were only detected by chromatography and/or mass spectrometry (MS) and to discuss the role of these biomarkers in the field of OSA.
We systemically reviewed relevant articles from PubMed and EMBASE referring to proteins and metabolite profiles of biological samples in patients with OSA. The analytical platforms in this review were focused on chromatography and/or MS.
In total, 30 studies evaluating biomarkers in patients with OSA using chromatography and/or MS methods were included. Numerous proteins and metabolites, including lipid profiles, adrenergic/dopaminergic biomarkers and derivatives, amino acids, oxidative stress biomarkers, and other micromolecules were identified in patients with OSA.
Applying chromatography and/or MS methods to detect biomarkers helps develop an understanding of OSA mechanisms. More proteomic and metabolomic studies are warranted to develop potential diagnostic and clinical monitoring methods for OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is one of the most common sleep disorders, affecting about 4% of middle-aged males and 2% of middle-aged females.1 Moreover, the frequency of OSA has increased along with the obesity epidemic and the changes in lifestyle that have occurred over the last 2 decades.2 OSA is presently characterized as recurrent episodes of a completely or partly obstructed upper airway during sleep.3 The immediate effects caused by episodes of apnea and hypopnea include large intra-thoracic pressure swings, intermittent hypoxia (IH), frequent micro-arousals, and fragmented sleep.4 The ensuing sympathetic activation, oxidative stress, and inflammatory cytokines are correlated closely with a range of sequelae (ie, hypertension, stroke, cardiovascular disease (CVD), diabetes mellitus, and cancer).5–11 Moreover, OSA is associated with all-cause mortality.12 Thus, OSA has become a major challenge for global public healthcare.
To date, the gold standard tool to diagnose OSA is overnight polysomnography (PSG).13 However, PSG itself can lead to sleep disturbance. Moreover, PSG is expensive and uncomfortable for patients with suspected OSA. In addition, it is labor-intensive for the polysomnographic technologist. Thus, more comfortable and easier tools as surrogates for PSG monitoring, or alternative tests, should be developed. Identifying potential biomarkers would facilitate the clinical decision-making process. Unfortunately, no ideal biomarkers for early diagnosis, severity, prognosis, or response to OSA treatment were found by reviewing the literature.14
Systems biology, including genomics, transcriptomics, proteomics, and metabolomics, have been extensively applied to various sleep diseases.15,16 Proteomics and metabolomics have been widely used to discover differences in small molecules downstream of genetic or environmental variations in chronic kidney disease,17,18 polycystic ovary syndrome,19,20 cancers,21,22 diabetes mellitus,23,24 and pulmonary diseases,25,26 and have shown promising initial results.
Proteomics is the large-scale study of the proteome, particularly analysis of its structure and function.27 Metabolomics refers to the study of complete sets of metabolites, which are context-dependent and vary depending on physiology and the developmental or pathological status of cells, tissues, and organisms.28 Perturbations in biological pathways and changes in protein and metabolite concentration occur when an organism or cell is in an abnormal state. Understanding altered proteomic and metabolic profiles in patients with OSA could improve diagnostic tests as well as uncover new approaches to treat or even prevent OSA.
To date, few studies have used proteomics and metabolomics to reveal differences in small molecules in patients with OSA.29–36 Other studies have identified several proteins and metabolites using chromatography and/or mass spectrometry (MS) methods. The purpose of this review is to summarize all identified biomarkers associated with OSA.
METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.37 Ethical approval was not necessary for this meta-analysis as each included study has already received ethics approval.
Search Strategy
A comprehensive search was performed in December 2014 using the PubMed and EMBASE electronic databases to identify relevant studies. The following Mesh terms and keywords were used: “sleep disordered breathing,” “SDB,” “obstructive sleep apnea,” “OSA,” and “sleep apnea” combined with relevant terms, such as “chromatography” and “MS.” In addition, the references of the included studies were also reviewed to increase our search yield. Only studies published in English were included. This search was performed by 2 authors separately.
Two reviewers (HX and XZ) screened titles and abstracts of studies identified through the search, respectively. Full-text articles of studies evaluating metabolic and protein profiles of plasma, urine, adipose tissue, saliva, and tonsillar tissue, among others, from patients with OSA using chromatography and/or MS were included. Exclusion criteria included: not a human study; and did not use overnight PSG to diagnose OSA.
Two reviewers (HX and XZ) independently extracted data from eligible studies using a standard form. The following information was collected: first author, publication year, types of analytical techniques, specimen types, participants, and main biomarkers. Discrepancies were resolved by group discussion. If the protein and metabolite chemical nomenclature differed among studies, their common names were described.
RESULTS
Search Results
We identified 350 citations (113 from PubMed and 237 from EMBASE) following the initial search. Of the 350 references, 71 were removed due to duplication. After screening titles and abstracts, 36 full-text articles were selected for further consideration. Six articles were further excluded for the following reasons: overlapping population,38,39 animal study,40–42 and book review.43 Thus, 30 studies that evaluated biomarkers using chromatography and/or MS in patients with OSA were included in this systematic review (Figure 1).
FIGURE 1.
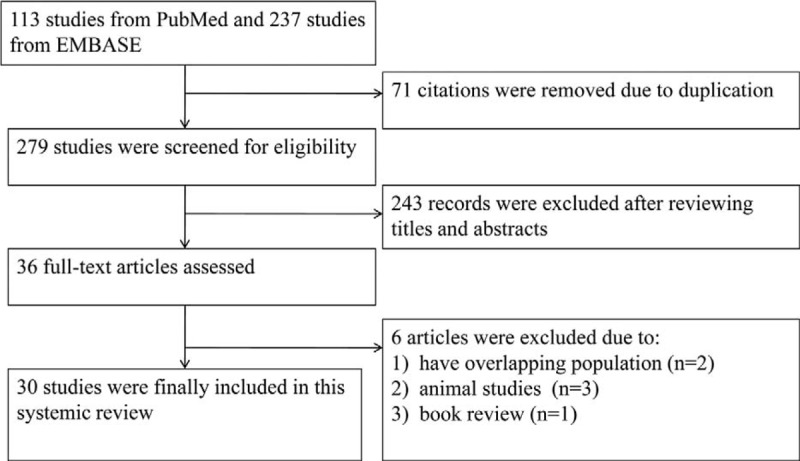
Flow chart of the studies included and excluded in this systemic review.
Study Characteristics
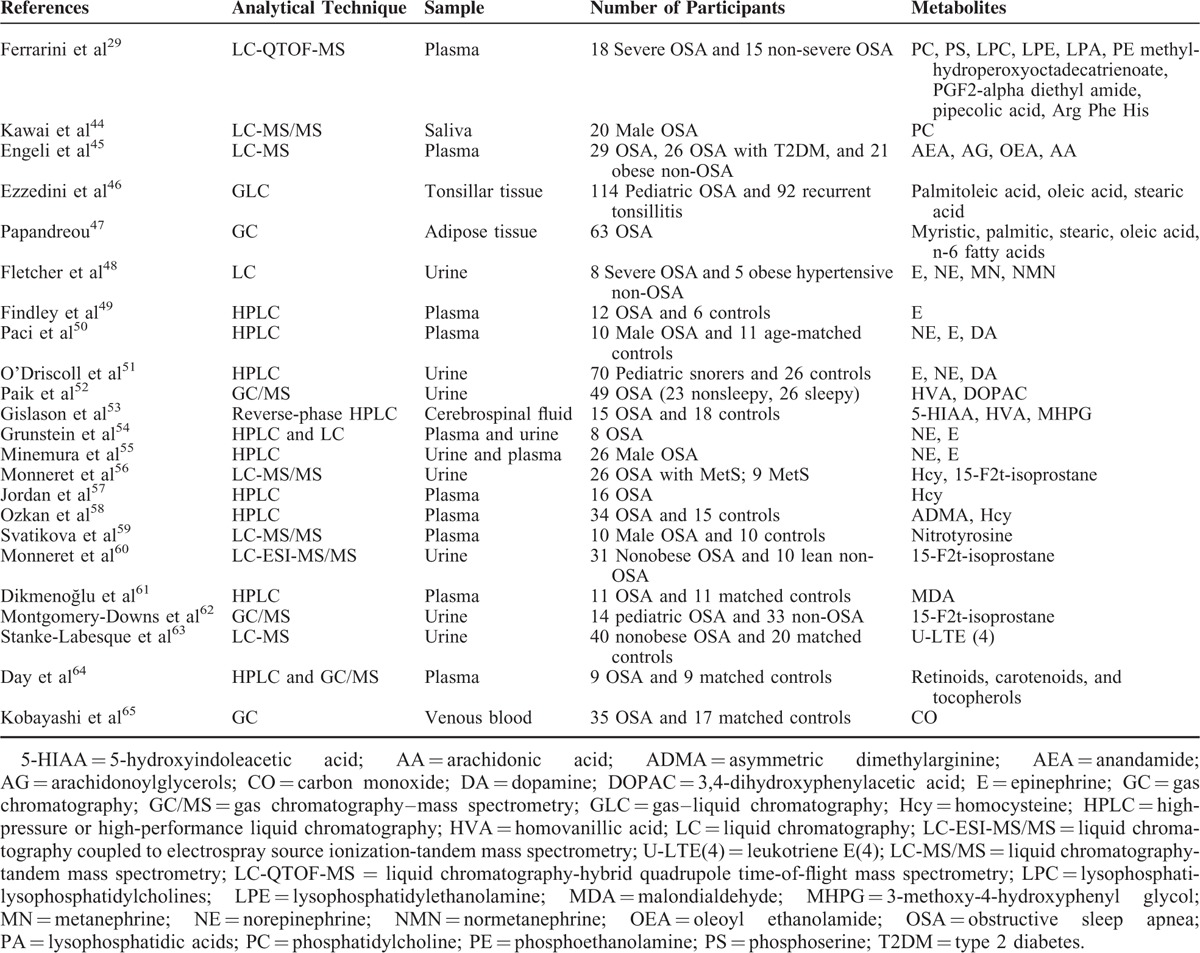
Of the 30 studies, 7 evaluated proteins and 23 evaluated metabolites in patients with OSA. The basic characteristics are shown in Tables 1 and 2.
TABLE 1.
Proteins Identified in Patients With Obstructive Sleep Apnea (OSA)
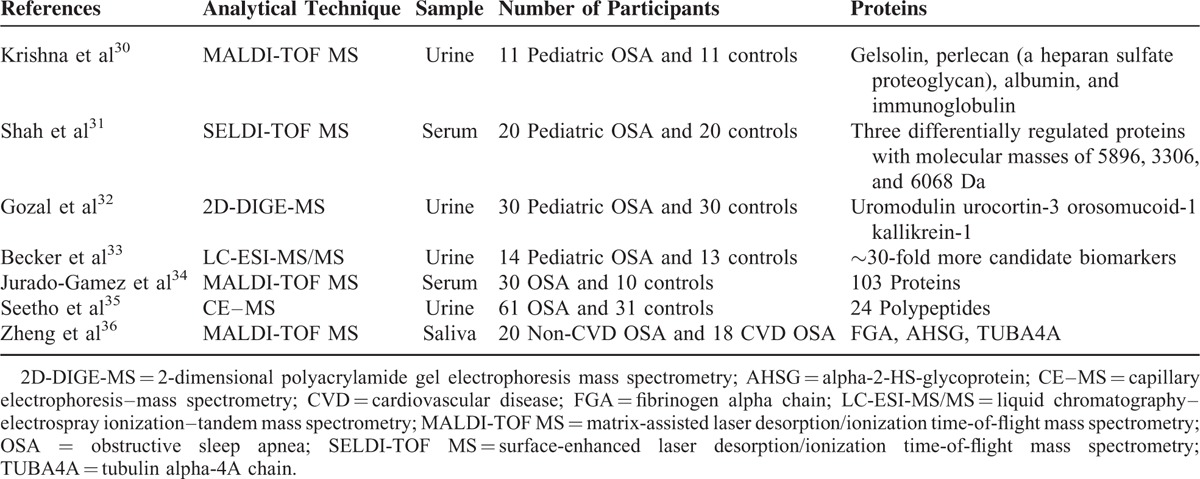
TABLE 2.
Metabolites Identified in Patients With Obstructive Sleep Apnea (OSA)
The analytical platforms used for detecting proteins and metabolites included gas chromatography (GC) (n = 2), liquid chromatography (LC) (n = 2), high-pressure or high-performance liquid chromatography (HPLC) (n = 8), reverse-phase HPLC (n = 1), gas–liquid chromatography (GLC) (n = 1), gas chromatography–mass spectrometry (GC/MS) (n = 4), liquid chromatography–mass spectrometry (LC/MS) (n = 2), liquid chromatography–tandem mass spectrometry (LC-MS/MS) (n = 2), liquid chromatography coupled with electrospray source ionization-tandem mass spectrometry (LC-ESI-MS/MS) (n = 2), liquid chromatography–hybrid quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) (n = 1), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (n = 3), surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (n = 1), and 2-dimensional polyacrylamide gel electrophoresis mass spectrometry (2D-DIGE-MS) (n = 1).
The biological samples utilized for the protein and metabolite analyses included urine (n = 13), saliva (n = 2), plasma (n = 11), tonsillar tissue (n = 1), adipose tissue (n = 1), venous blood (n = 1), cerebrospinal fluid (n = 1), and serum (n = 2).
Protein Profiles in Patients With OSA
Krishna et al30 performed the first study on urinary protein expression in children with OSA and revealed that the levels of particular proteins (gelsolin, perlecan, albumin, and immunoglobulin) were different from those of primary snorers. This preliminary study suggested that altered renal glomerular/tubular permeability and increased catabolism of certain proteins occur in patients with OSA.30 Almost at the same time, Shah et al31 determined that levels of 3 serum proteins with masses of 5896, 3306, and 6068 kDa in children with OSA were different from those of controls, and that these proteomic biomarkers could be used to diagnose OSA with 93% sensitivity and 90% specificity. However, identification of other related proteins may have been precluded due to a methodological deficiency in both studies. Gozal et al used 2D-DIGE-MS and found 16 differentially expressed proteins in patients with OSA. Seven of these were validated by either Western blot or enzyme-linked immunosorbent assay (ELISA).32 In addition, sensitivity was 95% and specificity was 100% using a combination of urinary uromodulin, urocortin-3, orosomucoid-1, and kallikrein.32 Becker et al developed a rigorous and reproducible proteomics workflow method using LC-MS/MS to explore deeper in the proteome and to detect proteins of lower abundance. As a result, more than 30 candidate biomarkers were identified in children with OSA.33 Two other studies used MALDI-TOF-MS and CE-MS analyses to reveal differentially expressed serum and urine proteins in adult patients with OSA and control subjects, respectively. Jurado-Gamez et al reported differential expression of 103 proteins between patients with OSA and controls. The differentially expressed proteins were associated with derangements in lipid and vascular metabolic pathways.34 Seetho et al profiled the expression of urinary peptides in OSA-obese and non-OSA obese subjects. As a result, 24 candidate polypeptides were identified, of which 8 were derived from collagen and fibrinogen alpha; however, no significant differences were obtained.35 Zheng et al identified 5 upregulated mass peaks and 6 downregulated mass peaks in patients with OSA and CVD and verified that the salivary alpha-2-HS-glycoprotein (AHSG) level was lower in patients with OSA and CVD compared with that in patients with OSA without CVD.36
Metabolite Profiles in Patients With OSA
Only 1 pilot metabolomics study explored and assessed potential plasma biomarkers in patients with nonsevere and severe OSA using LC-QTOF-MS. Fourteen statistically significant features were identified and highlighted.29 The biochemical categories of these metabolites included phosphatidylcholine (PC), lysophosphatidylcholines (LPE), phosphoethanolamine (PE), lysophosphatidylethanolamine (LPA), phosphoserine (PS), lysophosphatidic acids, fatty acids, eicosanoids, amino acid metabolites and derivatives, and peptides.29 Besides these metabolites were detected in metabolomics studies, metabolic biomarkers in patients with OSA detected by MS or chromatography alone should also be addressed.
Lipid Metabolites
Salivary PC concentrations increased in patients with OSA when detected by the LC-MS/MS method, and lower levels of PC in patients with OSA may decrease upper airway patency by reducing surface tension.44 Circulating anandamide (AEA), 1/2-arachidonoylglycerols (AG), and oleoyl ethanolamide (OEA) levels were higher in plasma of patients with OSA compared with controls, whereas arachidonic acid concentrations were similar between the 2 groups, suggesting the important role of the endocannabinoid system in blood pressure regulation in patients with OSA at high risk for hypertension and CVD.45 Ezzedini et al46 used GC-LC and found that palmitoleic and oleic acid levels were lower, whereas the stearic acid level was higher, in tonsillitis tissue than in hyperplastic tonsillar tissue, suggesting that fatty acid composition is altered in tonsillar tissue of children with OSA. In addition, Papandreou et al38,47 reported that saturated fatty acids (ie, myristic, palmitic, stearic, and oleic acids) and n-6 fatty acids were correlated with sleep parameters in adipose tissue of patients with OSA using GC. After controlling for confounding factors, the results revealed an independent association between n-3 fatty acids and sleep quality in patients with OSA.38,47
Catecholamine Metabolites and Derivatives
Fletcher et al48 found that norepinephrine (NE) and normetanephrine levels were significantly higher in 8 patients with OSA compared with those in 5 obese hypertensive controls. In another study, 12 patients with OSA had higher plasma epinephrine (E) levels than those of 6 normal volunteers.49 Paci et al examined E, NE, and dopamine (DA) levels in 10 male patients with OSA and 11 age-matched controls. The results showed that plasma NE levels in the patients with OSA were higher than those in controls.50 O’Driscoll et al51 used HPLC to reveal that all urinary catecholamines increase significantly in children with OSA, and that levels of full-night NE and E are related to the severity of OSA. Paik et al used GC-MS to detect urinary neurotransmitter metabolites and showed that the dopamine metabolites homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) increased in sleepy patients with OSA, indicating that excessive daytime sleepiness (EDS) in patients with OSA is probably caused by overnight upregulation of the dopaminergic system.52 These results indicate that increased sympathetic tone occurs in patients with OSA.
However, findings of several other studies are inconsistent with these aforementioned studies. E and metanephrine (MN) levels were not different between 8 patients with OSA and 5 obese hypertensive controls.48 Paci et al50 reported that E and DA levels were in the normal range in both patients with OSA and control subjects. Gislason et al53 detected 5-hydroxyindoleacetic acid (5-HIAA), HVA, and 3-methoxy-4-hydroxyphenyl glycol (MHPG) in the cerebrospinal fluid of 15 patients with OSA and 18 controls; however, levels of all of the aforementioned biomarkers were similar in patients with OSA and control subjects. Two studies evaluated the effects of continuous positive airway pressure (CPAP) treatment on urinary E and NE levels, which were confirmed in plasma. One of the studies found that withdrawing CPAP treatment led to no change in NE level.54 The other study detected no correlation among PSG parameters, but CPAP treatment reduced E levels 55
The inconsistency of catecholamine metabolites in patients with OSA may be due in part to use of different analytical platforms, various biological samples, small sample size, and different sample collection protocols. In addition, different study populations and unadjusted confounding factors would also result in inconsistencies.
Amino Acid Metabolites
Serum homocysteine (Hcy) levels in patients with OSA are significantly higher than those in controls,56 and CPAP effectively decreases Hcy levels in patients with OSA by almost 30%.57 Another study showed that plasma Hcy level is higher in patients with OSA, while asymmetric dimethylarginine (ADMA) not.58 OSA is not accompanied by increased circulating free nitrotyrosine either before or after sleep.59 This result suggests that chronic nocturnal IH does not increase systemic nitric oxide-mediated oxidative/nitrosative burden in patients with OSA.59
Oxidative Stress Metabolites
Urinary 15-F2t-isoprostane, one of the most sensitive and specific metabolites of lipid peroxidation, is positively correlated with carotid intima-media thickness and IH in nonobese patients with OSA,60 and both 15-F2t-isoprostane and the apnea-hypopnea index are independently associated with Hcy levels in patients with OSA and metabolic syndrome.56 Malondialdehyde (MDA) is another important oxidative stress biomarker that is significantly higher in patients with OSA than that in controls.61 However, 15-F2t-isoprostane is not a significant feature in children with OSA.62
Other Metabolites
Leukotriene E4 (U-LTE4) is a marker of proinflammatory cysteinyl leukotriene production, and increased urinary U-LTE4 has been demonstrated in patients with OSA.63 CPAP treatment reduces U-LTE4 concentration by 22% only in patients with OSA and a normal body mass index (BMI).63 Patients with mild OSA exhibit altered levels of specific retinoids, carotenoids, and tocopherols, which may be markers and/or mediators of increased susceptibility to vascular disease.64 Circulating carbon monoxide levels are enable assessment of the clinical severity of OSA.65
DISCUSSION
This systemic review pooled available studies that applied chromatography and/or MS-based methods to detect biomarkers in patients with OSA. Numerous proteins and metabolites, including lipid profiles, adrenergic/dopaminergic biomarkers and their derivatives, amino acids, and oxidative stress biomarkers in patients with OSA have been identified. However, the results were not always consistent and far from clinical application. These differences may have been caused by: the source, storage, handling, and preparation of the biological sample; interindividual differences due to the highly transient and sensitive nature of metabolic fluxes; the close interaction between the micro and macroenvironments, including diet and microflora; and lack of uniformity in the technology and data analysis across different analytical platforms. Further studies with larger populations grouped by different phenotypes for subclass analyses are necessary to validate these results. Additionally, there is lack of a clear understanding of the mechanisms of these biomarkers. More organized investigations in cell and animal models are necessary to determine the molecular mechanisms.
Many novel small metabolites and proteins are identified and quantified in biological samples from patients with specific human diseases, including OSA, with development of the bio-analytical approach. The most-used samples include bio-fluids, such as plasma, serum, saliva, and urine, as well as tissues. Two major high-throughput analytical spectroscopic approaches (MS and proton nuclear magnetic resonance spectroscopy [1NMR]) are commonly used and have become core technologies for metabolomics and proteomics to determine protein metabolic profiles. 1NMR spectroscopy-based methods provide high resolution but low sensitivity. To date, no study has used this analytical platform to detect biomarkers in biological samples from patients with OSA. MS-based methods have higher sensitivity and can simultaneously analyze a broader range of molecules.66,67 MS-based analyses focus on detecting ionized molecules and measuring their mass-to-charge ratio.68 The combination of MS and chromatography (GC or LC) is commonly used for metabolomics and proteomics. GC requires volatile and thermally stable analytics, and nonvolatile compounds must be derivatized before a GC–MS analysis.69 Public libraries have enabled reliable identification of compounds from GC–MS data. LC–MS-based methods are more universal techniques and enable separation of different types of compounds with the appropriate column and solvents.69,70 Thus, each platform has its own strengths and limitations in terms of assessing samples and identifying compounds. A combination of different analytical platforms would be an ideal approach to OSA biomarker research.
The characteristics of OSA include chronic IH and sleep fragmentation, both of which can activate the sympathetic nervous system, followed by oxidative stress/systemic inflammation. Levels of various biomarkers fluctuate during this progression. Proteic biomarkers: in pediatric OSA, gelsolin, perlecan, albumin, immunoglobulin, uromodulin, urocortin-3, orosomucoid-1 and kallikrein alter in urine.30,32 In rats exposed to IH, reduced kallikrein may play an important role in preservation of renal function.71 Becker et al map identified urinary proteins to specific functional pathways (ie, acute-phase proteins, angiogenesis, hemostasis, leukocyte immunity, and lipid binding).33 In adult OSA, the identified proteins in serum are primarily associated with derangements in lipid and vascular metabolic pathways.34 In obese OSA, some of the identified peptides in urine comprise collagen alpha chain subtypes and fibrinogen; these peptides seem to be associated with increased metabolic syndrome.35 In OSA with CVD, the salivary AHSG decreases in CVD group.36 AHSG plays an important role protecting against vascular calcification, as vascular calcification is a strong predictor of CVD.72,73 Thus, we speculate that proteic biomarkers in pediatric OSA may be associated with both kidney dysfunction (ie, altered glomerulotubular permeability) and disease-related pathological processes, while the proteic biomarkers in adult OSA seem to be associated with cardiovascular and metabolic alterations. Two proteomic studies use immunoblotting/ELISA or Western blot method to further verified the identified peptides, the results are consistent with proteomic approaches.32,36 Only 1 proteomic study explores differential serum protein expression and OSA severity in adults.34 The underexpressed complement component 4-binding alpha and thrombospondin and overexpressed fibronectin, apolipoprotein B (ApoB)-100 and apolipoprotein D alter with OSA severity.34Lipidic biomarkers: Compared with recurrent tonsillitis, hyperplastic tonsillar tissue in pediatric OSA contains more proportions of monounsaturated fatty acids and fewer proportions of saturated fatty acids.46 In adult OSA, significant positive correlations are found between sleep parameters and lipid (ie, myristic, palmitic, stearic, saturated fatty, oleic, and polyunsaturated fatty acids) metabolism in buttock subcutaneous tissue.38 Plasma PC, LPE, PE, LPA, PS, AEA, 1/2-AG, and OEA are higher in adult OSA.29,45 Salivary PC is also higher in patients with OSA.44 Altered state of lipid metabolism in tonsillar tissue in pediatric OSA may be linked to glucose metabolism and membrane permeability.46 Thus the lipid metabolism disorder might be correlated with pediatric OSA occurrence. In adult OSA, the lipid metabolism disorder is simply a sign of metabolic disorders in general condition. Catecholamine metabolites and derivatives: In pediatric OSA, increased sympathetic tone can increasingly release of catecholamines (eg, E, NE, and DA).51 And, the E, NE, and DA are all related to the severity of OSA in children. In earlier studies, catecholamines and dopamine metabolites are similar between adult OSA and controls. That may partly be explained by small enrolled subjects and strict case selection. In later studies, similar to results in children, E, NE, and DA in adult OSA are all higher than those in controls. Thus, catecholamines seem to be ideal biomarkers reflecting sympathetic overactivity.74Amino acid metabolites: Hcy level increased in adult OSA,56,58 and CPAP treatment could effectively lower serum Hcy.57 However, no significant change of nitrotyrosine and ADMA was witnessed in OSA. These might indicate that reactive oxygen species, not reactive nitrogen species is the most important free radicals in adult OSA. Recent meta-analyses also reveal the similar results of Hcy level in OSA and effect of CPAP therapy despite various methods employed in included studies.75,76 Significantly increased Hcy level is observed in the severe OSA group, thus the Hcy could be recognized as a representative biomarker in severe OSA. Markers of oxidative stress: In pediatric OSA, Montgomery-Downs et al find the 15-F2t-isoprostane is not elevated in children with OSA.62 The authors ascribe this phenomenon to intrinsic antioxidant capacity and small sample size in this study. However, the metabolic intermediates of enhanced oxidative stress (eg, 15-F2t-isoprostane and MDA) are elevated in adult OSA.56,60,61 The 15-F2t-isoprostane elevates with OSA severity in adults and could add the notion that oxidative stress may contribute to OSA-related cardiovascular complications.60 The MDA, which measures lipid peroxidation, is also verified to be elevated in OSA by numerous methods.77 For quantifying isoprostane, the ELISA method has often been used. However, due to cross-reactivity interference, it gives only an approximate estimation.60 Thus, the ELISA overestimates by twofold of the isoprostane level when compared with GC/MS.78
Interestingly, gut microbiota participate in regulating many metabolic pathways, and the comprehensive interaction between the host and microbiota and metabolism has been hotly debated.79,80 One study found that IH alters the composition and diversity of fecal microbiota in a murine model.81 Levels of Prevotella, Paraprevotella, Desulfovibrio, and Lachnospiraceae increase, whereas those of Bacteroides, Odoribacter, Turicibacter, Peptococcaceae, and Erysipelotrichaceae decrease.81 Evidence from another murine model initially demonstrated that sleep fragmentation also has a significant impact on microbial community structure in mice.82 Lipopolysaccharide-binding protein (LBP) is a biomarker of potential low-cascade endotoxemia, formed in response to microbial translocation; LBP is elevated in children with OSA, including those who are not obese.83 All of these studies directly or indirectly reported that OSA influenced the gut microbiome composition. Interestingly, several biomarkers discussed in this systemic review associated with an altered gut microbiome are noteworthy. An aromatic amino acid (ie, tryptophan and tyrosine) metabolic disorder is always persistent in intestinal dysbacteriosis, so the metabolites of tryptophan (5-HIAA and HVA) and tyrosine (DA and NE) are altered due to the imbalance in the intestinal flora. Endocannabinoid system activity is involved in the control of glucose and energy metabolism, and endocannabinoids (ie, AEA, 1/2-AG, and OEA) can be up- or down-regulated by specific gut microbes, such as Akkermansia muciniphila.84,85 Thus, we infer that alterations in particular gut microflora species in patients with OSA affect downstream metabolites. Alterations in these metabolites could cause low-grade systemic inflammation, resulting in insulin resistance and increased CVD risk. Integrating metabolomics and metagenomics could facilitate development of potential preventive and therapeutic interventions for OSA-related complications.
We must recognize some inevitable limitations in our systemic review. Eligible studies that were not indexed or published, and so were not included, may have resulted in publication bias. The marked heterogeneity in the included studies was due to relatively small sample sizes, use of different techniques, and different biological samples. Thus, a meta-analysis of the results of these studies was not feasible. Additionally, not all of the studies controlled for confounding factors, such as age and BMI. Last, few metabolomic studies have been performed to identify diagnostic biomarkers in adult patients with OSA, although some studies speculated that a particular protein might be a predictor of pediatric OSA. Additionally, the results of these proteomics studies are preliminary and should be validated in larger independent cohorts. Despite these limitations, advances in quantitative proteomics and metabolomics will enable more in-depth analyses of cellular systems. There are a number of advantages of quantitative proteins and metabolites compared with traditional chemometric approaches, including improved statistical robustness, simpler biological interpretation, and detailed information on specific protein and metabolite identities; hence, they show great potential for clinical application.
In summary, many novel OSA-related biomarkers have been identified by chromatography and/or MS-based methods. In addition, applying the proteomics and metabolomics approach helps us better understand OSA pathophysiology, and will enable early detection and development of personalized treatment strategies. However, well-matched case–control proteomic and metabolomic studies in community-based populations are warranted to confirm these findings. Then, proteins and metabolites identified using these translational and personalized approaches will be considered biomarkers for OSA screening.
Footnotes
Abbreviations: 1NMR = proton nuclear magnetic resonance spectroscopy, 2D-DIGE-MS = 2-dimensional polyacrylamide gel electrophoresis mass spectrometry, 5-HIAA = 5-hydroxyindoleacetic acid, AA = arachidonic acid, ADMA = asymmetric dimethylarginine, AEA = anandamide, AG = arachidonoylglycerols, AHSG = alpha-2-HS-glycoprotein, ApoB = apolipoprotein B, CO = carbon monoxide, CPAP = continuous positive airway pressure, CVD = cardiovascular disease, DA = dopamine, DOPAC = 34-dihydroxyphenylacetic acid, Eepinephrine, ELISA = enzyme-linked immunosorbent assay, FGA = fibrinogen alpha chain, GC = gas chromatography, GC/MS = gas chromatography–mass spectrometry, GLC = gas–liquid chromatography, Hcy = homocysteine, HPLC = high-pressure or high-performance liquid chromatography, HVA = homovanillic acid, HVA = homovanillic acid, IH = intermittent hypoxia, LBP = lipopolysaccharide-binding protein, LC = liquid chromatography, LC/MS = liquid chromatography–mass spectrometry, LC-ESI-MS/MS = liquid chromatography coupled with electrospray source ionization–tandem mass spectrometry, LC-MS/MS = liquid chromatography–tandem mass spectrometry, LC-QTOF-MS = liquid chromatography–hybrid quadrupole time-of-flight mass spectrometry, LPA = lysophosphatidic acids, LPC = lysophosphatidylcholines, LPE = lysophosphatidylethanolamine, MALDI-TOF-MS = matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, MDA = malondialdehyde, MHPG = 3-methoxy-4-hydroxyphenyl glycol, MN = metanephrine, MS = mass spectrometry, NE = norepinephrine, NMN = normetanephrine, OEA = oleoyl ethanolamide, OSA = obstructive sleep apnea, PC = phosphatidylcholine, PE = phosphoethanolamine, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PS = phosphoserine, PSG = polysomnography, T2DM = type 2 diabetes, TUBA4A = tubulin alpha-4A chain, U-LTE(4) = leukotriene E(4).
HX and XZ have contributed equally to this article.
Authors’ contributions: YSK and XHJ provided the conceptual design of the project, writing and editing final version of the manuscript. JW and ZXJ participated in writing and editing final version of the manuscript. All listed authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 117:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strollo PJ, Jr, Rogers RM. Obstructive sleep apnea. N Engl J Med 1996; 334:99–104. [DOI] [PubMed] [Google Scholar]
- 4.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 2008; 5:207–217. [DOI] [PubMed] [Google Scholar]
- 5.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 6.Dyken ME, Somers VK, Yamada T, et al. Investigating the relationship between stroke and obstructive sleep apnea. Stroke 1996; 27:401–407. [DOI] [PubMed] [Google Scholar]
- 7.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290:1906–1914. [DOI] [PubMed] [Google Scholar]
- 8.Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 2014; 190:218–225. [DOI] [PubMed] [Google Scholar]
- 9.Christensen AS, Clark A, Salo P, et al. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep 2013; 36:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilat H, Vinker S, Buda I, et al. Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine 2014; 93:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie W, Zheng F, Song X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: a PRISMA-compliant systematic review and meta-analysis. Medicine 2014; 93:e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montesi SB, Bajwa EK, Malhotra A. Biomarkers of sleep apnea. Chest 2012; 142:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnardottir ES, Mackiewicz M, Gislason T, et al. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 2009; 32:447–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan HL, Kheirandish-Gozal L, Gozal D. The promise of translational and personalised approaches for paediatric obstructive sleep apnoea: an “Omics” perspective. Thorax 2014; 69:474–480. [DOI] [PubMed] [Google Scholar]
- 17.Zhao YY. Metabolomics in chronic kidney disease. Clin Chim Acta 2013; 422:59–69. [DOI] [PubMed] [Google Scholar]
- 18.Konvalinka A, Scholey JW, Diamandis EP. Searching for new biomarkers of renal diseases through proteomics. Clin Chem 2012; 58:353–365. [DOI] [PubMed] [Google Scholar]
- 19.Murri M, Insenser M, Escobar-Morreale HF. Metabolomics in polycystic ovary syndrome. Clin Chim Acta 2014; 429:181–188. [DOI] [PubMed] [Google Scholar]
- 20.Insenser M, Escobar-Morreale HF. Proteomics and polycystic ovary syndrome. Expert Rev Proteomics 2013; 10:435–447. [DOI] [PubMed] [Google Scholar]
- 21.Abbassi-Ghadi N, Kumar S, Huang J, et al. Metabolomic profiling of oesophago-gastric cancer: a systematic review. Eur J Cancer 2013; 49:3625–3637. [DOI] [PubMed] [Google Scholar]
- 22.Shukla HD, Vaitiekunas P, Cotter RJ. Advances in membrane proteomics and cancer biomarker discovery: current status and future perspective. Proteomics 2012; 12:3085–3104. [DOI] [PubMed] [Google Scholar]
- 23.Zhang AH, Qiu S, Xu HY, et al. Metabolomics in diabetes. Clin Chim Acta 2014; 429:106–110. [DOI] [PubMed] [Google Scholar]
- 24.Sundsten T, Ortsäter H. Proteomics in diabetes research. Mol Cell Endocrinol 2009; 297:93–103. [DOI] [PubMed] [Google Scholar]
- 25.Snowden S, Dahlén SE, Wheelock CE. Application of metabolomics approaches to the study of respiratory diseases. Bioanalysis 2012; 4:2265–2290. [DOI] [PubMed] [Google Scholar]
- 26.O’Neil SE, Lundbäck B, Lötvall J. Proteomics in asthma and COPD phenotypes and endotypes for biomarker discovery and improved understanding of disease entities. J Proteomics 2011; 75:192–201. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins MR, Pasquali C, Appel RD, et al. From proteins to proteomes: large scale protein identification by two-dimensional electrophoresis and amino acid analysis. Biotechnology (N Y) 1996; 14:61–65. [DOI] [PubMed] [Google Scholar]
- 28.Xie G, Li X, Li H, et al. Toward personalized nutrition: comprehensive phytoprofiling and metabotyping. J Proteome Res 2013; 12:1547–1559. [DOI] [PubMed] [Google Scholar]
- 29.Ferrarini A, Rupérez FJ, Erazo M, et al. Fingerprinting-based metabolomic approach with LC-MS to sleep apnea and hypopnea syndrome: a pilot study. Electrophoresis 2013; 34:2873–2881. [DOI] [PubMed] [Google Scholar]
- 30.Krishna J, Shah ZA, Merchant M, et al. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med 2006; 7:221–227. [DOI] [PubMed] [Google Scholar]
- 31.Shah ZA, Jortani SA, Tauman R, et al. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatr Res 2006; 59:466–470. [DOI] [PubMed] [Google Scholar]
- 32.Gozal D, Jortani S, Snow AB, et al. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. Am J Respir Crit Care Med 2009; 180:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker L, Kheirandish-Gozal L, Peris E, et al. Contextualised urinary biomarker analysis facilitates diagnosis of paediatric obstructive sleep apnoea. Sleep Med 2014; 15:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jurado-Gamez B, Gomez-Chaparro JL, Muñoz-Calero M, et al. Serum proteomic changes in adults with obstructive sleep apnoea. J Sleep Res 2012; 21:139–146. [DOI] [PubMed] [Google Scholar]
- 35.Seetho IW, Siwy J, Albalat A, et al. Urinary proteomics in obstructive sleep apnoea and obesity. Eur J Clin Invest 2014; 44:1104–1115. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Li R, Zhang J, et al. Salivary biomarkers indicate obstructive sleep apnea patients with cardiovascular diseases. Sci Rep 2014; 4:7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papandreou C. Gluteal adipose tissue fatty acids and sleep quality parameters in obese adults with OSAS. Sleep Breath 2013; 17:1315–1317. [DOI] [PubMed] [Google Scholar]
- 39.Papandreou C, Schiza SE, Tsibinos G, et al. Gluteal adipose-tissue polyunsaturated fatty-acids profiles and depressive symptoms in obese adults with obstructive sleep apnea hypopnea syndrome: a cross-sectional study. Pharmacol Biochem Behav 2011; 98:316–319. [DOI] [PubMed] [Google Scholar]
- 40.Tang S, Machaalani R, Kashem MA, et al. Intermittent hypercapnic hypoxia induced protein changes in the piglet hippocampus identified by MALDI-TOF-MS. Neurochem Res 2009; 34:2215–2225. [DOI] [PubMed] [Google Scholar]
- 41.Klein JB, Gozal D, Pierce WM, et al. Proteomic identification of a novel protein regulated in CA1 and CA3 hippocampal regions during intermittent hypoxia. Respir Physiol Neurobiol 2003; 136:91–103. [DOI] [PubMed] [Google Scholar]
- 42.Thongboonkerd V, Gozal E, Sachleben LR, Jr, et al. Proteomic analysis reveals alterations in the renal kallikrein pathway during hypoxia-induced hypertension. J Biol Chem 2002; 277:34708–34716. [DOI] [PubMed] [Google Scholar]
- 43.Snow A, Gozal D, Valdes R, et al. Urinary proteins for the diagnosis of obstructive sleep apnea syndrome. Methods Mol Biol 2010; 641:223–241. [DOI] [PubMed] [Google Scholar]
- 44.Kawai M, Kirkness JP, Yamamura S, et al. Increased phosphatidylcholine concentration in saliva reduces surface tension and improves airway patency in obstructive sleep apnoea. J Oral Rehabil 2013; 40:758–766. [DOI] [PubMed] [Google Scholar]
- 45.Engeli S, Blüher M, Jumpertz R, et al. Circulating anandamide and blood pressure in patients with obstructive sleep apnea. J Hypertens 2012; 30:2345–2351. [DOI] [PubMed] [Google Scholar]
- 46.Ezzedini R, Darabi M, Ghasemi B, et al. Tissue fatty acid composition in obstructive sleep apnea and recurrent tonsillitis. Int J Pediatr Otorhinolaryngol 2013; 77:1008–1012. [DOI] [PubMed] [Google Scholar]
- 47.Papandreou C. Independent associations between fatty acids and sleep quality among obese patients with obstructive sleep apnoea syndrome. J Sleep Res 2013; 22:569–572. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher EC, Miller J, Schaaf JW, et al. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep 1987; 10:35–44. [DOI] [PubMed] [Google Scholar]
- 49.Findley LJ, Boykin M, Fallon T, et al. Plasma adenosine and hypoxemia in patients with sleep apnea. J Appl Physiol 1988; 64:556–561. [DOI] [PubMed] [Google Scholar]
- 50.Paci A, Marrone O, Lenzi S, et al. Endogenous digitalis like factors in obstructive sleep apnea. Hypertens Res 2000; 23 (Suppl):S87–S91. [DOI] [PubMed] [Google Scholar]
- 51.O’Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med 2011; 12:483–488. [DOI] [PubMed] [Google Scholar]
- 52.Paik MJ, Kim DK, Nguyen DT, et al. Correlation of daytime sleepiness with urine metabolites in patients with obstructive sleep apnea. Sleep Breath 2014; 18:517–523. [DOI] [PubMed] [Google Scholar]
- 53.Gislason T, Hedner J, Terenius L, et al. Substance P, thyrotropin-releasing hormone, and monoamine metabolites in cerebrospinal fluid in sleep apnea patients. Am Rev Respir Dis 1992; 146:784–786. [DOI] [PubMed] [Google Scholar]
- 54.Grunstein RR, Stewart DA, Lloyd H, et al. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep 1996; 19:774–782. [DOI] [PubMed] [Google Scholar]
- 55.Minemura H, Akashiba T, Yamamoto H, et al. Acute effects of nasal continuous positive airway pressure on 24-hour blood pressure and catecholamines in patients with obstructive sleep apnea. Intern Med 1998; 37:1009–1013. [DOI] [PubMed] [Google Scholar]
- 56.Monneret D, Tamisier R, Ducros V, et al. The impact of obstructive sleep apnea on homocysteine and carotid remodeling in metabolic syndrome. Respir Physiol Neurobiol 2012; 180:298–304. [DOI] [PubMed] [Google Scholar]
- 57.Jordan W, Berger C, Cohrs S, et al. CPAP-therapy effectively lowers serum homocysteine in obstructive sleep apnea syndrome. J Neural Transm 2004; 111:683–689. [DOI] [PubMed] [Google Scholar]
- 58.Ozkan Y, Firat H, Simşek B, et al. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath 2008; 12:149–154. [DOI] [PubMed] [Google Scholar]
- 59.Svatikova A, Wolk R, Wang HH, et al. Circulating free nitrotyrosine in obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol 2004; 287:R284–R287. [DOI] [PubMed] [Google Scholar]
- 60.Monneret D, Pepin JL, Godin-Ribuot D, et al. Association of urinary 15-F2t-isoprostane level with oxygen desaturation and carotid intima-media thickness in nonobese sleep apnea patients. Free Radic Biol Med 2010; 48:619–625. [DOI] [PubMed] [Google Scholar]
- 61.Dikmenoğlu N, Ciftçi B, Ileri E, et al. Erythrocyte deformability, plasma viscosity and oxidative status in patients with severe obstructive sleep apnea syndrome. Sleep Med 2006; 7:255–261. [DOI] [PubMed] [Google Scholar]
- 62.Montgomery-Downs HE, Krishna J, Roberts LJ, et al. Urinary F2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep Breath 2006; 10:211–215. [DOI] [PubMed] [Google Scholar]
- 63.Stanke-Labesque F, Bäck M, Lefebvre B, et al. Increased urinary leukotriene E4 excretion in obstructive sleep apnea: effects of obesity and hypoxia. J Allergy Clin Immunol 2009; 124:364–370.370.e361–362. [DOI] [PubMed] [Google Scholar]
- 64.Day RM, Matus IA, Suzuki YJ, et al. Plasma levels of retinoids, carotenoids and tocopherols in patients with mild obstructive sleep apnoea. Respirology 2009; 14:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi M, Miyazawa N, Takeno M, et al. Circulating carbon monoxide level is elevated after sleep in patients with obstructive sleep apnea. Chest 2008; 134:904–910. [DOI] [PubMed] [Google Scholar]
- 66.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev 2007; 26:51–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shushan B. A review of clinical diagnostic applications of liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev 2010; 29:930–944. [DOI] [PubMed] [Google Scholar]
- 68.Rankin NJ, Preiss D, Welsh P, et al. The emergence of proton nuclear magnetic resonance metabolomics in the cardiovascular arena as viewed from a clinical perspective. Atherosclerosis 2014; 237:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Putri SP, Yamamoto S, Tsugawa H, et al. Current metabolomics: technological advances. J Biosci Bioeng 2013; 116:9–16. [DOI] [PubMed] [Google Scholar]
- 70.Yin P, Xu G. Current state-of-the-art of nontargeted metabolomics based on liquid chromatography-mass spectrometry with special emphasis in clinical applications. J Chromatogr A 2014; 1374:1–13. [DOI] [PubMed] [Google Scholar]
- 71.Chen CF, Chen LW, Chien CT, et al. Renal kallikrein in chronic hypoxic rats. Clin Exp Pharmacol Physiol 1996; 23:819–824. [DOI] [PubMed] [Google Scholar]
- 72.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 2004; 15:2959–2964. [DOI] [PubMed] [Google Scholar]
- 73.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003; 112:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jullian-Desayes I, Joyeux-Faure M, Tamisier R, et al. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med Rev 2015; 21:23–38. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Niu X, Xiao Y, et al. Effect of continuous positive airway pressure on homocysteine levels in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath 2014; 18:687–694. [DOI] [PubMed] [Google Scholar]
- 76.Niu X, Chen X, Xiao Y, et al. The differences in homocysteine level between obstructive sleep apnea patients and controls: a meta-analysis. PLoS ONE 2014; 9:e95794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Archontogeorgis K, Nena E, Papanas N, et al. Biomarkers to improve diagnosis and monitoring of obstructive sleep apnea syndrome: current status and future perspectives. Pulm Med 2014; 2014:930535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J Lipid Res 2007; 48:1607–1617. [DOI] [PubMed] [Google Scholar]
- 79.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science 2012; 336:1262–1267. [DOI] [PubMed] [Google Scholar]
- 80.Xie G, Zhang S, Zheng X, et al. Metabolomics approaches for characterizing metabolic interactions between host and its commensal microbes. Electrophoresis 2013; 34:2787–2798. [DOI] [PubMed] [Google Scholar]
- 81.Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 82.Carreras A, Leone V, Peris E, et al. Sleep fragmentation alters gut microbiota: potential association with metabolic alterations in mice. Am J Respir Crit Care Med 2014; 189:2414. [Google Scholar]
- 83.Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab 2014; 99:656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cani PD, Geurts L, Matamoros S, et al. Glucose metabolism: focus on gut microbiota, the endocannabinoid system and beyond. Diabetes Metab 2014; 40:246–257. [DOI] [PubMed] [Google Scholar]
- 85.Cani PD. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clin Microbiol Infect 2012; 18 (Suppl 4):50–53. [DOI] [PubMed] [Google Scholar]


