Supplemental Digital Content is available in the text
Abstract
Currently 2 difference classes of cyclooxygenase (COX)-2 inhibitors, coxibs and relatively selective COX-2 inhibitors, are available for patients requiring nonsteroidal anti-inflammatory drug (NSAID) therapy; their gastroprotective effect is hardly directly compared.
The aim of this study was to compare the gastroprotective effect of relatively selective COX-2 inhibitors with coxibs.
MEDLINE, EMBASE, and the Cochrane Library (from their inception to March 2015) were searched for potential eligible studies.
We included randomized controlled trials comparing coxibs (celecoxib, etoricoxib, parecoxib, and lumiracoxib), relatively selective COX-2 inhibitors (nabumetone, meloxicam, and etodolac), and nonselective NSAIDs with a study duration ≥4 weeks.
Comparative effectiveness and safety data were pooled by Bayesian network meta-analysis. The primary outcomes were ulcer complications and symptomatic ulcer. Summary effect-size was calculated as risk ratio (RR), together with the 95% confidence interval (CI).
This study included 36 trials with a total of 112,351 participants. Network meta-analyses indicated no significant difference between relatively selective COX-2 inhibitors and coxibs regarding ulcer complications (RR, 1.38; 95% CI, 0.47–3.27), symptomatic ulcer (RR, 1.02; 95% CI, 0.09–3.92), and endoscopic ulcer (RR, 1.18; 95% CI, 0.37–2.96). Network meta-analyses adjusting potential influential factors (age, sex, previous ulcer disease, and follow-up time), and sensitivity analyses did not reveal any major change to the main results. Network meta-analyses suggested that relatively selective COX-2 inhibitors and coxibs were associated with comparable incidences of total adverse events (AEs) (RR, 1.09; 95% CI, 0.93–1.31), gastrointestinal AEs (RR, 1.04; 95% CI, 0.87–1.25), total withdrawals (RR, 1.00; 95% CI, 0.74–1.33), and gastrointestinal AE-related withdrawals (RR, 1.02; 95% CI, 0.57–1.74).
Relatively selective COX-2 inhibitors appear to be associated with similar gastroprotective effect and tolerability as coxibs. Owing to the indirectness of the comparisons, future research is required to confirm the study conclusion.
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most highly prescribed drugs, commonly used for musculoskeletal conditions such as rheumatoid arthritis and osteoarthritis. However, the use of NSAIDs is often limited by the gastrointestinal toxicity.1,2 It has been reported that NSAID-induced gastrointestinal complications such as ulcer bleeding, perforation, and obstruction may occur in approximately 2% to 4% of NSAID users.3,4 Worse still, NSAIDs lead to considerable mortality worldwide. In the United States5,6 and the United Kingdom,7 NSAIDs are thought to cause at least 7000 and 1000 deaths every year, respectively.
It has been recognized that both the efficacy and toxicity of NSAIDs result from their inhibition of cyclooxygenase (COX), which primarily has 2 structurally and functionally distinct isoforms, COX-1 and COX-2.8,9 COX-1 is the constitutive isoform expressed throughout the body and plays an important role in gastrointestinal protection and platelet aggregation.8,9 While COX-2 is an inducible COX that is involved in the inflammatory response.9,10 The discovery of COX-2 has led to the important development of therapeutic COX-2 inhibitors. Strong evidence indicates that COX-2 inhibitors are associated with significantly lower incidence of gastrointestinal adverse effects than nonselective NSAIDs.11,12
Currently there are 2 classes of COX-2 inhibitors, including coxibs and relatively selective COX-2 inhibitors, available for prescription.9,11 Coxibs, including celecoxib, etoricoxib, parecoxib, and lumiracoxib, are a relatively new class of NSAIDs and their gastrointestinal safety has been systematically evaluated.10,11 Clinical guidelines now recommend coxibs for patients with high gastrointestinal and low cardiovascular risk.13 However, coxibs are much more expensive than conventional NSAIDs.9,14,15 In contrast, relatively selective COX-2 inhibitors, including nabumetone, meloxicam, and etodolac, are a group of traditional NSAIDs that were retrospectively found to have COX-2 selectivity.9,11 They are structurally dissimilar with coxibs and cheaper, but their selective COX-2 properties have not been rigorously evaluated. So far a large number of clinical trials have been performed to evaluate the gastroprotective effectiveness of coxibs and relatively selective COX-2 inhibitors; however, these studies often took nonselective NSAIDs as control and there are hardly any trials directly compared the 2 different classes of COX-2 inhibitors. Network meta-analysis, in the context of a systematic review, is a meta-analysis in which multiple treatments are compared using both direct comparisons of interventions within trials and indirect comparisons across trials based on a common comparator.16,17 In this study, we carried out a network meta-analysis to indirectly compare the gastroprotective effect of relatively selective COX-2 inhibitors with coxibs
METHODS
This study was carried out according to the Cochrane handbook for systematic reviews of interventions,18 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19 Because this is a secondary literature based study, ethic approval is not necessary.
Literature Search
We searched the Cochrane Library, MEDLINE, and EMBASE from their inception to March 2015. The search strategy included the following combined texts and MeSH terms: “Nonsteroidal anti-inflammatory drugs,” “coxibs,” “COX-2 inhibitors,” “celecoxib,” “etoricoxib,” “parecoxib,” “lumiracoxib,” “nabumetone,” “meloxicam,” “etodolac,” “peptic ulcer,” “bleeding,” “perforation,” “obstruction,” “randomized controlled trial,” and “clinical trial.” All searches were restricted to human studies and there was no limitation on publication language. We manually searched reference lists of the included studies and related review articles to identify additional trials.
Study Selection
We included randomized controlled trials (RCTs) comparing coxibs (celecoxib, etoricoxib, parecoxib, and lumiracoxib), relatively selective COX-2 inhibitors (nabumetone, meloxicam, and etodolac), and nonselective NSAIDs in patients with chronic musculoskeletal conditions or health people. The classification of NSAIDs in this study is as same as previous reports.9,20 Some selective COX-2 inhibitors which have been withdrawn from the market, like rofecoxib, valdecoxib, and nimesulide, were not included. Eligible studies should report at least 1 outcome for this systematic review and the follow-up time should be equal or longer than 4 weeks.
Two investigators independently selected the potentially eligible studies and extracted the data, disagreement was resolved by discussion. Duplicate citations were removed by reference management software, and the remaining records were evaluated by examining the titles, abstracts, and full articles sequentially. We only included the publication with the most relevant data if 2 or more papers were published for a same trial.
Data Extraction and Quality Assessment
Data from eligible studies were extracted using a standard form for this study. The data extracted from eligible studies included study information, patient characteristics, intervention, outcomes, and study methods. We consulted the authors of original studies to collect missing information as necessary. The methodological quality of the included studies was evaluated using the Jadad scale.21 The Jadad scale provides an overall evaluation of the methodological quality by assessing the risk of bias in randomization, blinding, and withdrawals/dropouts. Data extraction and quality assessment were independently carried out by 2 authors, and disagreements were resolved by discussion with a third author.
Outcome
The primary outcomes for this systematic review included ulcer complications (bleeding, perforation, and obstruction) and symptomatic ulcers. Secondary outcomes included endoscopic ulcers, gastrointestinal adverse events (AEs), total AEs, total withdrawals, and withdrawals due to gastrointestinal AEs.
Statistical Analysis
In order to account for the expected clinical and methodological heterogeneity, we used Bayesian random-effects models to evaluate the effect between coxibs and relatively selective COX-2 inhibitors. Summary effect-size was presented as risk ratio (RR) together with the 95% confidence intervals (CIs). Comparisons between coxibs and relatively selective COX-2 inhibitors were indirectly calculated through nonselective NSAIDs which is a common reference for both of the 2 different COX-2 inhibitors in original trials.
Like traditional meta-analysis, network meta-analysis also holds the assumption of heterogeneity across studies in the available direct comparisons. We tested the heterogeneity by Q-statistic and I2-index statistic.18 In addition, network meta-analyses require that a valid indirect comparison of 2 treatments by way of 2 direct comparisons with common reference must include trials that are sufficiently similar in important clinical and methodological characteristics.22,23 This assumption ensures that indirect comparisons from network meta-analysis are not influenced by potential effect modifiers. In our study, the assumption of similarity was tested using meta-regression analyses by adding potential effect modifiers as covariates to the network meta-analysis models.22,23 The potential effect modifiers assessed in our data analysis included average age, proportion of females, proportion of patients with previous ulcer disease, and length of follow-up. We would plan to evaluate other factors including Helicobacter pylori infection and ulcer risk, but such analyses were not performed due to insufficient data. Lastly, network meta-analyses assume that the direct and indirect estimated effects should be consistent when both are available in the network meta-analysis.24 Because direct comparison between coxibs and relatively selective COX-2 inhibitors were not available for all study outcomes, a test for assumption of consistency was not required in our study.
The primary results reported in our study were based on the network meta-analyses including all available trial data. In addition, we also reported the results from the network meta-analyses which adjusted other potential effect modifiers. Sensitivity analyses were performed for the primary outcomes by excluding small studies with <100 participants, excluding studies with poor mythological quality (Jadad <3), and excluding studies of diclofenac. Data analysis was carried out by RevMan version 5.3 and WinBUGS version 1.4.
RESULTS
Study Characteristics and Risk of Bias
Our search strategy identified 503 citations from electronic databases and 62 citations from other resources. We excluded 497 citations after excluding duplicates and screening titles/abstracts. The full texts of the remaining 68 records were screened and 36 trials including 112,351 participants from 35 publications were finally included3,25–58 (there are 2 trials reported in a same paper,28 see the flowchart of study selection in Figure 1).
FIGURE 1.
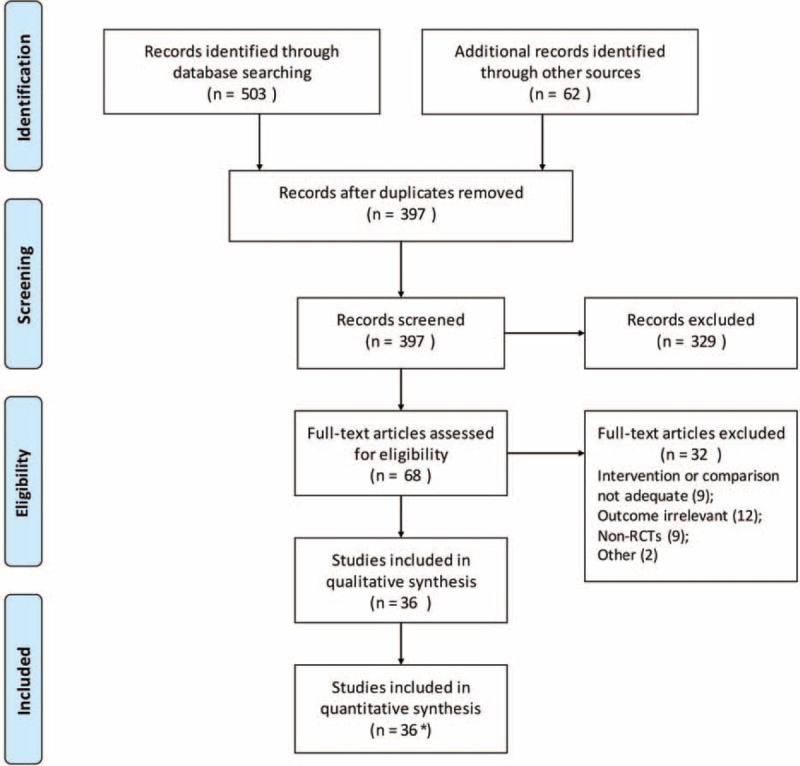
Flowchart of study selection. ∗One publication reported 2 trials in the same paper.
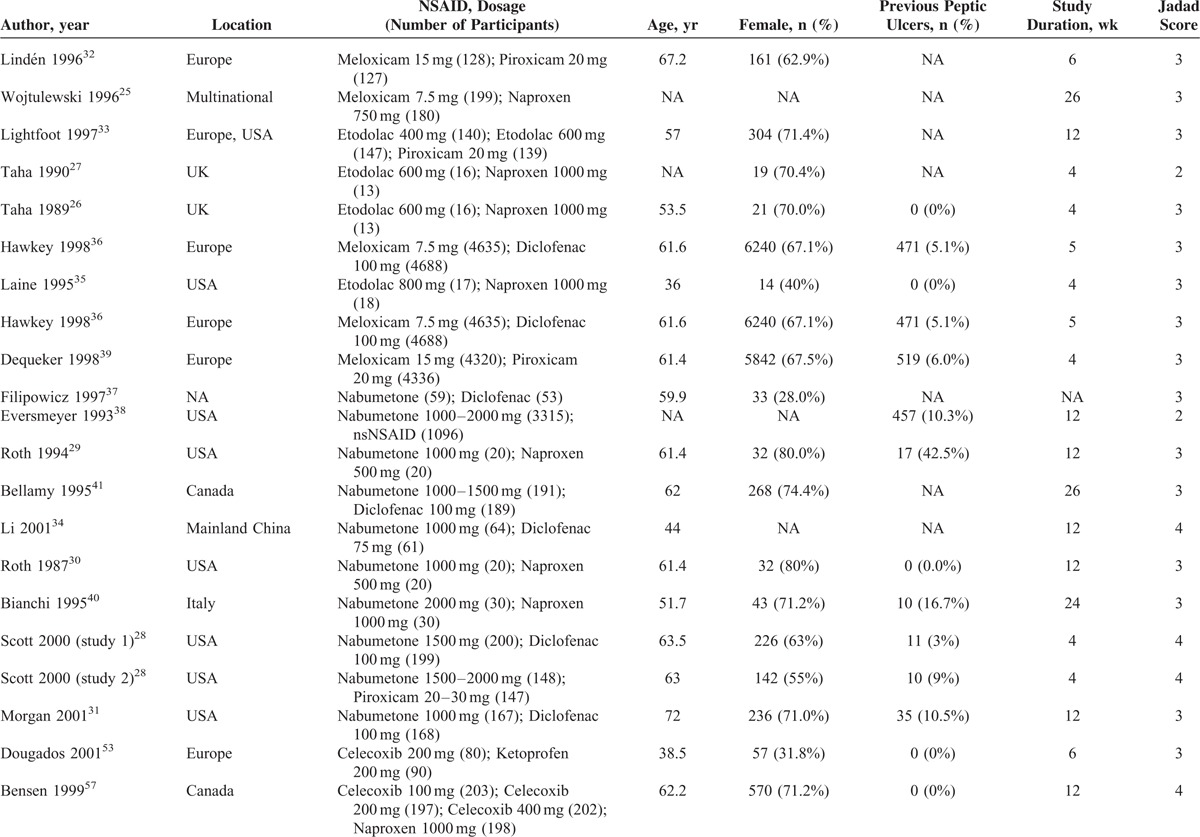
Table 1 presents the characteristics and risk of bias of included studies. Of 36 included RCTs, 18 trials25–41 compared relatively selective COX-2 inhibitors with nonselective NSAIDs, 18 trials3,42–58 compared coxibs with nonselective NSAIDs. We did not find any study directly compared coxibs with relatively selective COX-2 inhibitors. Thirty-five included trials were performed in patients with chronic musculoskeletal conditions and 135 included healthy people. The mean age of studies ranged from 36 to 72 years (median: 61.4 years). The study duration ranged from 4 to 156 weeks (median: 12 weeks). The mythological quality of most included trials was assessed as moderate or high. Eighteen studies had a Jadad scale >4 points, 16 studies had 3 points, and 3 studies had 2 points.
TABLE 1.
Characteristics of Included Studies
Ulcer Complications
Twenty studies including 59,717 participants and 145 events contributed to the analyses of ulcer complications. Pairwise meta-analyses comparing relatively selective COX-2 inhibitors with nonselective NSAIDs (10 trials; P = 0.46, I2 = 0%), and comparing coxibs with nonselective NSAIDs (10 trials; P = 0.48, I2 = 0%) showed no significant heterogeneity across included studies. Network meta-regression analyses testing the assumption of similarity did not reveal any prespecified factors that significantly modified the estimated effects (Supplemental digital content—Table S1, http://links.lww.com/MD/A428).
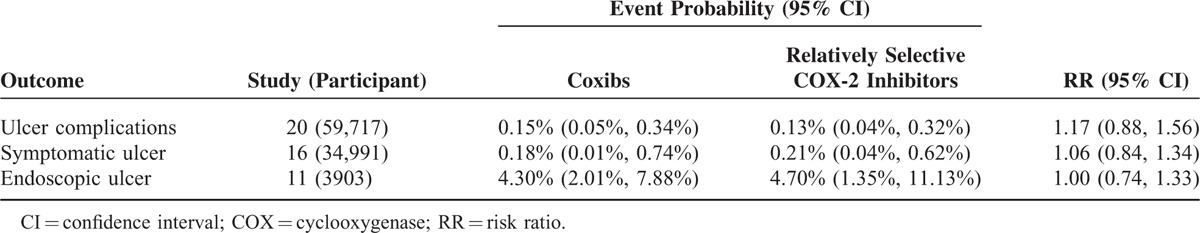
Network meta-analysis indicated that the event probability was 0.13% (95% CI, 0.04–0.32) for relatively selective COX-2 inhibitors and 0.15% (95% CI, 0.05–0.34) for coxibs. There was no significant difference between relatively selective COX-2 inhibitors and coxibs (RR, 1.38; 95% CI, 0.47–3.27) (Table 2). Network meta-analysis adjusting for the prespecified factors showed very similar RRs as the primary estimated effect (Table 3).
TABLE 1 (Continued).
Characteristics of Included Studies
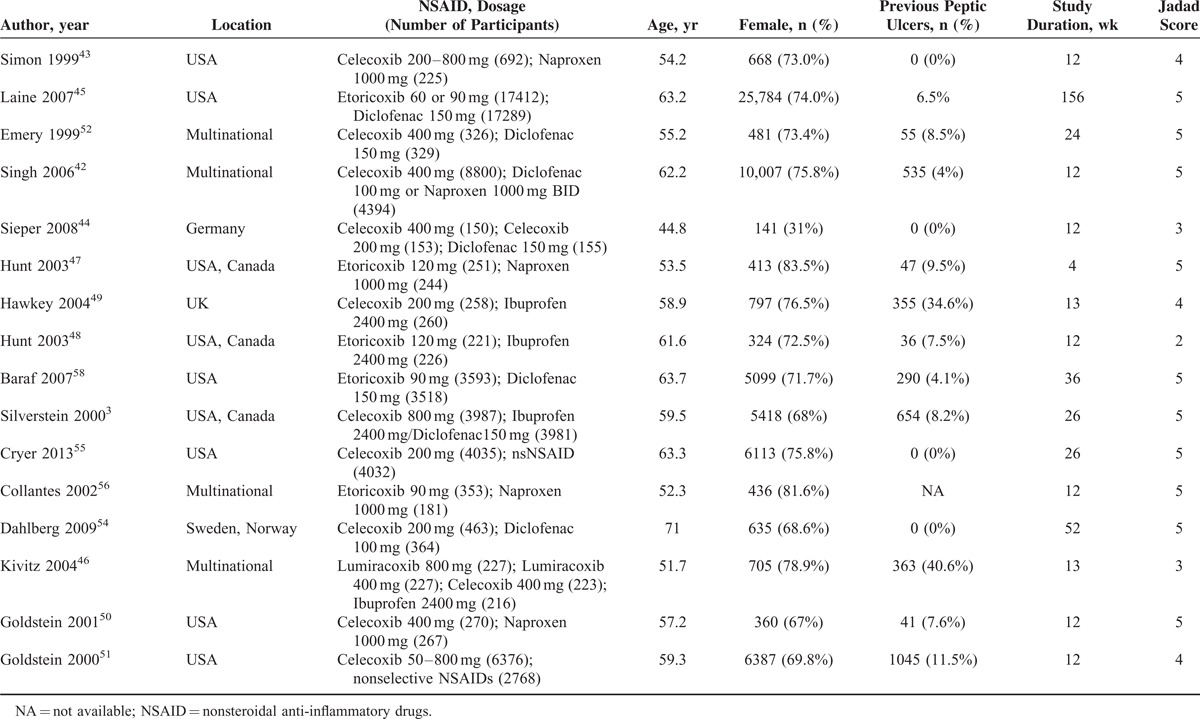
TABLE 2.
Network Meta-Analyses Comparing Coxibs With Relatively Selective Cyclooxygenase-2 Inhibitors in Preventing Ulcer Complications, Symptomatic Ulcer, and Endoscopic Ulcer
Symptomatic Ulcer
Sixteen trials including 34,991 participants and 66 events contributed to the analyses of symptomatic ulcer. Pairwise meta-analyses comparing relatively selective COX-2 inhibitors with nonselective NSAIDs (11 trials; P = 0.86, I2 = 0%), and comparing coxibs with nonselective NSAIDs (5 trials; P = 0.95, I2 = 0%) showed no significant heterogeneity across included trials. Network meta-regression analyses testing the assumption of similarity did not reveal any prespecified factors that significantly modified the estimated effects (Supplemental digital content—Table S1, http://links.lww.com/MD/A428).
Network meta-analysis indicated that the event probability was 0.21% (95% CI, 0.04–0.62) for relatively selective COX-2 inhibitors and 0.18% (95% CI, 0.01–0.74) for coxibs. There was no significant difference between relatively selective COX-2 inhibitors and coxibs (RR, 1.02; 95% CI, 0.09–3.92) (Table 2). The crude RR was consistent with those from network meta-analyses adjusting for the prespecified factors (Table 3).
Endoscopic Ulcer
Network meta-analyses evaluating the risk of endoscopic ulcer involved 3903 participants and 465 events from 11 trials. Pairwise meta-analysis comparing relatively selective COX-2 inhibitors with nonselective NSAIDs show no significant heterogeneity across included trials (5 trials; P = 0.91, I2 = 0%), while substantial heterogeneity was shown in the meta-analysis comparing coxibs with nonselective NSAIDs (6 trials; P = 0.006, I2 = 69%). Meta-regression indicated that average age, proportion of females, and study duration did not significantly modify the estimated effects (Supplemental digital content—Table S1, http://links.lww.com/MD/A428).
Network meta-analysis indicated that the event probability was 4.70% (95% CI, 1.35–11.13) for relatively selective COX-2 inhibitors and 4.30% (95% CI, 2.01–7.88) for coxibs. There was no significant difference between relatively selective COX-2 inhibitors and coxibs (RR, 1.18; 95% CI, 0.37–2.92) (Table 2). The crude RR was consistent with those from network meta-analyses adjusting for the prespecified factors (Table 3).
Overall Safety and Tolerability
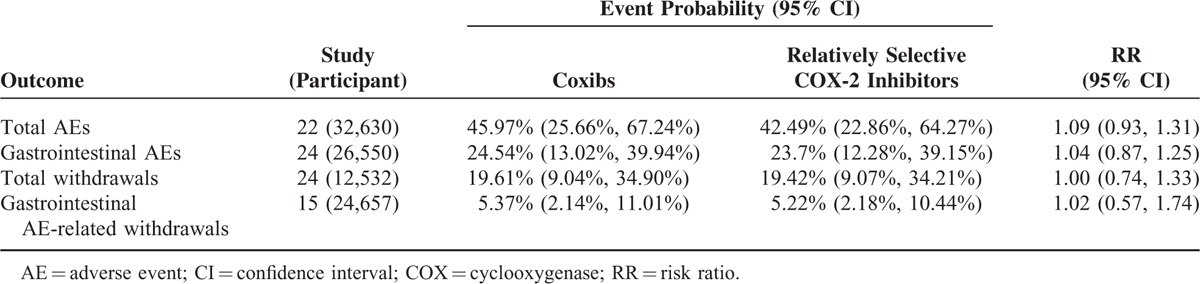
Table 4 presents the network meta-analyses about the safety of relatively selective COX-2 inhibitors and coxibs taking nonselective NSAIDs as the reference. Network meta-analysis indicated that relatively selective COX-2 inhibitors and coxibs were associated with comparable incidences of total AEs (RR, 1.09; 95% CI, 0.93–1.31), gastrointestinal AEs (RR, 1.04; 95% CI, 0.87–1.25), total withdrawals (RR, 1.00; 95% CI, 0.74–1.33), and gastrointestinal AE-related withdrawals (RR, 1.02; 95% CI, 0.57–1.74).
TABLE 3.
Network Meta-Analyses Comparing Coxibs With Relatively Selective Cyclooxygenase-2 Inhibitors After Adjusting for Potential Influential Factors
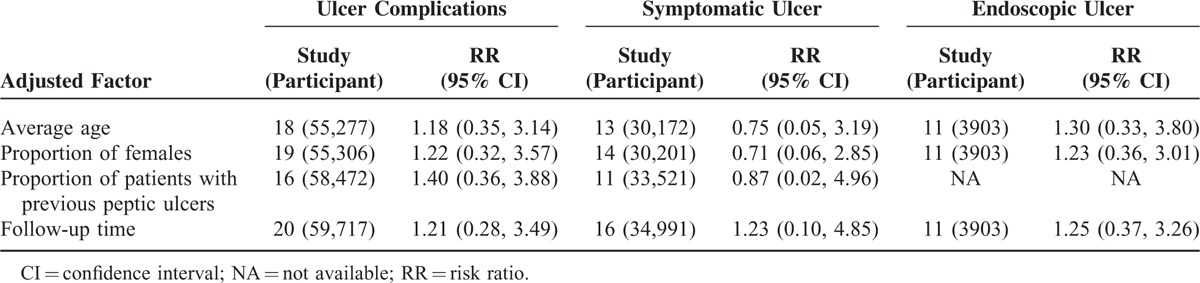
TABLE 4.
Network Meta-Analysis Comparing the Safety and Tolerability of Coxibs vs Relatively Selective Cyclooxygenase-2 Inhibitors
Sensitivity Analysis
The results of sensitivity analyses are presented in Supplemental digital content-Table S2, http://links.lww.com/MD/A428. The estimated effects for ulcer complications, symptomatic ulcer, and endoscopic ulcer were robust and we did not indicate any major influence to the estimated effects between relatively selective COX-2 inhibitors and coxibs by sensitivity analyses.
DISCUSSION
It has been widely accepted that the NSAIDs with COX-2 selectivity are associated with less gastrointestinal harms. However, the comparative gastroprotective effect of relatively selective COX-2 inhibitors and coxibs remains unclear due to lack of head-to-head trail. In this systematic review, we indirectly compared the gastroprotective effect of relatively selective COX-2 inhibitors and coxibs by Bayesian network meta-analysis. Our study suggested that relatively selective COX-2 inhibitors were associated with comparable risk of ulcer complications, symptomatic ulcer, and endoscopic ulcer as compared with coxibs. Additionally, these 2 different classes of COX-2 inhibitors also showed a similar safety and tolerability profile. These findings provide evidence supporting the use of selective COX-2 inhibitors as an alternative strategy to reduce the gastrointestinal adverse effects in patients requiring NSAID therapy.
Based on the predefined criteria, we did not identify any eligible study directly compared selective COX-2 inhibitors with coxibs. Some trials comparing selective COX-2 inhibitors with coxibs were excluded because the time of follow-up was <4 weeks,59,60 and the evaluated coxibs (rofecoxib, which has been withdrawn from market) was not covered by this systematic review.61 Results from these studies suggested that selective COX-2 inhibitors and coxibs were well tolerated59–61 and showed comparable tolerability profile,61 which are consistent with our findings. In addition, the study by Truitt et al61 also suggested that rofecoxib and nabumeton were associated with similar risk of serious gastrointestinal complications, but the estimate effect was based on only 1 event and 289 participants.11,61
Since selective COX-2 inhibitors and coxibs showed comparable gastrointestinal safety, the costs and cardiovascular safety become the primary influential factors for selecting the most suitable COX-2 inhibitors for patients. The high cost is still a limitation for coxibs.9,14,15 Data from the Irish General Medical Services Prescription Database suggested that coxibs were up to 10-fold more expensive, with a median monthly costs of €34.61 for coxibs, compared to €3.26 for nonselective NSAIDs.15 In addition, coxibs may be associated with increased risk of cardiovascular adverse events, and this has led to the withdrawal of many coxibs, including rofecoxib and valdecoxib. However, not only COX-2 inhibitors but also traditional NSAIDs are associated with increased cardiovascular adverse events. US FDA has announced warnings about cardiovascular safety for COX-2 selective and most nonselective NSAIDs.62 Future research is expected to directly compare the cost-effectiveness and cardiovascular safety between these agents.
Our study only considered nonselective NSAIDs as the comparator for network meta-analysis because they are the most common control used in the related original trails. Theoretically it is possible to consider other gastroprotective strategies as the comparators in the network meta-analysis models, but including more nodes may increase the complexity and risk of inconsistency in network. Our estimated effects of selective COX-2 inhibitors and coxibs as compared with nonselective NSAID were consistent with previous meta-analyses.11,14,63
To the best of our knowledge, this is the first network meta-analysis evaluating the gastroprotective effectiveness between selective COX-2 inhibitors and coxibs. Network meta-analysis provides a coherent and methodologically robust evaluation of comparative effectiveness for multiple interventions, which may help guide clinicians and patients to make informed treatment decisions.64 This is particularly important as direct comparison between the 2 difference classes of COX2-inhibitors is lacking so clinicians and patients have to make clinical decision based on indirect evidence. In addition, the sample size of this study is large, which make it possible to get a precise estimation of important but rare clinical outcomes, and to control potential influential factors. Lastly, low level of heterogeneity, consistent results from different models adjusting for various influential factors, and stable sensitivity analyses further increased our confidence in the results.
This study need to be explained with caution due to some limitations. Firstly, clinical outcomes like ulcer complications and symptomatic ulcer were sparsely reported and often obtained from drug safety information in the original trials. The definition of clinical outcomes might not be consistent across included studies. Secondly, indirect comparisons from network meta-analyses are observational in nature, which may be biased due to study-level confounding factors.65,66 Though we have controlled age, sex, patients with previous ulcer disease, and length of follow-up, other factors including H pylori infection and ulcer risk were not adjusted due to insufficient data. Lastly, the results may be affected as the mythological quality of some original trials is low. However, the potential influence is unlikely to be substantial because sensitivity analyses by study quality did not show major change to the primary estimated effects.
In conclusion, this systematic review suggested that relatively selective COX-2 inhibitors appear to have a similar gastroprotective effect and tolerability as coxibs. Relatively selective COX-2 inhibitors may be used as an alternative strategy to reduce the gastrointestinal adverse effects in patients requiring NSAID therapy. Because our findings are based on indirect comparisons, future research, particularly head-to-head trials, is required to confirm the conclusion.
Footnotes
Abbreviations: AE = adverse event, CI = confidence interval, COX = cyclooxygenase, NSAID = nonsteroidal anti-inflammatory drug, RCT = randomized controlled trial, RR = risk ratio.
MY and H-YW contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Soll AH, Weinstein WM, Kurata J, et al. Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann Intern Med 1991; 114:307–319. [DOI] [PubMed] [Google Scholar]
- 2.Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994; 343:769–772. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1995; 123:241–249. [DOI] [PubMed] [Google Scholar]
- 4.Laine L. Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am 1996; 6:489–504. [PubMed] [Google Scholar]
- 5.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 1999; 340:1888–1899. [DOI] [PubMed] [Google Scholar]
- 6.Cryer B. NSAID-associated deaths: the rise and fall of NSAID-associated GI mortality. Am J Gastroenterol 2005; 100:1694–1695. [DOI] [PubMed] [Google Scholar]
- 7.Langman MJ. Ulcer complications associated with anti-inflammatory drug use. What is the extent of the disease burden? Pharmacoepidemiol Drug Saf 2001; 10:13–19. [DOI] [PubMed] [Google Scholar]
- 8.Marnett LJ. Structure, function and inhibition of cyclo-oxygenases. Ernst Schering Res Found Workshop 2000. 65–83. [DOI] [PubMed] [Google Scholar]
- 9.Hawkey CJ. COX-1 and COX-2 inhibitors. Best Pract Res Clin Gastroenterol 2001; 15:801–820. [DOI] [PubMed] [Google Scholar]
- 10.McCormack PL. Celecoxib A review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. Drugs 2011; 71:2457–2489. [DOI] [PubMed] [Google Scholar]
- 11.Brown TJ, Hooper L, Elliott RA, et al. A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling. Health Technol Assess 2006; 10:1–183. [DOI] [PubMed] [Google Scholar]
- 12.Hooper L, Brown TJ, Elliott RA, et al. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced lay non-steroidal anti-inflammatory drugs: systematic review. Br Med J 2004; 329:948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanza FL, Chan FK, Quigley EM, et al. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009; 104:728–738. [DOI] [PubMed] [Google Scholar]
- 14.Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 2008; 12:1–278. [DOI] [PubMed] [Google Scholar]
- 15.Teeling M, Bennett K, Feely J. Have COX-2 inhibitors influenced the co-prescription of anti-ulcer drugs with NSAIDs? Br J Clin Pharmacol 2004; 57:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005; 331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23:3105–3124. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. www.cochrane-handbook.org [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown TJ, Hooper L, Elliott RA, et al. A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling. Health Technol Assess 2006; 10:1. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Dias S, Sutton AJ, Welton NJ, et al. NICE DSU Technical Support Document 3: Heterogeneity: subgroups, meta-regression, bias and bias-adjustment (last updated April 2012); 2011. [PubMed] [Google Scholar]
- 23.Song F, Loke YK, Walsh T, et al. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009; 338:b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials (last updated April 2012); 2011. [PubMed] [Google Scholar]
- 25.Wojtulewski JA, Schattenkirchner M, Barcelo P, et al. A six-month double-blind trial to compare the efficacy and safety of meloxicam 7.5 mg daily and naproxen 750 mg daily in patients with rheumatoid arthritis. Br J Rheumatol 1996; 35 (Suppl 1):22–28. [DOI] [PubMed] [Google Scholar]
- 26.Taha AS, McLaughlin S, Sturrock RD, et al. Evaluation of the efficacy and comparative effects on gastric and duodenal mucosa of etodolac and naproxen in patients with rheumatoid arthritis using endoscopy. Br J Rheumatol 1989; 28:329–332. [DOI] [PubMed] [Google Scholar]
- 27.Taha AS, McLaughlin S, Holland PJ, et al. Effect on gastric and duodenal mucosal prostaglandins of repeated intake of therapeutic doses of naproxen and etodolac in rheumatoid arthritis. Ann Rheum Dis 1990; 49:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott DL, Palmer RH. Safety and efficacy of nabumetone in osteoarthritis: emphasis on gastrointestinal safety. Aliment Pharmacol Ther 2000; 14:443–452. [DOI] [PubMed] [Google Scholar]
- 29.Roth SH, Bennett R, Caldron P, et al. A longterm endoscopic evaluation of patients with arthritis treated with nabumetone vs naproxen. J Rheumatol 1994; 21:1118–1123. [PubMed] [Google Scholar]
- 30.Roth SH. Endoscopy-controlled study of the safety of nabumetone compared with naproxen in arthritis therapy. Am J Med 1987; 83:25–30. [DOI] [PubMed] [Google Scholar]
- 31.Morgan GJ, Kaine J, DeLapp R, et al. Treatment of elderly patients with nabumetone or diclofenac—gastrointestinal safety profile. J Clin Gastroenterol 2001; 32:310–314. [DOI] [PubMed] [Google Scholar]
- 32.Linden B, Distel M, Bluhmki E. A double-blind study to compare the efficacy and safety of meloxicam 15 mg with piroxicam 20 mg in patients with osteoarthritis of the hip. Br J Rheumatol 1996; 35:35–38. [DOI] [PubMed] [Google Scholar]
- 33.Lightfoot R. Comparison of the efficacy and safety of etodolac and piroxicam in patients with rheumatoid arthritis. Etodolac Study 326 Rheumatoid Arthritis Investigators Group. J Rheumatol Suppl 1997; 47:10–16. [PubMed] [Google Scholar]
- 34.Li S. Comparison of the efficacy and safety of nabumetone and diclofenac sodium in the treatment of patients with rheumatoid arthritis. Zhonghua Yi Xue Za Zhi 2001; 81:557–560. [PubMed] [Google Scholar]
- 35.Laine L, Sloane R, Ferretti M, et al. A randomized double-blind comparison of placebo, etodolac, and naproxen on gastrointestinal injury and prostaglandin production. Gastrointest Endosc 1995; 42:428–433. [DOI] [PubMed] [Google Scholar]
- 36.Hawkey C, Kahan A, Steinbruck K, et al. Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients. International MELISSA Study Group. Meloxicam Large-scale International Study Safety Assessment. Br J Rheumatol 1998; 37:937–945. [DOI] [PubMed] [Google Scholar]
- 37.Filipowicz S, Maldyk H, Bernacka K, et al. Relifex in treatment of coxarthrosis and gonarthrosis. Reumatologia 1997; 35:39–50. [Google Scholar]
- 38.Eversmeyer W, Poland M, DeLapp RE, et al. Safety experience with nabumetone versus diclofenac, naproxen, ibuprofen, and piroxicam in osteoarthritis and rheumatoid arthritis. Am J Med 1993; 95:10S–18S. [DOI] [PubMed] [Google Scholar]
- 39.Dequeker J, Hawkey C, Kahan A, et al. Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis. Br J Rheumatol 1998; 37:946–951. [DOI] [PubMed] [Google Scholar]
- 40.Bianchi Porro G, Montrone F, Petrillo M, et al. Gastroduodenal tolerability of nabumetone versus naproxen in the treatment of rheumatic patients. Am J Gastroenterol 1995; 90:1485–1488. [PubMed] [Google Scholar]
- 41.Bellamy N, Bensen WG, Beaulieu A, et al. A multicenter study of nabumetone and diclofenac SR in patients with osteoarthritis. J Rheumatol 1995; 22:915–920. [PubMed] [Google Scholar]
- 42.Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am J Med 2006; 119:255–266. [DOI] [PubMed] [Google Scholar]
- 43.Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999; 282:1921–1928. [DOI] [PubMed] [Google Scholar]
- 44.Sieper J, Klopsch T, Richter M, et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis 2008; 67:323–329. [DOI] [PubMed] [Google Scholar]
- 45.Laine L, Curtis SP, Cryer B, et al. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 2007; 369:465–473. [DOI] [PubMed] [Google Scholar]
- 46.Kivitz AJ, Nayiager S, Schimansky T, et al. Reduced incidence of gastroduodenal ulcers associated with lumiracoxib compared with ibuprofen in patients with rheumatoid arthritis. Aliment Pharmacol Ther 2004; 19:1189–1198. [DOI] [PubMed] [Google Scholar]
- 47.Hunt RH, Harper S, Watson DJ, et al. The gastrointestinal safety of the COX-2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal events. Am J Gastroenterol 2003; 98:1725–1733. [DOI] [PubMed] [Google Scholar]
- 48.Hunt RH, Harper S, Callegari P, et al. Complementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxib. Aliment Pharmacol Ther 2003; 17:201–210. [DOI] [PubMed] [Google Scholar]
- 49.Hawkey CC, Svoboda P, Fiedorowicz-Fabrycy IF, et al. Gastroduodenal safety and tolerability of lumiracoxib compared with ibuprofen and celecoxib in patients with osteoarthritis. J Rheumatol 2004; 31:1804–1810. [PubMed] [Google Scholar]
- 50.Goldstein JL, Silverstein FE, Agrawal NM, et al. Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitor. Am J Gastroenterol 2000; 95:1681–1690. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein JL, Correa P, Zhao WW, et al. Reduced incidence of gastroduodenal ulcers with celecoxib, a novel cyclooxygenase-2 inhibitor, compared to naproxen in patients with arthritis. Am J Gastroenterol 2001; 96:1019–1027. [DOI] [PubMed] [Google Scholar]
- 52.Emery P, Zeidler H, Kvien TK, et al. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet 1999; 354:2106–2111. [DOI] [PubMed] [Google Scholar]
- 53.Dougados M, Behier JM, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum 2001; 44:180–185. [DOI] [PubMed] [Google Scholar]
- 54.Dahlberg LE, Holme I, Hoye K, et al. A randomized, multicentre, double-blind, parallel-group study to assess the adverse event-related discontinuation rate with celecoxib and diclofenac in elderly patients with osteoarthritis. Scand J Gastroenterol 2009; 38:133–143. [DOI] [PubMed] [Google Scholar]
- 55.Cryer B, Li C, Simon LS, et al. GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded endpoint (PROBE) trial. Am J Gastroenterol 2013; 108:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collantes E, Curtis SP, Lee KW, et al. A multinational randomized, controlled, clinical trial of etoricoxib in the treatment of rheumatoid arthritis (ISRCTN25142273). BMC Fam Pract 2002; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999; 74:1095–1105. [DOI] [PubMed] [Google Scholar]
- 58.Baraf HSB, Fuentealba C, Greenwald M, et al. Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the etoricoxib versus diclofenac sodium gastrointestinal tolerability and effectiveness (EDGE) trial. J Rheumatol 2007; 34:408–420. [PubMed] [Google Scholar]
- 59.Bianchi M, Broggini M. A randomised, double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee. Drugs 2003; 63 (Suppl 1):37–46. [DOI] [PubMed] [Google Scholar]
- 60.Bianchi M, Broggini M, Balzarini P, et al. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract 2007; 61:1270–1277. [DOI] [PubMed] [Google Scholar]
- 61.Truitt KE, Sperling RS, Ettinger WH, Jr, et al. A multicenter, randomized, controlled trial to evaluate the safety profile, tolerability, and efficacy of rofecoxib in advanced elderly patients with osteoarthritis. Aging (Milano) 2001; 13:112–121. [DOI] [PubMed] [Google Scholar]
- 62.FDA US. Public Health Advisory—FDA Announces Important Changes and Additional Warnings for COX-2 Selective and Non-Selective Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htm Accessed June 2015. [Google Scholar]
- 63.Hooper L, Brown TJ, Elliott R, et al. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ 2004; 329:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells G, Sultan S, Chen L, et al. Indirect Evidence: Indirect Treatment Comparisons in Meta-Analysis. Ottawa:Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 65.Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014; 349:g5630. [DOI] [PubMed] [Google Scholar]
- 66.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomized controlled trials (last updated April 2012); 2011. [PubMed] [Google Scholar]


