Abstract
The association between dialysate calcium (DCa) concentration and mortality in hemodialysis (HD) patients is controversial. In this study, we evaluated the impact of DCa concentration on mortality in incident HD patient.
Incident HD patients were selected from the Clinical Research Center registry—a prospective cohort study on dialysis patients in Korea. Patients were categorized into 3 groups according to the prescribed DCa concentration at the time of enrollment. High DCa was defined as a concentration of 3.5 mEq/L, mid-DCa as 3.0 mEq/L, and low DCa as 2.5 to 2.6 mEq/L. The primary outcome was all-cause mortality and secondary outcomes were cardiovascular or infection-related hospitalization.
A total of 1182 patients with incident HD were included. The number of patients in each group was 182 (15.4%) in high DCa group, 701 (59.3%) in the mid-DCa group, and 299 (25.3%) in the low DCa group. The median follow-up period was 16 months. The high DCa group had a significantly higher risk of all-cause mortality compared with the mid-DCa group (hazard ratio [HR] 2.23, 95% confidence interval [CI] 1.28–3.90, P = 0.005) and the low DCa group (HR 3.67, 95% CI 1.78–7.55, P < 0.001) after adjustment for clinical variables. The high DCa group was associated with higher risk of cardiovascular and infection-related hospitalization compared with the low DCa group (HR 3.25, 95% CI 1.53–6.89, P = 0.002; and HR 2.77, 95% CI 1.29–5.94, P = 0.009, respectively). Of these 1182 patients, 163 patients from each group were matched by propensity scores. In the propensity score matched analysis, the high DCa group had a significantly higher risk of all-cause mortality compared with the mid-DCa group (HR 2.52, 95% CI 1.04–6.07, P = 0.04) and the low DCa group (HR 4.25, 95% CI 1.64–11.03, P = 0.003) after adjustment for clinical variables.
Our data showed that HD using a high DCa was a significant risk factor for all-cause mortality and cardiovascular or infection-related hospitalization in incident HD patients.
INTRODUCTION
Abnormal bone and mineral metabolism are highly prevalent and are associated with mortality in patients with end-stage renal disease (ESRD).1–3 Abnormal bone and mineral metabolism can potentially be modified by the prescription of phosphate binders, active vitamin D compounds, and calcimimetics, and by controlling the dialysate calcium (DCa) concentration. And, such modifications may be associated with improvement of mortality in hemodialysis (HD) patients.
Although current guidelines recommended a DCa either 2.5 mEq/L or between 2.5 and 3.0 mEq/L,4–7 a wide variation in prescribed DCa concentration actually exists among different countries.2,8–10 Moreover, the association between DCa concentration and mortality is controversial.11 The Dialysis Outcome Practice Pattern Study (DOPPS) showed that a higher DCa was associated with significantly increased all-cause mortality.2 However, the Association Régionale des Néphrologues OStéodystrophie database (ARNOS) French cohort demonstrated that high DCa (3.5 mEq/L) was not associated with increased mortality compared to low DCa (2.5 mEq/L).8 Furthermore, another study reported that very low DCa <2.5 mEq/L was associated with an increased risk of sudden cardiac arrest.12
Differences in the study design or study populations may contribute to this discrepancy. Most of the previous studies included patients with prevalent HD, which may cause early mortality bias (so-called selection of survivors) and a carryover effect of previous exposure to dialysate with various ranges of calcium concentration. Therefore, in this study, we included only patients with incident HD and investigated the impact of DCa concentration on mortality.
The aim of this study was to determine the association between DCa concentration and mortality in the incident HD population in the Clinical Research Center (CRC) registry for ESRD, a prospective cohort study on dialysis patients in Korea.
METHODS
Study Population
All patients in this study participated in the CRC registry for ESRD. This is an ongoing observational prospective cohort study in patients with ESRD from 31 centers in Korea. The cohort started in April 2009 and was followed up to July 2014. This study included adult (>18 years of age) dialysis patients. A total of 1517 incident patients undergoing HD were enrolled in this cohort from April 2009 to May 2014. Enrollment was done at the time of initiation of dialysis. After exclusion of patients for whom information about the dialysis calcium concentration was not available (n = 335), 1182 patients with incident HD were included in the final analysis.
Demographic data and clinical data were collected at enrollment. Assessment of dialysis characteristics and measurements of health were performed every 6 months until follow-up was complete. Dates and causes of mortality were reported throughout the follow-up period.
Ethics
The study was approved by the institutional review board at each center (The Catholic University of Korea, Bucheon St Mary's Hospital; The Catholic University of Korea, Incheon St Mary's Hospital; The Catholic University of Korea, Seoul St Mary's Hospital; The Catholic University of Korea, St Mary's Hospital; The Catholic University of Korea, St Vincent's Hospital; The Catholic University of Korea, Uijeongbu St Mary's Hospital; Cheju Halla General Hospital; Chonbuk National University Hospital; Chonnam National University Hospital; Chung-Ang University Medical Center; Chungbuk National University Hospital; Chungnam National University Hospital; Dong-A University Medical Center; Ehwa Womens University Medical Center; Fatima Hospital, Daegu; Gachon University Gil Medical Center; Inje University Pusan Paik Hospital; Kyungpook National University Hospital; Kwandong University College of Medicine, Myongji Hospital; National Health Insurance Corporation Ilsan Hospital; National Medical Center; Pusan National University Hospital; Samsung Medical Center, Seoul; Seoul Metropolitan Government, Seoul National University, Boramae Medical Center; Seoul National University Hospital; Seoul National University, Bundang Hospital; Yeungnam University Medical Center; Yonsei University, Severance Hospital; Yonsei University, Gangnam Severance Hospital; Ulsan University Hospital; Wonju Christian Hospital [in alphabetical order]) and performed in accordance to the Declaration of Helsinki. Written informed consent was obtained from all patients.
Clinical and Dialysis Parameters
In the CRC for ESRD study, baseline demographic and clinical data including age, sex, height, weight, body mass index (BMI), causes of ESRD, comorbidity, laboratory investigations, and therapeutic characteristics were recorded. Cardiovascular disease was defined as presence of one or more of the coronary heart disease, congestive heart failure, peripheral vascular disease, and cerebrovascular disease. Serum hemoglobin, serum albumin, serum creatinine, blood urea nitrogen, serum potassium, serum total cholesterol, serum calcium, serum phosphorous, high-sensitivity C-reactive protein (hsCRP) and serum intact-parathyroid hormone (iPTH) were measured. The estimated glomerular filtration rate (eGFR) was calculated at the time of dialysis initiation using the Modification of Diet in Renal Disease 4-variable equation: eGFR = 175 × serum creatinine − 1.154 × age − 0.203 × 1.212 (if black) × 0.742 (if female).
Serum calcium levels were measured by using the chromophore 5-nitro-5′-methyl-(1,2-bis(o-aminophenoxy)ethan-N,N,N′,N′-tetraacetic acid (NM-BAPTA), and serum phosphorus levels were measured using Roche modular P analyzer. The corrected calcium was calculated using the following equation: corrected calcium (mg/dL) = 0.8 × (4-serum albumin level [g/L]) + serum calcium (mg/dL). The single-pool Kt/V (spKt/V) was determined by a 2-point urea modeling based on the intradialytic reduction in blood urea and intradialytic weight loss. The composition of the dialysate that was prescribed at the day of enrollment was recorded. Detailed data on medication, such as calcium-containing or noncalcium-containing phosphate binders, active vitamin D compounds, calcimimetics, angiotensin-converting enzyme inhibitor (ACEi), and angiotensin receptor blocker (ARB), were recorded in the clinical database.
For analysis of the effect of DCa concentration on mortality, the patients were categorized into 3 groups according to the postdiluted DCa concentration: high DCa was defined as a concentration of 3.5 mEq/L, mid-DCa as 3.0 mEq/L, and low DCa as 2.5 to 2.6 mEq/L.
Outcomes
The primary outcome of this study was all-cause mortality and the secondary outcome was cardiovascular and infection-related hospitalization. For each death and hospitalization, the clinical center principal investigator completed a form that included cause of death and hospitalization according to the CRC for ESRD study classification.
Statistical Analyses
Data with continuous variables and normal distribution are presented as mean ± SD, and those without normal distribution are presented as the median with ranges, as appropriate for the type of variable. The Student t test, Mann–Whitney U test, one-way analysis of variance (ANOVA), or Kruskal–Wallis test were used as appropriate to determine the significance of differences among continuous variables. Categorical variables are presented as percentages. The Pearson chi-square test or Fisher exact test were used to analyze the differences among categorical variables.
Since patients in this study were not randomly assigned to high, mid, or low DCa, we used the propensity score to reduce potential confounding and selection biases. Propensity scores were calculated using multivariate logistic regression to estimated probability of using high DCa versus mid or low DCa. The covariates of age, sex, BMI, diabetes mellitus, cardiovascular disease, serum levels of hemoglobin, albumin, albumin-corrected calcium, and phosphorus were included in the propensity score model. Propensity scores were then used to match patients with high DCa to patients with mid and low DCa using a greedy nearest-neighbor matching algorithm. Patients without a corresponding match were excluded. We analyzed all available data without imputation of missing values. Propensity score matching was performed with SAS (version 9.2, SAS Inc., Cary, NC).
Absolute mortality rates were calculated per 100 person-years of follow-up. The primary outcome was all-cause mortality. All patients were followed until death (event) or the end of the study, with censoring of data at the time that a patient underwent renal transplantation or was lost to follow-up because of refusal of further participation or transfer to a nonparticipating hospital. The survival curves were estimated by the Kaplan–Meier method and compared by the log-rank test among low, mid, and high DCa groups. The Cox proportional-hazard regression model was used to calculate hazard ratio (HR) with 95% confidence interval (CI) for all-cause mortality. The Cox models were adjusted for significant or nearly significant (P < 0.1) predictor for all-cause mortality in univariate Cox regression analysis including age, cardiovascular diseases, BMI, diastolic blood pressure (BP), eGFR, serum levels of albumin, albumin-corrected serum calcium, phosphorus and intact PTH, the use of calcitriol or vitamin D analogs, the use of calcium-containing phosphate binder, and types of vascular access. We checked the relevant interactions between the variables such as those between serum albumin and albumin-corrected calcium, and no interaction between them were seen. A linear mixed model was used to compare the impact on the albumin-corrected serum calcium levels, serum phosphorus levels, and serum-intact PTH levels over time after initiation of HD, using the mid-DCa as the reference category. A value of P < 0.05 was considered as statistically significant. All statistical analyses were performed using the SPSS 11.5 software (Chicago, IL).
RESULTS
Patients Characteristics
Of the 1182 incident HD patients, 25.3 % (299 patients) were in the low DCa group, 59.3% (701 patients) were in the mid-DCa group, and 15.4% (182 patients) were in the high DCa group.
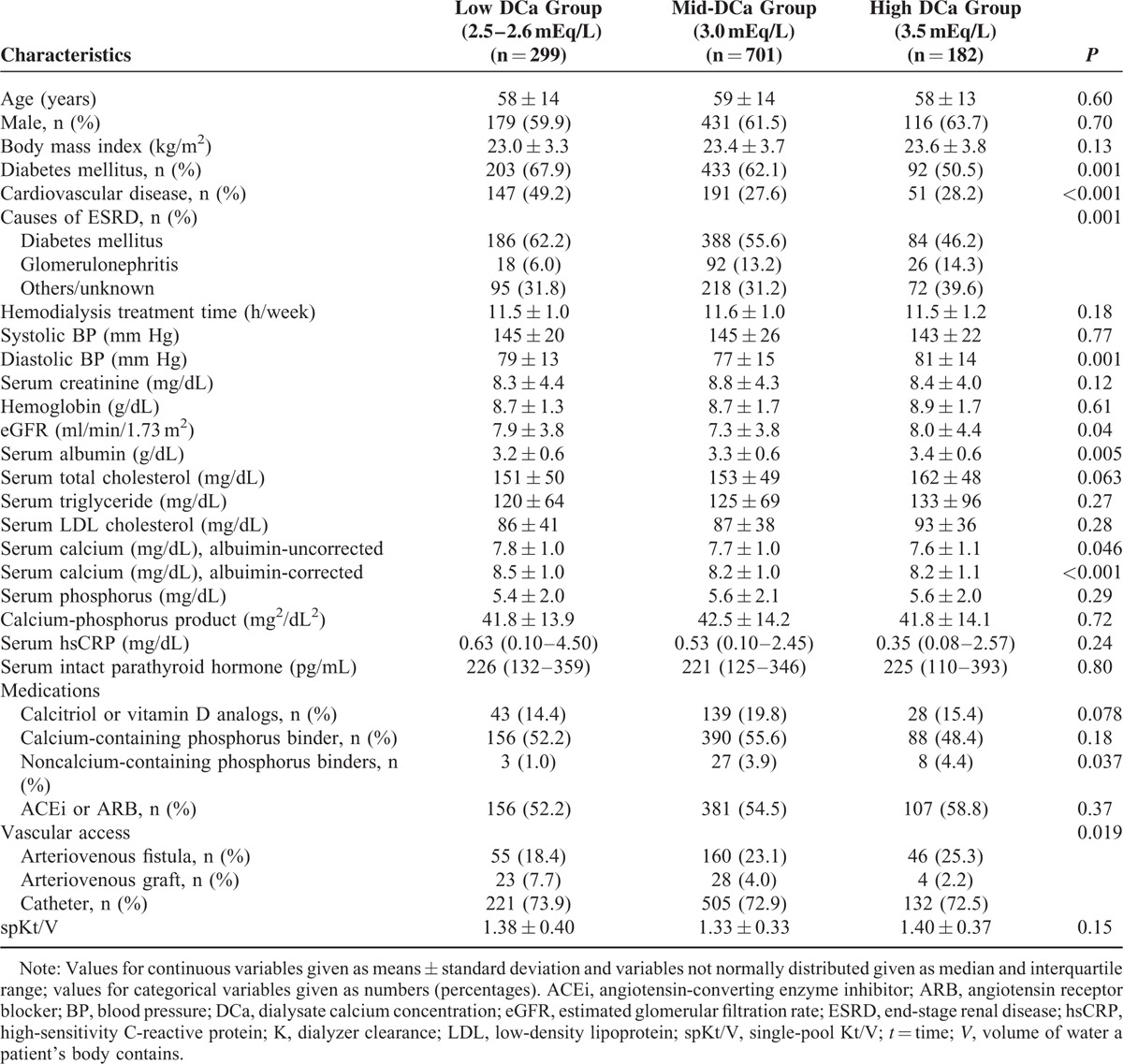
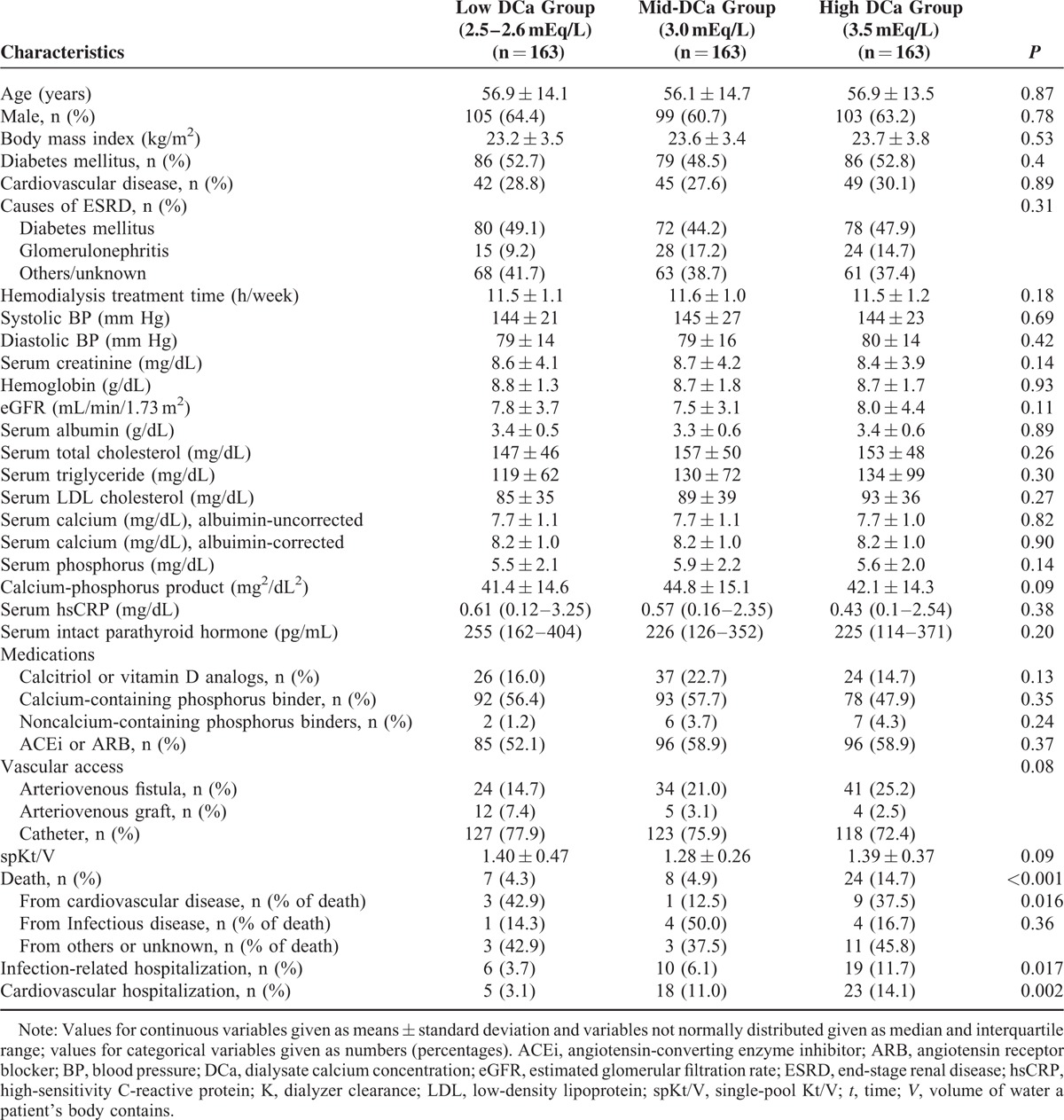
Table 1 shows the baseline characteristics of participants. There were no significant differences in age, sex, and BMI among the groups with low DCa, mid-DCa, and high DCa. There was a significant difference in the prevalence of the comorbidities among the groups. The low DCa group had higher prevalence of diabetes mellitus and cardiovascular disease compared with the other groups. The main causes of ESRD were diabetes mellitus (55.8 %) and glomerulonephritis (11.5%). Diabetes mellitus as a primary cause of ESRD was more prevalent in the low DCa group (62.2%). There was no significant difference in mean HD treatment time and systolic BP among the groups. The mid-DCa group had lower diastolic BP and eGFR at the commencing of HD compared with the other groups. The low DCa group had lower serum albumin levels. Serum levels of albumin-corrected and uncorrected calcium were higher in the low DCa group, compared with the groups with mid-DCa and high DCa. There were no significant differences among the 3 groups in the levels of serum hemoglobin, serum creatinine, serum total cholesterol, serum triglyceride, serum low-density lipoprotein cholesterol (LDL-C), serum phosphorus, hsCRP, and serum iPTH. There was no difference in the use of calcitriol or vitamin D analogs, the use of ACEi or ARB, and calcium-containing phosphorus binder at the time of enrollment among the 3 groups. The use of noncalcium-containing phosphorus binder in the low DCa group is lower than in the mid-DCa and high DCa group. Calcimimetics were not used in patients in this study. The use of arteriovenous fistula as vascular access was more prevalent in the high DCa group. There were no significant differences in spKt/V among the groups.
TABLE 1.
Baseline Characteristics of Incident HD Patients According to the Dialysate Calcium Concentration
Effect of Dialysate Calcium Concentration on All-cause Mortality
The median follow-up period was 16 months (interquartile range 5–32 months) in the whole cohort. The median follow-up period in each group was 17 months (interquartile range 7–29 months) in the low DCa group, 15 months (interquartile range 5–32 months) in the mid-DCa group, and 19 months (interquartile range 4–39 months) in the high DCa group. There was no difference in the follow-up period among the 3 groups (P = 0.22). During the follow-up period, 446 patients left the study for reasons other than death. The reasons for censoring included kidney transplantation (n = 53), transfer to a nonparticipating hospital (n = 221), refusal of further participation (n = 76), and others (n = 96). There were 93 deaths during the follow-up period. The leading causes of death were cardiovascular diseases (34 deaths, 36.6% of all deaths) and infectious diseases (26 deaths, 28.0% of all deaths). Table 2 shows the causes of deaths in each group. There was no significant difference in the causes of death among the 3 groups (P = 0.14). The absolute mortality during the follow-up period was 4.8 deaths per 100 person-years.
TABLE 2.
Causes of Deaths in Each Group
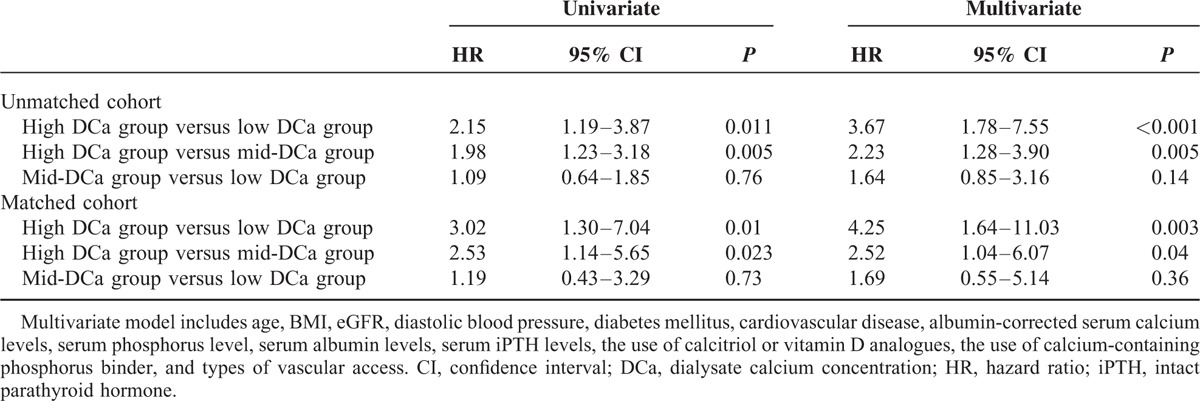
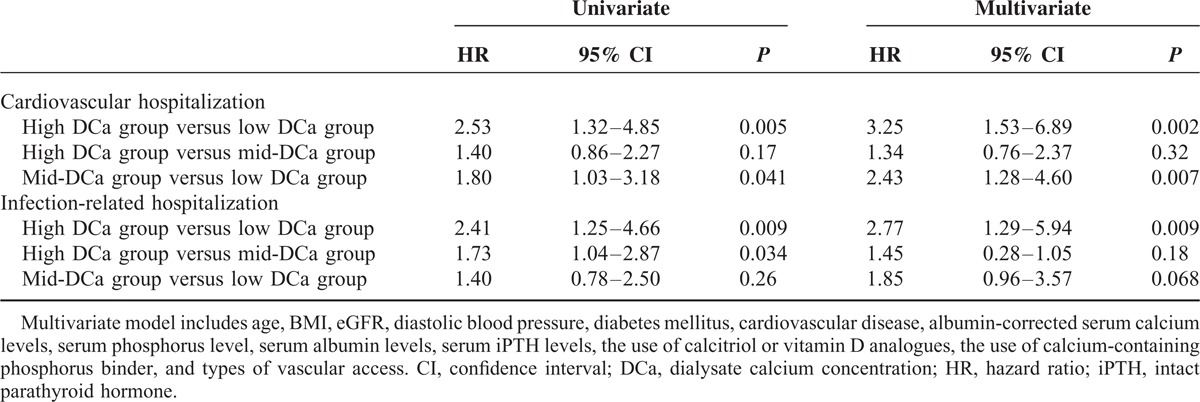
Figure 1 shows the Kaplan–Meier plot of patient survival according to the DCa group. As shown, survival was decreased in patients in the high DCa group compared with those in the low DCa group and mid-DCa group (P = 0.007 by log-rank test). Table 3 shows the univariate Cox regression analysis for mortality. In univariate Cox proportional-hazard models, age, BMI, diabetes mellitus, cardiovascular disease, serum albumin level, serum iPTH level, albumin-corrected serum calcium levels, serum phosphorus levels, the use of calcium-containing phosphorus binder, and type of vascular access and high DCa were associated with all-cause mortality. Table 4 shows the univariate and multivariate Cox regression analysis of mortality according to DCa concentrations. In univariate analysis, the HR for all-cause mortality of the high DCa group was 2.15 (95% CI 1.19–3.87, P = 0.011) using the low DCa group as the reference category and 1.98 (95% CI 1.23–3.18, P = 0.005) using the mid-DCa group as the reference category. There was no significant difference in all-cause mortality between the low DCa group and the mid-DCa group (HR 1.09, 95% CI 0.64–1.85, P = 0.76). In multivariate analysis, the high DCa group had independently significant association with increased all-cause mortality compared with the low DCa group (HR 3.67, 95% CI 1.78–7.55, P < 0.001) and compared with the mid-DCa group (HR 2.23, 95% CI 1.28–3.90, P = 0.005) after adjusting for age, BMI, diastolic BP, diabetes mellitus, cardiovascular disease, eGFR, albumin-corrected serum calcium levels, serum phosphorus level, serum albumin levels, serum iPTH levels, the use of calcitriol or vitamin D analogs, the use of calcium-containing phosphorus binder, and types of vascular access. There was no significant difference in all-cause mortality between the low DCa group and the mid-DCa group after adjustment for the potential confounders (HR 1.64, 95% CI 0.85–3.16, P = 0.14).
FIGURE 1.
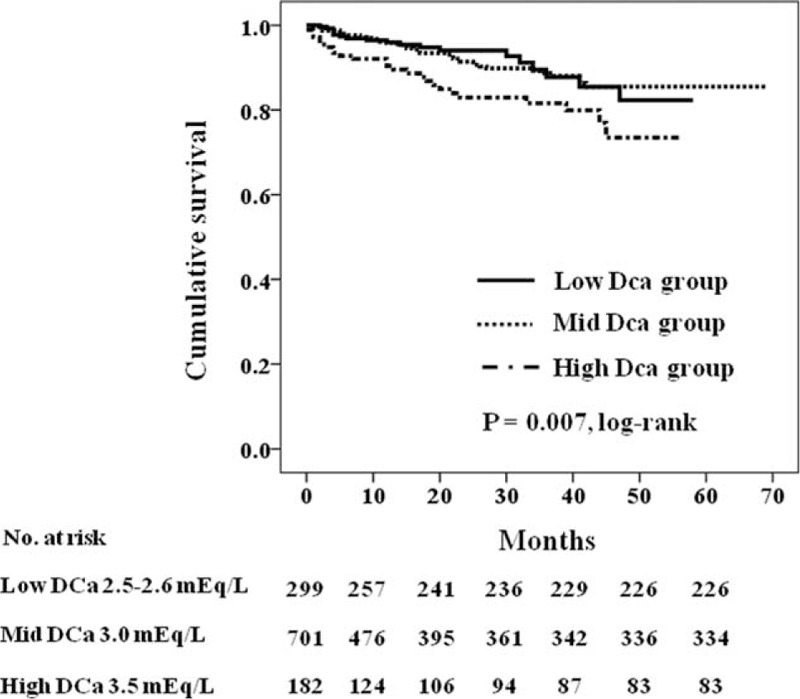
Kaplan–Meier survival curve for mortality according to dialysate calcium concentration (P = 0.007 by log-rank test).
TABLE 3.
Univariate Cox Regression Analysis for Mortality
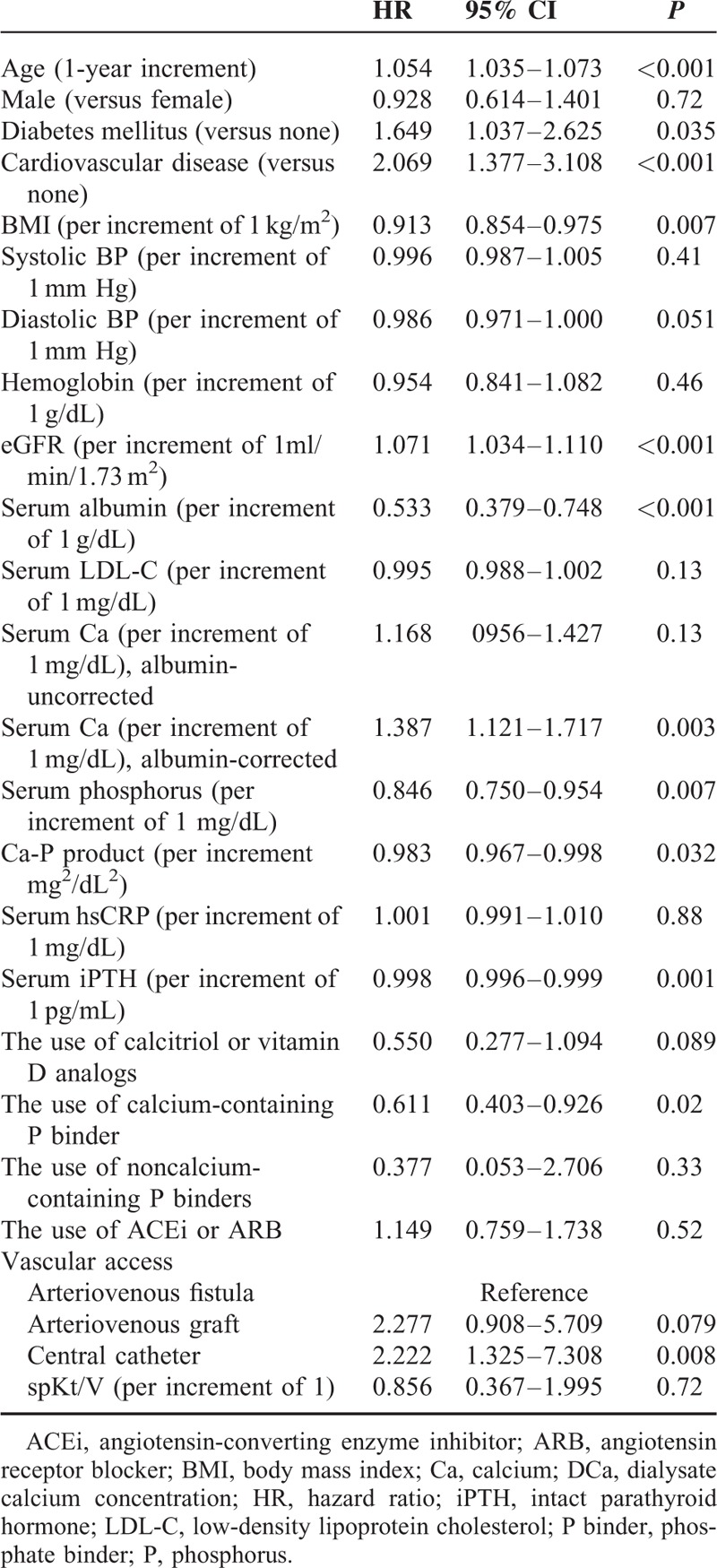
TABLE 4.
Univariate and Multivariate Cox Regression Analysis of All-cause Mortality According to Dialysate Calcium Concentrations
Of the 1182 patients, 163 patients in the high DCa group were matched with 163 patients in the mid-DCa group and the low DCa group. Following propensity score matching, the 3 groups were well matched and no significant differences in demographic characteristics or underlying diseases were observed among the 3 groups (Table 5). In the propensity score-matched analysis, the high DCa group had a significantly higher risk of all-cause mortality compared with the low DCa group (HR 4.25, 95% CI 1.64–11.03, P = 0.003) and compared to the mid-DCa group (HR 2.52, 95% CI 1.04–6.07, P = 0.04) after adjustment for age, BMI, diastolic BP, diabetes mellitus, cardiovascular disease, eGFR, albumin-corrected serum calcium levels, serum phosphorus level, serum albumin levels, serum iPTH levels, the use of calcitriol or vitamin D analogs, the use of calcium-containing phosphorus binder, and types of vascular access (Table 4).
TABLE 5.
Baseline Characteristics and Clinical Outcome of Incident HD Patients According to the Dialysate Calcium Concentration After Propensity Score Matching
Effect of Dialysate Calcium Concentration on Cardiovascular and Infection-related Hospitalization
We investigated the impact of DCa concentration on cardiovascular and infection-related hospitalization. Table 6 shows the distribution of causative diseases in patients with cardiovascular and infection-related hospitalization during the follow-up period. Ischemic heart disease and congestive heart failure were common causes of cardiovascular hospitalization, and respiratory infection was the most common cause of infection-related hospitalization. Figures 2 and 3 show the Kaplan–Meier plot of cardiovascular and infection-related hospitalization according to the DCa concentration. The log-rank test showed cardiovascular and infection-related hospitalization rates were significantly increased in the high DCa group (P = 0.017 and 0.018, respectively). Table 7 shows the univariate and multivariate Cox regression analysis for cardiovascular and infection-related hospitalization. In the crude model, the HRs for cardiovascular and infection-related hospitalization of the high DCa group were 2.53 (95% CI 1.32–4.85, P = 0.005) and 2.41 (95% CI 1.25–4.66, P = 0.009), using the low DCa group as the reference category. In multivariate Cox regression analysis, the high DCa group was associated with higher risk of cardiovascular and infection-related hospitalization compared with the low DCa group (HR 3.25, 95% CI 1.53–6.89, P = 0.002; and HR 2.77, 95% CI 1.29–5.94, P = 0.009, respectively). There was no significant difference in cardiovascular and infection-related hospitalization between high DCa group and the mid-DCa group.
TABLE 6.
Distribution of Causative Diseases in Patients With Cardiovascular and Infection-related Hospitalization During the Follow-up Period
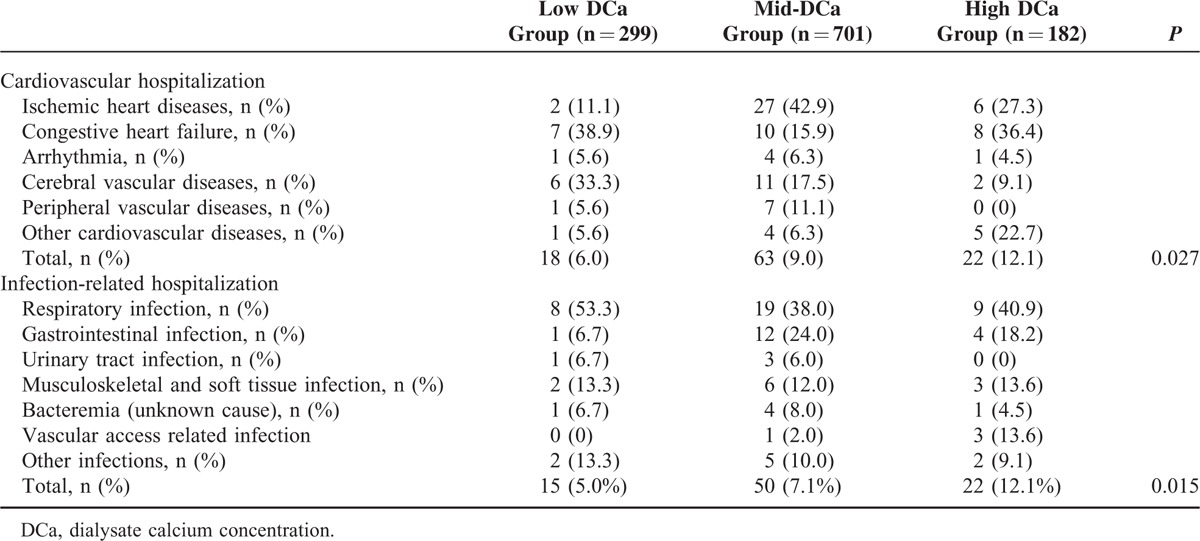
FIGURE 2.
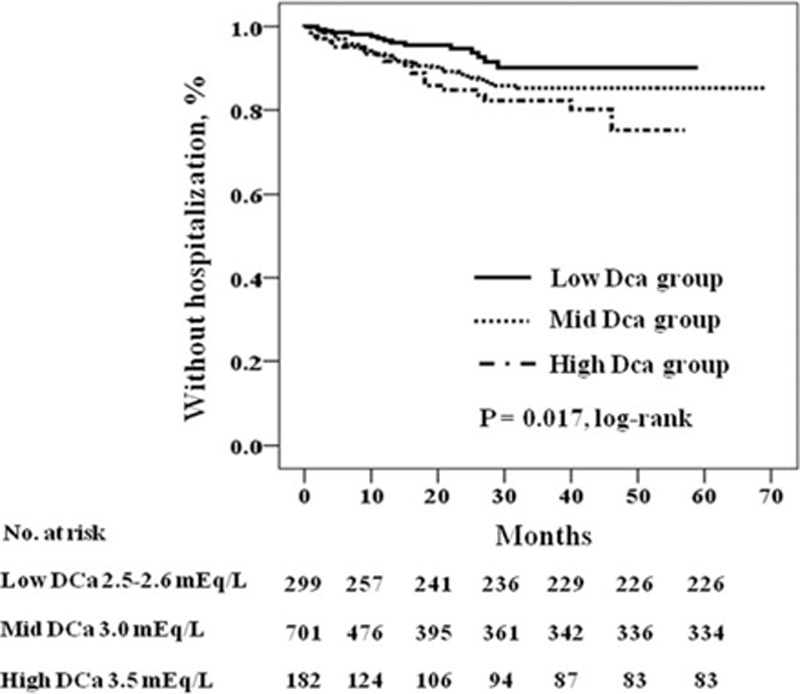
Kaplan–Meier plot of cardiovascular hospitalization according to dialysate calcium concentration (P = 0.017 by log-rank test).
FIGURE 3.
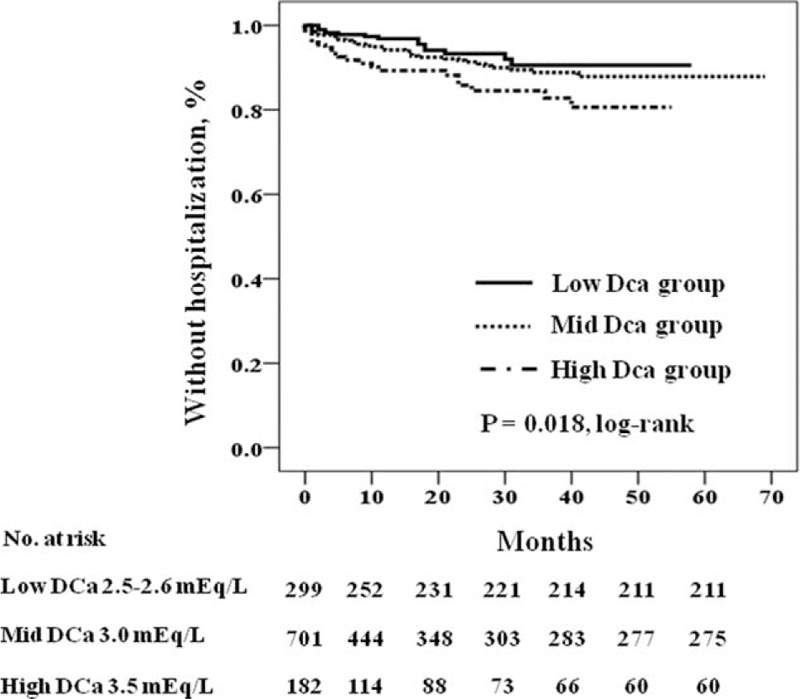
Kaplan–Meier plot of infection-related hospitalization according to dialysate calcium concentration (P = 0.018 by log-rank test).
TABLE 7.
Univariate and Multivariate Cox Regression Analysis of Cardiovascular and Infection-Related Hospitalization According to Dialysate Calcium Concentrations
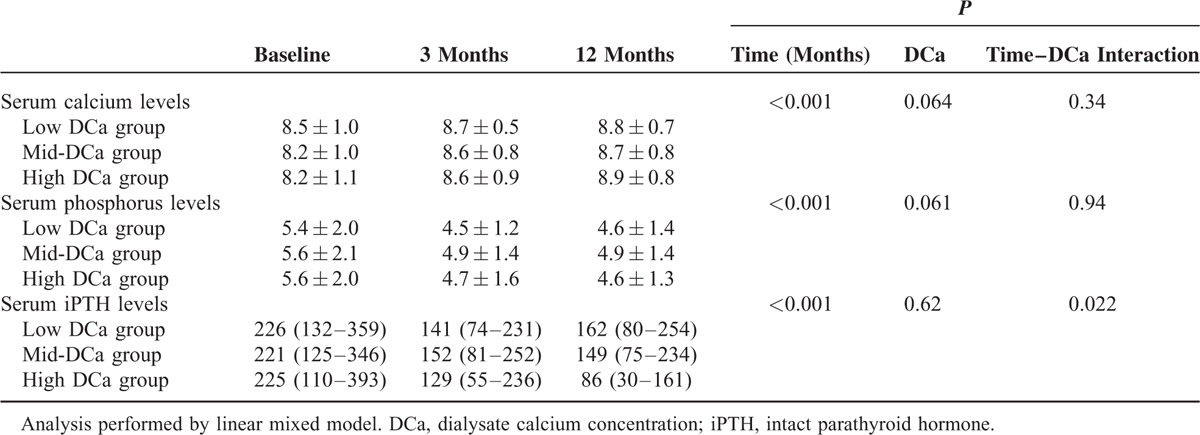
Changes in Serum Levels of Calcium, Phosphorus, and iPTH Over Time
The changes of serum levels of calcium, phosphorus, and iPTH over time among DCa groups may influence mortality and morbidity. Therefore, we determined whether baseline DCa affects the change of serum levels of calcium, phosphorus, and iPTH over time after initiation of HD. Table 8 shows that there is significant effect of DCa on changes of serum iPTH levels over time (P = 0.022 for time–DCa interaction). However, there was no significant effect of DCa on change of serum calcium levels (P = 0.34 for time–DCa interaction) and serum phosphorus levels over time (P = 0.94 for time–DCa interaction).
TABLE 8.
Changes of Albumin-Corrected Serum Calcium Levels, Serum Phosphorus Levels and iPTH Levels Over Time According to Dialysate Calcium Concentration
DISCUSSION
In this multicenter prospective observational study, we showed that high DCa concentration was associated with increased all-cause mortality, compared with mid and low DCa concentrations in the whole study cohort, and also propensity score-matched cohort. Furthermore, high DCa concentration had higher risk of cardiovascular and infection-related hospitalization.
These findings are consistent with those of the DOPPS2 and Hsu et al's13 study, but not with those of the regional ARNOS French cohort.8 The strength of our study compared with previous studies is that only incident HD patients were included. Previous studies included either only prevalent HD patients or both incident and prevalent HD patients.2,8,13 Through the enrollment of only incident HD patients, our study could avoid early mortality bias and carryover effects of previous exposure to dialysates with various ranges of calcium concentration.
The exact mechanisms underlying the association between mortality and high DCa are unclear. However, a plausible explanation is that a positive calcium balance (increased calcium load) resulting from a high DCa may contribute to increased mortality. Dialysis calcium balance, defined as dialysis calcium mass transfer from and toward the dialysate fluid during the dialysis treatment, is mainly determined by the gradient between the dialysate and serum calcium concentrations and the ultrafiltration volume.11,14 It is generally accepted that dialysis calcium balance is consistently positive when a DCa concentration is at least 3.0 mEq/L.14–17 In this study, DCa concentration did not influence the change of serum levels of calcium and phosphorus over time. Therefore, it may be assumed that increased mortality and morbidity in the high DCa group was not related to changes in serum calcium and phosphorus levels over time (Table 8). Repeated exposure to increased calcium load has been associated with arterial calcification and stiffness.18,19 Arterial calcification and stiffness are consistently linked to an increased risk of mortality in HD patients.20,21 Thus, it may be postulated that the transient increase of serum calcium level just after each HD session in the high DCa group may result in arterial calcification and stiffness, which may contribute to increased mortality and morbidity. Interestingly, high DCa excessively decreases serum iPTH levels compared with mid-DCa or low DCa in this study. In consideration that very low iPTH levels are associated with increased risk of vascular calcification and mortality,22 excessive decrease of serum iPTH levels may also contribute to higher risk of mortality and morbidity. Further study is needed to clarify the influence of high DCa on vascular calcification as an explanation for the association between high DCa and mortality. Additionally, increased calcium load is associated with accelerated apoptosis, impaired phagocytic function, and an attenuated oxidative burst in blood neutrophils, which may contribute to the increased incidence of infection-related complications and mortality in dialysis patients.23,24 In our study, high DCa concentration was associated with higher risk of infection-related hospitalization.
Another interesting finding of our study is that there was no significant difference in all-cause mortality and infection-related hospitalization between the low DCa and mid-DCa groups. But, mid-DCa concentration was associated with higher risk of cardiovascular hospitalization compared with low DCa concentration. The prescription of mid-DCa or low DCa varies widely among different countries. In general, low DCa is predominantly used in the United States and mid-DCa is predominantly used in the European countries and Japan.2,9,12 In our study, HD using mid-DCa was more prevalent than HD using low DCa (65.6% vs. 17.6 %, respectively).
Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines have offered an opinion-based recommendation of low DCa as a means to maintain neutral calcium balance and prevent vascular calcification.4 However, there are few data comparing mortality between low DCa and mid-DCa, and it is not clear which type of DCa is more beneficial for survival.
DOPPS showed that lower DCa had survival benefit.2 However, The Japan dialysis outcomes and practice patterns study (J-DOPPS) reported that the all-cause mortality risk was higher with low DCa than with mid-DCa, although it was significant.9 Furthermore, Pun et al12 recently reported that very low DCa (DCa <2.5 mEq/L) is associated with increased risk of sudden cardiac arrest. Our findings suggest that low DCa and mid-DCa have equivalent impact on all-cause mortality. Considering this controversy, the prescription of DCa might depend on the specific conditions of individual patients rather than fixing one single type of DCa.
Therapeutic regimens such as calcium-containing phosphate binder, noncalcium-containing phosphate binder, and calcitriol or vitamin D analogs may affect a patient's calcium mass balance and mortality in patients with HD treatment.25 In our study, at the time of enrollment, there was no difference in the use of calcium-containing phosphate binder or calcitriol or vitamin D analogs among the groups (Table 1). The use of noncalcium-containing phosphate binder was more prevalent in the mid-DCa group and high DCa group than in the low DCa. When these regimens were adjusted in our study, the mortality among the low DCa, mid-DCa, and high DCa groups still remained (Table 4). Therefore, we cautiously considered that baseline prescription of regimens may not influence the association between DCa and mortality. However, these interpretations may be limited because of the lack of data for the change of prescription of the regimens.
Our study has some potential limitations. First, the design of our study was not a randomized, controlled study. In this study, patients were prescribed DCa concentrations according to the treatment policy of each center and the preference of treating physician, which may influence the results of our study. To evaluate the “center effect,” we compared all-cause mortality with and without adjusting for center. No significant center effect on all-cause mortality was observed (data not shown), which suggests that the center effect was not significant in this study. We performed a complementary analysis by propensity score methods to reduce potential confounding and selection biases. In the study of propensity score-matched cohort, the high DCa group had a significantly higher risk of all-cause mortality compared with the mid-DCa group and the low DCa group, which was consistent with the result of the unmatched cohort. Second, despite the multicenter nature of the study, the cohort consisted of only Korean patients and all were Asian. Thus, it is uncertain whether our results can be generalized to other ethnic groups. Third, data on vascular calcification, bone turnover markers, ionized calcium level, overall calcium balance, and vitamin D level, which may be helpful for investigating the mechanism for the association between DCa and mortality, were not available in this study. Fourth, data for the changes in DCa prescription in each dialysis center were not available. The lack of an accurate reflection of changing therapeutic practice on DCa prescription may be criticized. However, most dialysis centers have used a fixed DCa concentration, and most patients have been prescribed a fixed DCa concentration for a long time.8,26
In conclusion, our study demonstrated that HD using high DCa is independently associated with increased all-cause mortality compared with HD using low DCa or mid-DCa, and there was no significant difference in all-cause mortality between low DCa and mid-DCa in incident HD patients. Although the prescription of DCa should be individualized to meet the specific condition of HD patients, our findings cautiously suggest that careful attention is necessary in the use of high DCa and efforts to reduce the overuse of high DCa may be helpful to decrease mortality.
Acknowledgments
We thank the study coordinators Hye Young Lim, Nam Hee Kim, Mi Joung Moon, Hwa Young Lee, Mi Joung Kwon, Su Yeon An, Su Joung Oh, and Hye Young Kwak for contribution to this study, and Jung Wha Hong (Biostatistics Collaboration Unit, Yonsei University College of Medicine) for statistical consultation.
Footnotes
Abbreviations: ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor blocker, BMI = body mass index, BP = blood pressure, Ca-P product = calcium–phosphorus product, CI = confidence interval, CKD-MBD = chronic kidney disease-mineral and bone disorder, CRC = Clinical Research Center, DCa = dialysate calcium concentration, DOPPS = Dialysis Outcome Practice Pattern Study, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, HD = hemodialysis, HR = hazard ratio, hsCRP = high-sensitivity C-reactive protein, iPTH = intact parathyroid hormone, J-DOPPS = Japan dialysis outcomes and practice patterns study, K = dialyzer clearance, K/DIGO = Kidney Disease Improving Global Outcomes, K/DOQI = Kidney Disease Outcomes Quality Initiative, LDL-C = low-density lipoprotein cholesterol, spKt/V = single-pool Kt/V, t = time, V = volume of water a patient's body contains.
Funding: This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2014; 15:2208–2218. [DOI] [PubMed] [Google Scholar]
- 2.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2005; 67:1179–1187. [DOI] [PubMed] [Google Scholar]
- 3.London GM, Guérin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003; 18:1731–1740. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42 (4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; 113:S1–S130. [DOI] [PubMed] [Google Scholar]
- 6.Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 2010; 55:773–799. [DOI] [PubMed] [Google Scholar]
- 7.Kooman J, Basci A, Pizzarelli F, et al. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant 2007; 22 (Suppl 2):ii22–ii44. [DOI] [PubMed] [Google Scholar]
- 8.Jean G, Lataillade D, Genet L, et al. Higher dialysate calcium is not associated with mortality in hemodialysis patients: results from the French ARNOS study. Nephrol Ther 2013; 9:103–107. [DOI] [PubMed] [Google Scholar]
- 9.Kimata N, Albert JM, Akiba T, et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int 2007; 11:340–348. [DOI] [PubMed] [Google Scholar]
- 10.Kong X, Zhang L, Zhang L, et al. Mineral and bone disorder in Chinese dialysis patients: a multicenter study. BMC Nephrol 2012; 13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messa P. The ups and downs of dialysate calcium concentration in haemodialysis patients. Nephrol Dial Transplant 2013; 28:3–7. [DOI] [PubMed] [Google Scholar]
- 12.Pun PH, Horton JR, Middleton JP. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu CW, Lin JL, Lin-Tan DT, et al. High-calcium dialysate: a factor associated with inflammation, malnutrition and mortality in non-diabetic maintenance haemodialysis patients. Nephrology (Carlton) 2010; 15:313–320. [DOI] [PubMed] [Google Scholar]
- 14.Malberti F, Ravani P. The choice of the dialysate calcium concentration in the management of patients on haemodialysis and haemodiafiltration. Nephrol Dial Transplant 2003; 18 (Suppl 7):vii37–vii40. [DOI] [PubMed] [Google Scholar]
- 15.Bosticardo G, Malberti F, Basile C, et al. Optimizing the dialysate calcium concentration in bicarbonate haemodialysis. Nephrol Dial Transplant 2012; 27:2489–2496. [DOI] [PubMed] [Google Scholar]
- 16.Hou SH, Zhao J, Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 1991; 18:217–224. [DOI] [PubMed] [Google Scholar]
- 17.Sigrist M, McIntyre CW. Calcium exposure and removal in chronic hemodialysis patients. J Ren Nutr 2006; 16:41–46. [DOI] [PubMed] [Google Scholar]
- 18.Kyriazis J, Katsipi I, Stylianou K, et al. Arterial stiffness alterations during haemodialysis: the role of dialysate calcium. Nephron Clin Pract 2007; 106:c34–c42. [DOI] [PubMed] [Google Scholar]
- 19.LeBoeuf A, Mac-Way F, Utescu MS, et al. Impact of dialysate calcium concentration on the progression of aortic stiffness in patients on haemodialysis. Nephrol Dial Transplant 2011; 26:3695–3701. [DOI] [PubMed] [Google Scholar]
- 20.Noordzij M, Cranenburg EM, Engelsman LF. NECOSAD Study Group Progression of aortic calcification is associated with disorders of mineral metabolism and mortality in chronic dialysis patients. Nephrol Dial Transplant 2011; 26:1662–1669. [DOI] [PubMed] [Google Scholar]
- 21.Verbeke F, Van Biesen W, Honkanen E. CORD Study Investigators Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol 2011; 6:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jean G, Lataillade D, Genet L, et al. ARNOS study investigators Association between very low PTH levels and poor survival rates in haemodialysis patients: results from the French ARNOS cohort. Nephron Clin Pract 2011; 118:c211–c216. [DOI] [PubMed] [Google Scholar]
- 23.Rivara MB, Ravel V, Kalantar-Zadeh K, et al. Uncorrected and albumin-corrected calcium, phosphorus, and mortality in patients undergoing maintenance dialysis. J Am Soc Nephrol 2015; 26:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hörl WH, Haag-Weber M, Mai B, et al. Verapamil reverses abnormal [Ca2+]i and carbohydrate metabolism of PMNL of dialysis patients. Kidney Int 1995; 47:1741–1745. [DOI] [PubMed] [Google Scholar]
- 25.Gotch FA, Kotanko P, Thijssen S, et al. The KDIGO guideline for dialysate calcium will result in an increased incidence of calcium accumulation in hemodialysis patients. Kidney Int 2010; 78:343–350. [DOI] [PubMed] [Google Scholar]
- 26.Zhang DL, Wang LY, Sun F, et al. Is the dialysate calcium concentration of 1.75 mmol/L suitable for Chinese patients on maintenance hemodialysis? Calcif Tissue Int 2014; 94:301–310. [DOI] [PubMed] [Google Scholar]


