Abstract
The fatty liver index (FLI), which is an algorithm based on waist circumference, body mass index (BMI), triglyceride, and gamma-glutamyl-transferase (GGT), was initially developed to detect fatty liver in Western countries. Our study aimed to evaluate the accuracy and optimal cut-off point of the FLI for predicting nonalcoholic fatty liver disease (NAFLD) in middle-aged and elderly Chinese.
This cross-sectional study included 8626 Chinese adults aged 40 years or above recruited from Jiading District, Shanghai, China. Anthropometric and biochemical features were collected by a standard protocol. NAFLD was diagnosed by hepatic ultrasonography. The accuracy and cut-off point of the FLI to detect NAFLD were evaluated by area under the receiver operator characteristic curve (AUROC) and the maximum Youden index analysis, respectively.
The AUROC of the FLI for NAFLD was 0.834 (95% confidence interval: 0.825–0.842), and larger than that of its each individual component [0.786 (0.776–0.796), 0.783 (0.773–0.793), 0.727 (0.716–0.739), and 0.707 (0.695–0.719) for waist circumference, BMI, triglyceride, and GGT, respectively] (all P < 0.001). The optimal cut-off point of the FLI for diagnosing NAFLD was 30 with the maximum Youden Index of 0.51, achieving a high sensitivity of 79.89% and a specificity of 71.51%. The FLI-diagnosed NAFLD individuals were in worse metabolic characteristics (waist circumference, BMI, blood pressure, serum lipids, and aminotransferases) than ultrasonography-diagnosed NAFLD patients (all P < 0.05).
The FLI could accurately identify NAFLD and the optimal cut-off point was 30 in middle-aged and elderly Chinese. As FLI-diagnosed NAFLD patients were in worse metabolism, much attention should be paid to the metabolic controls and managements of NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), defined as hepatic steatosis in the absence of excessive alcohol use and any other obvious damage factors, is becoming the most common chronic liver disease in the Western world, affecting up to 46% adults.1,2 With the increasing prevalence of obesity, type 2 diabetes, and related metabolic disorders, NAFLD has become an important public health concern in Asia. The prevalence of NAFLD in China has nearly doubled in the last 10 to 15 years.3,4 NAFLD is traditionally thought to be an initial state, and prospective studies have demonstrated that NAFLD will develop toward nonalcoholic steatohepatitis and fibrosis,5–7 leading to cirrhosis.8,9 Recently, accumulating evidence suggested that NAFLD may play an important role in the progression of cardiovascular disease and chronic kidney disease.10,11 Therefore, making more efforts to detect NAFLD early is necessary. Simultaneously, a simple and effective diagnostic method would be useful for early detection and better management of NAFLD patients.
The fatty liver index (FLI) is an algorithm based on waist circumference, body mass index (BMI), triglyceride, and gamma-glutamyl-transferase (GGT) for the prediction of fatty liver,12 and is easy to employ as its each individual component is a routine measurement in clinical practice. It has been validated as a practical, reliable, and economic technique to diagnose NAFLD in large epidemiology studies.13 However, to our best knowledge, it has never been externally validated in Chinese adults.
The present study aimed to validate the accuracy and the optimal cut-off point of the FLI for predicting NAFLD in Chinese adults aged 40 years or above, and compare the metabolic characteristics between ultrasonography (US)-diagnosed and FLI-diagnosed NAFLD patients.
METHODS
Study Population
The participants of this cross-sectional study were recruited in Jiading District, Shanghai, China, from March to August, 2010. The study design and data collection were described previously.14,15 In brief, a total of 10,569 residents aged 40 years or above were invited and 10,375 of them signed the consent form and agreed to take part in the survey, with a participation rate of 98.2%. For the current analysis, we excluded participants with missing data on hepatic ultrasonography (n = 44), participants with excessive alcohol use (more than 140 g/week in men and 70 g/week in women, n = 975), participants with viral or autoimmune hepatitis or hepatitis caused by drugs (n = 335), participants with more than 3 times of normal serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), or GGT level of the study population (n = 88),16 participants seropositive for hepatitis B surface antigen (n = 303), participants with missing data on waist circumference (n = 4). Eventually, a total of 8626 participants were included in the current analysis.
The study protocol was in accordance with the principle of Helsinki Declaration II and approved by the Institutional Review Board of Rui-Jin Hospital affiliated with Shanghai Jiao-Tong University School of Medicine. Written informed consent was obtained from each participant before data collection.
Data Collection
A standard questionnaire was used to collect detailed information on sociodemographic characteristics, medical history, and lifestyle by trained physicians with face-to-face interview. Current smokers were defined as those who smoked at least 1 cigarette per day or 7 cigarettes per week in the past 6 months. Current drinkers were defined as those who consumed alcohol at least once per week over the past 6 months. Physical activity at leisure time was collected and categorized as low, moderate, and high levels using the short form of the International Physical Activity Questionnaire (IPAQ).17
Height and weight were measured to the nearest 0.1 cm and 0.1 kg, with participants wearing no shoes and light weight clothing. BMI was calculated as weight (kg) divided by squared height (m2). Waist circumference was also measured to the nearest 0.1 cm at the umbilical level in a standing position. Blood pressure was measured on the nondominant arm 3 times with 1 minute interval in a sitting position after at least 5 minutes rest, by an automated electronic device (OMRON Model HEM-752, FUZZY, Omron Company, Dalian, China). The average of 3 measurements was used for analysis.
Biochemical Evaluation
After an overnight fast of at least 10 hours, venous blood samples were collected for biochemical evaluation. Fasting serum insulin, triglyceride, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), total cholesterol (TC), ALT, AST, GGT, and serum creatinine were measured using an autoanalyzer (Modular E170; Roche, Basel, Switzerland). A standard 75-g oral glucose tolerance test (OGTT) was performed and blood glucose levels including fasting plasma glucose (FPG) and 2-h postprandial blood glucose (PBG) were measured with the use of the glucose oxidase method on an autoanalyzer (Modular P800; Roche). Hemoglobin A1c (HbA1c) was determined by high-performance liquid chromatography (HPLC, BIO-RAD D-10, USA). The insulin resistance index (homeostasis model assessment of insulin resistance (HOMA-IR)) was calculated as fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5.18 Estimated glomerular filtration rate (eGFR) was calculated with the abbreviated Modification of Diet in Renal Disease (MDRD) formula recalibrated for Chinese: eGFR = 186 × (serum creatinine × 0.011)−1.154 × (age)−0.203 × (0.742 if female) × 1.233, where serum creatinine is expressed in μmol/L and 1.233 is the adjusting coefficient for Chinese population.19
Evaluation of NAFLD
Ultrasonography
The hepatic US method was used to diagnose NAFLD for each participant after exclusion of alcohol abuse and other liver diseases. It was performed by 2 trained and certified ultrasonographists, using a high-resolution B-mode tomographic ultrasound system (Esaote Biomedica SpA, Gevona, Italy) with a 3.5-MHz probe. NAFLD was defined by the presence of at least 2 of 3 of the following abnormal findings: diffusely increased echogenicity of the liver relative to the right kidney; attenuation of the ultrasound beam; poor visualization of intrahepatic architectural details.14,15,20
Fatty Liver Index
The FLI, calculated in each participant, is a noninvasive method of assessing hepatic steatosis and is calculated by the following formula:12 FLI = (e0.953×loge(triglycerides)+0.139×BMI+0.718×loge(GGT)+0.053×waistcircumference−15.745)/ (1 + e0.953×loge(triglycerides)+0.139×BMI+0.718×loge(GGT)+0.053×waistcircumference−15.745) × 100.
Statistical Analysis
All statistical analyses were conducted by using SAS version 9.3 (SAS Institute Inc, Cary, NC). Continuous variables were presented as means ± standard deviation (SD) for normal distributed variables, or medians (interquartile range) for skewed variables. Categorical variables were presented as numbers (proportion).
Area under the receiver operator characteristic curve (AUROC) was used to indicate the predictive values of the FLI and its each individual component for diagnosing NAFLD. Comparisons between the AUROC of the FLI and that of its each individual component were conducted by using the method described by De Long et al.21 The Kappa value was calculated for evaluating the agreement of the FLI or its components compared with the hepatic US method for diagnosis of NAFLD. Degrees of agreement were categorized into very poor, poor, moderate, good, and excellent agreement according to Kappa values following: 0.00 to 0.20, 0.21 to 0.40, 0.41 to 0.60, 0.61 to 0.80, 0.81 to 1.00, respectively.22 The sensitivity (SN), specificity (SP), positive likelihood ratio (LR + ), negative likelihood ratio (LR−), positive predictive value (PPV), negative predictive value (NPV), and Youden index of 10-value intervals of the FLI were calculated, and the point with the maximum Youden index was used as the cut-off point of the FLI for detecting NAFLD.
Participants were categorized into 4 groups by 4 different diagnostic methods: non-NAFLD diagnosed based on both the FLI and hepatic US method [FLI (−) and US (−)], NAFLD diagnosed only based on the US method [FLI (−) and US (+)], NAFLD diagnosed only based on the FLI [FLI (+) and US (−)], and NAFLD diagnosed based on both the FLI and US method [FLI (+) and US (+)]. Comparisons of metabolic characteristics between participants with [FLI (−) and US (+)] and those with [FLI (+) and US (−)] were performed using t test for continuous variables and χ2 test for categorical variables.
Significant tests were 2-tailed and a P value <0.05 was considered statistically significant.
RESULTS
Characteristics of Participants
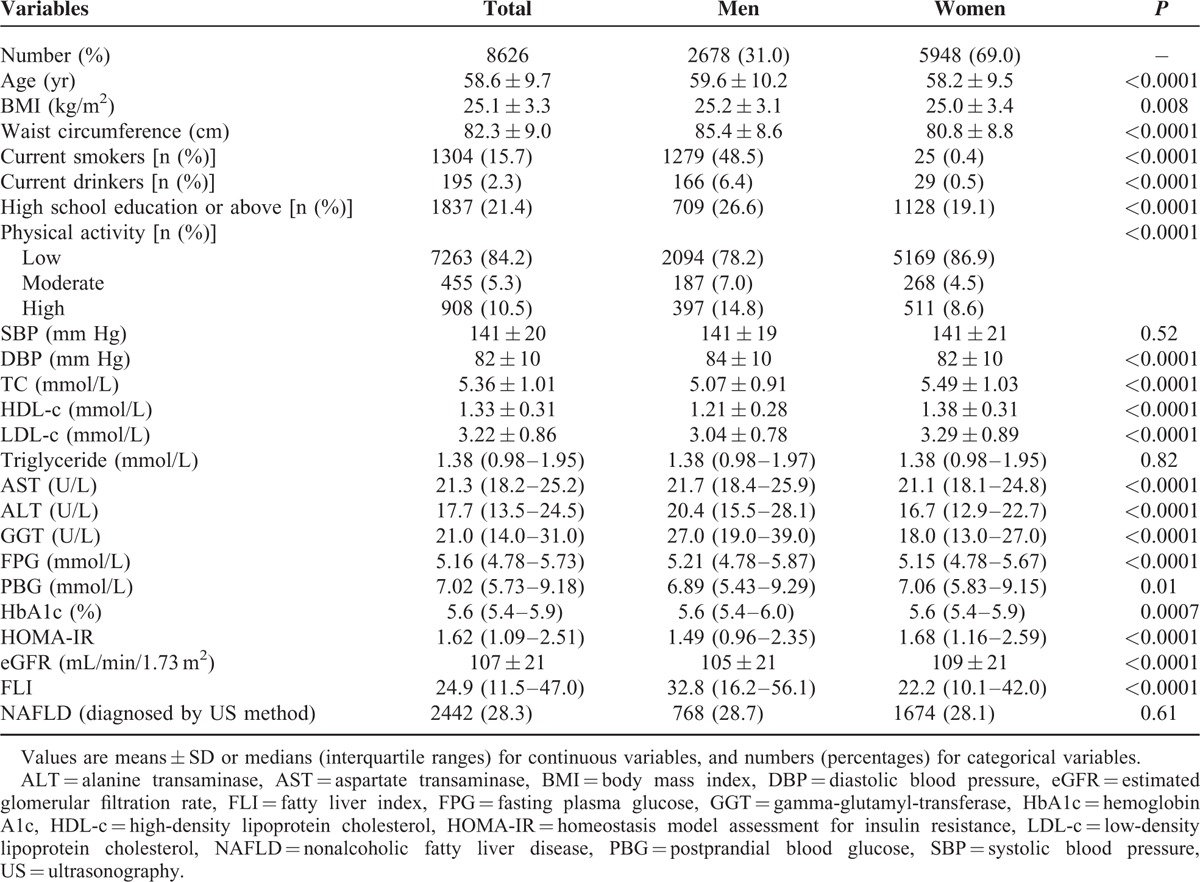
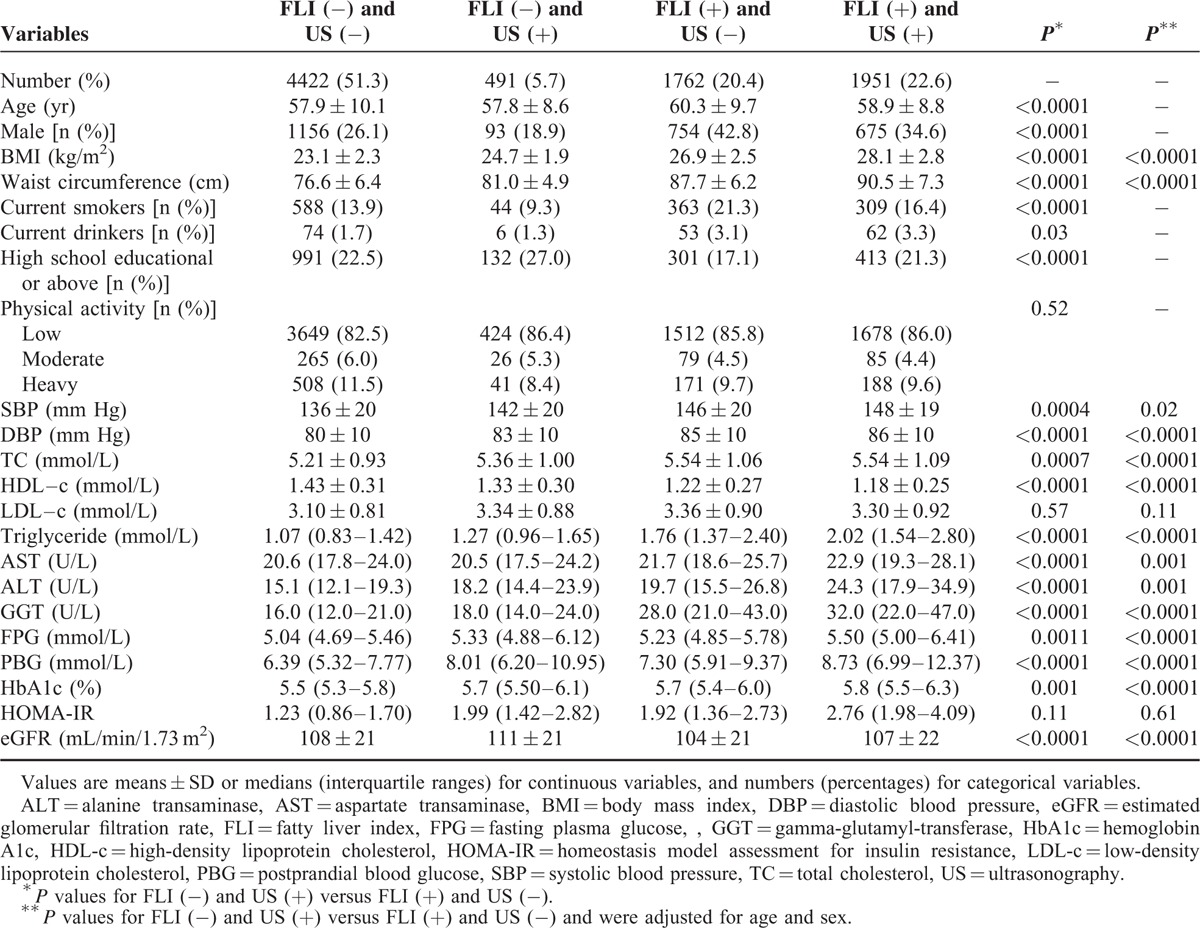
Table 1 shows the demographic and clinical characteristics of the participants. The mean age of all participants was 58.6 ± 9.7 years old, 69.0% were women. Women were younger, less likely to be current smokers, current drinkers, physically active, and received high school education or above, had lower levels of waist circumference, BMI, diastolic blood pressure (DBP), FPG, HbA1c, serum aminotransferases, FLI, but higher levels of PBG, HOMA-IR, HDL-c, LDL-c, TC, and eGFR (all P < 0.05). No significant difference was found in systolic blood pressure (SBP), triglyceride, or the percentage of ultrasonographically defined NAFLD between men and women (28.7% vs 28.1%).
TABLE 1.
Characteristics of the Study Participants
Associations Between the FLI and NAFLD
The median (interquartile range) of the FLI was 51.5 (33.4–69.3) and 17.6 (8.5–32.7) for NAFLD patients and non-NAFLD participants, respectively. Adjusting for age, sex, BMI, current drinking and smoking status, physical activity, blood pressure, FPG, and PBG, the risk of NAFLD significantly increased with each unit increment of the FLI (odds ratio (OR), 1.048; 95% confidence interval (CI), 1.044–1.052).
Diagnostic Accuracy of the FLI and Its Each Individual Component for NAFLD
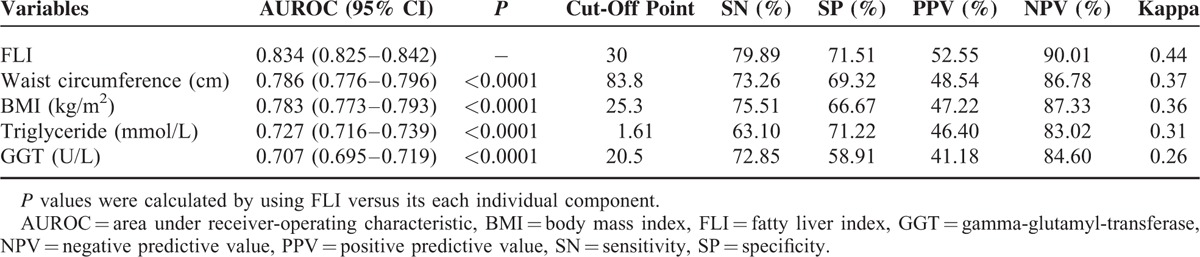
The AUROC of the FLI for predicting NAFLD was 0.834 (95% CI: 0.825–0.842) (Figure 1). In addition, the sex-specific and age-specific AUROCs of the FLI were 0.841 (95% CI: 0.831–0.851) for women, and 0.834 (95% CI: 0.818–0.850) for men, and 0.840 (95% CI: 0.830–0.850) for those aged <65 years old, and 0.819 (95% CI: 0.800–0.838) for those aged ≥65 years old, respectively. The optimal cut-off point was FLI ≥30, with the maximum Youden index of 0.51 and high sensitivity and specificity (79.89% SN, 71.51% SP, 52.55% PPV, and 90.01% NPV) (Table 2). AUROCs of each individual component of the FLI were all significantly lower than that of the FLI (AUROCs (95% CI) for waist circumference, BMI, triglyceride, and GGT were 0.786 (0.776–0.796), 0.783 (0.773–0.793), 0.727 (0.716–0.739), and 0.707 (0.695–0.719), respectively) (all P < 0.001). Furthermore, the Kappa value represented the agreement between the ability of the FLI and the US method to predict NAFLD was 0.44, indicating a moderate agreement; while poor agreements of the ability to detect NAFLD existed between each individual component of the FLI and the US method (Table 3).
FIGURE 1.
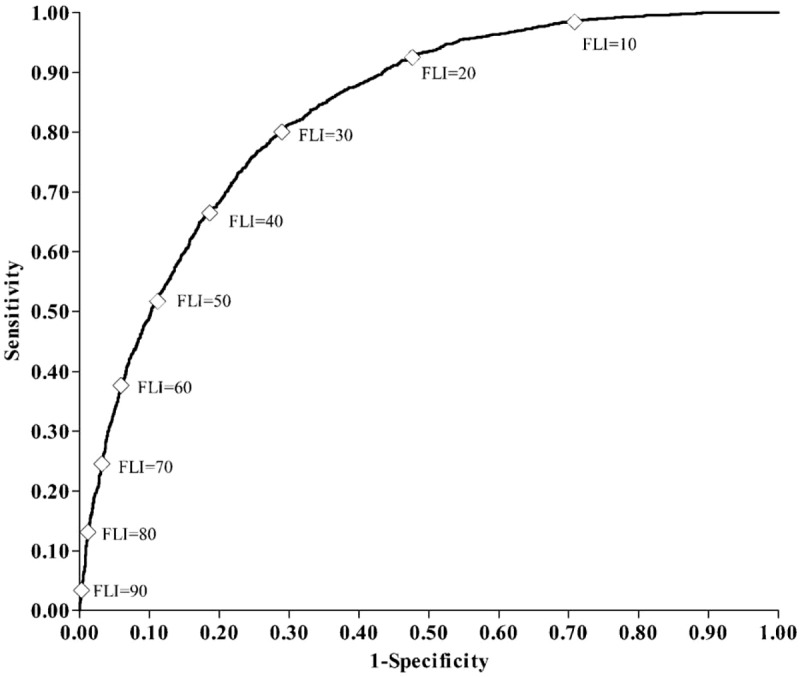
The receive-operating characteristic curve for fatty liver index (FLI) as a diagnostic indicator for nonalcoholic fatty liver disease (NAFLD), ⋄ Each 10-unit interval of the FLI.
TABLE 2.
Diagnostic Accuracy of the Fatty Liver Index
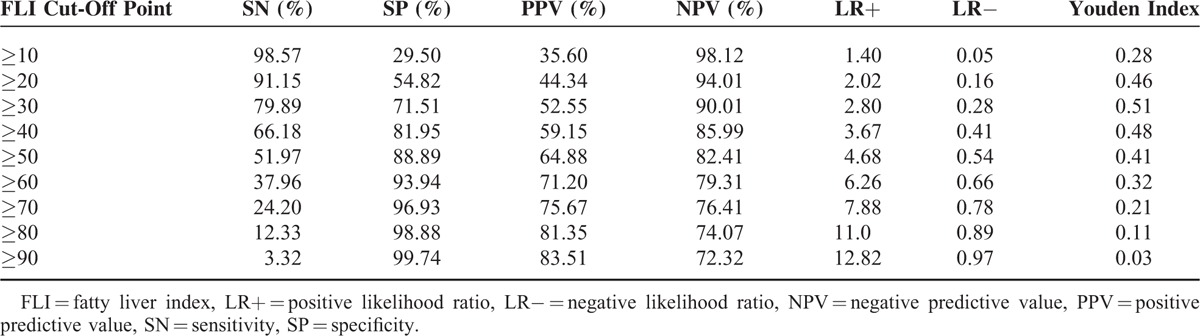
TABLE 3.
Areas Under the Receiver-Operating Characteristic Curves (AUROCs) for the Detection of Nonalcoholic Fatty Liver Disease (NAFLD) Using FLI and Its Each Separate Component
Comparisons of Characteristics Between US- and FLI-Diagnosed NAFLD Patients
In total, 1951 (22.6%) NAFLD patients and 4422 (51.3%) non-NAFLD participants were defined by using both the FLI and US methods. As compared with the NAFLD patients diagnosed by the US method but not the FLI [FLI (−) and US (+)], those defined by the FLI but not US method [FLI (+) and US (−)] were older, more likely to be men, current smokers and drinkers, higher levels of waist circumference, BMI, blood pressure, triglyceride, TC and serum aminotransferases, but lower educational attainment, lower levels of HDL-c, FPG, PBG, HbA1c, and eGFR (all P < 0.05). No significant differences existed in levels of physical activity, LDL-c and HOMA-IR between the 2 groups. Further adjustment for age and sex distribution did not statistically change the relationships (Table 4).
TABLE 4.
General Demographic and Biochemical Characteristics of Participants by Diagnostic Categories
DISCUSSION
In the present study, we demonstrated that the FLI could detect NAFLD accurately with a good AUROC of 0.834 (0.825–0.842) and the optimal cut-off point of the FLI for diagnosing NAFLD (defined by the maximum Youden index) was 30 with high sensitivity of 79.89% and specificity of 71.51% in middle-aged and elderly Chinese. The FLI was suggested to be a useful noninvasive method for the diagnosis of NAFLD. The FLI-diagnosed NAFLD patients were with worse metabolic characteristics than the US-diagnosed NAFLD patients.
In Chinese population, another fatty liver disease (FLD) index based on BMI, triglyceride, ALT to AST ratio, and hyperglycemia was proposed to predict the presence of NAFLD.23 It was performed in adults who visited the hospital for a routine health check, with AUROC of 0.819 for detecting NAFLD. The FLD index was first proposed in Chinese, and has never been validated in other population, particularly in other Asians. Comparably, in the present study, the FLI was validated in a community-based general Chinese population. The accuracy of the FLI for detecting NAFLD has been validated in Western populations, as well as in Korean population. The FLI, to a contain content, was a more unanimous approval and broadly applied method for detecting NAFLD.
The FLI was first proposed by Bedogni et al for the prediction of fatty liver in a Dionysos population (216 subjects with and 280 subjects without suspected liver disease) aged 18 to 75 years with a good AUROC of 0.84 (95% CI: 0.81–0.87).12 The accuracy of the FLI in comparison with the US method for detection and quantification of hepatic steatosis has been validated in several other studies. Koehler et al reported that the AUROCs of the FLI was 0.807 for fatty liver and 0.813 for NAFLD in a large population of white elderly persons, similar to that reported by Bedogni et al, while the sensitivity of the recommended cut-offs of the FLI (FLI <30 ruled out and FLI ≥60 ruled in fatty liver) was comparably lower, probably due to elder participants aged 76.3 years average.24 In Korean adults, although the FLI was a useful index for predicting fatty liver, it was not superior to waist circumference and BMI.25 It may be attributable to the small sample size and higher GGT and triglyceride levels than that of Dionysos population.12 In the present study, the validation of the FLI for NAFLD was conducted in a large number of Chinese adults aged 40 years old or above. The AUROC of 0.834 was similar to that of Dionysos population and significantly greater than that of its each individual component, involving waist circumference and BMI. Additionally, the AUROCs were fairly consistent, no matter in females or males, individuals aged <65 or ≥65 years old. Moreover, we found that the optimal cut-off point of the FLI for NAFLD was with high sensitivity and specificity. It might ascribe to the large sample size and analogous age, levels of triglyceride, and GGT of participants in our study to those of the Dionysos population. It should be noted that although the PPV of 52.55% in the present study was relatively low, it was affected by the low prevalence of NAFLD, while the AUROC of the FLI for predicting NAFLD was good which was based on high sensitivity and specificity, independent of prevalence of NAFLD.
The accuracy of the FLI to detect NAFLD presented in the present study showed that the FLI may be a simple and accurate method to detect NAFLD patients in Chinese adults, implicating its application in clinical practice. In addition, the present study found that NAFLD patients diagnosed by the FLI were in worse metabolic statuses. This might be due to the algorithm of the FLI based on metabolism-related parameters of waist circumference, BMI, triglyceride, and GGT. It indicated that as NAFLD patients identified only based on the FLI, more attention should be paid to the metabolic controls and the managements of NAFLD, in order to prevent the progression of nonalcoholic steatohepatitis, firbrosis, and other complications of NAFLD.
To the best of our knowledge, this is the first study to validate the accuracy of the FLI for detecting NAFLD in a large general Chinese population and to discuss differences between FLI-diagnosed NAFLD patients and US-diagnosed NAFLD patients. However, several limitations should be considered. First, the US method was used as the standard diagnostic one for NAFLD, lacking data of liver biopsy in our study. However, previous studies showed that the US method was the most practical option to detect NAFLD in clinical practice and epidemiological studies.26,27 Second, we did not have the information on the severity of hepatic steatosis, which restrained us to find specific cutoffs for steatosis quantification in the general population.
In conclusion, we validated the accuracy of the FLI for predicting NAFLD in a large general Chinese population, and determined that the optimal cut-off point of the FLI for NAFLD was 30 in middle-aged and elderly Chinese. Furthermore, FLI-diagnosed NAFLD patients were in worse metabolism-related situations than those with US-diagnosed NALFD.
Acknowledgment
We thank all the participants for their contribution and participation.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUROC = area under the receiver operator characteristic curve, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FLI = fatty liver index, FPG = fasting plasma glucose, GGT = gamma-glutamyl-transferase, HbA1c = hemoglobin A1c, HDL-c = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, LDL-c = low-density lipoprotein cholesterol, LR = negative likelihood ratio, LR = positive likelihood ratio, NAFLD = nonalcoholic fatty liver disease, NPV = negative predictive value, OR = odds ratio, PBG = 2-h postprandial blood glucose, PPV = positive predictive value, SBP = systolic blood pressure, SD = standard deviation, SN = sensitivity, SP = specificity, TC = total cholesterol, US = ultrasonography.
XH and MX contributed equally to this work. This work was supported by grants 2013BAI09B13 from the China National Clinical Research Center for Metabolic Diseases, 2015CB553600 from 973 Program, 2012ZX09303006-001 from the National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology, 2013ZYJB1002 from the Joint Research Program for Important Disease of the Shanghai Municipal Commission of Health and Family Planning, 2012AA02A509 from the National High Technology Research and Development Program of China (863 Program), and 81321001, 81390350, 81222008, 81270877, and 81130016 from the National Natural Science Foundation of China.
There are no conflicts of interest.
REFERENCES
- 1.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140:124–131. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34:274–285. [DOI] [PubMed] [Google Scholar]
- 3.Amarapurkar DN, Hashimoto E, Lesmana LA, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol 2007; 22:788–793. [DOI] [PubMed] [Google Scholar]
- 4.Fan JG, Saibara T, Chitturi S, et al. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol 2007; 22:794–800. [DOI] [PubMed] [Google Scholar]
- 5.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol 2003; 98:2042–2047. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116:1413–1419. [DOI] [PubMed] [Google Scholar]
- 7.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010; 59:969–974. [DOI] [PubMed] [Google Scholar]
- 8.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis 2009; 13:511–531. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999; 29:664–669. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008; 51:444–450. [DOI] [PubMed] [Google Scholar]
- 12.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Festi D, Schiumerini R, Marzi L, et al. Review article: the diagnosis of non-alcoholic fatty liver disease—availability and accuracy of non-invasive methods. Aliment Pharmacol Ther 2013; 37:392–400. [DOI] [PubMed] [Google Scholar]
- 14.Sun K, Lu J, Jiang Y, et al. Low serum potassium level is associated with nonalcoholic fatty liver disease and its related metabolic disorders. Clin Endocrinol (Oxf) 2014; 80:348–355. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Bi Y, Xu M, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol 2012; 32:2321–2326. [DOI] [PubMed] [Google Scholar]
- 16.Neuman MG, Cohen LB, Nanau RM. Biomarkers in nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2014; 28:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The IPAQ group. International physical activity questionnaire. 2014. http://www.ipaq.ki.se/scoring.pdf (accessed 6 January 2015). [Google Scholar]
- 18.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21:2191–2192. [DOI] [PubMed] [Google Scholar]
- 19.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 20.Palmentieri B, de Sio I, La Mura V, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis 2006; 38:485–489. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics 1988; 44:837–845. [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 23.Fuyan S, Jing L, Wenjun C, et al. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig Dis Sci 2013; 58:3326–3334. [DOI] [PubMed] [Google Scholar]
- 24.Koehler EM, Schouten JN, Hansen BE, et al. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol 2013; 11:1201–1204. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Kwon SY, Lee SW, et al. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int 2011; 31:1600–1601. [DOI] [PubMed] [Google Scholar]
- 26.Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009; 51:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwenzer NF, Springer F, Schraml C, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009; 51:433–445. [DOI] [PubMed] [Google Scholar]


